Investigation of Hydrogen Storage Characteristics of MgH2 Based Materials with Addition of Ni and Activated Carbon
Abstract
1. Introduction
2. Results
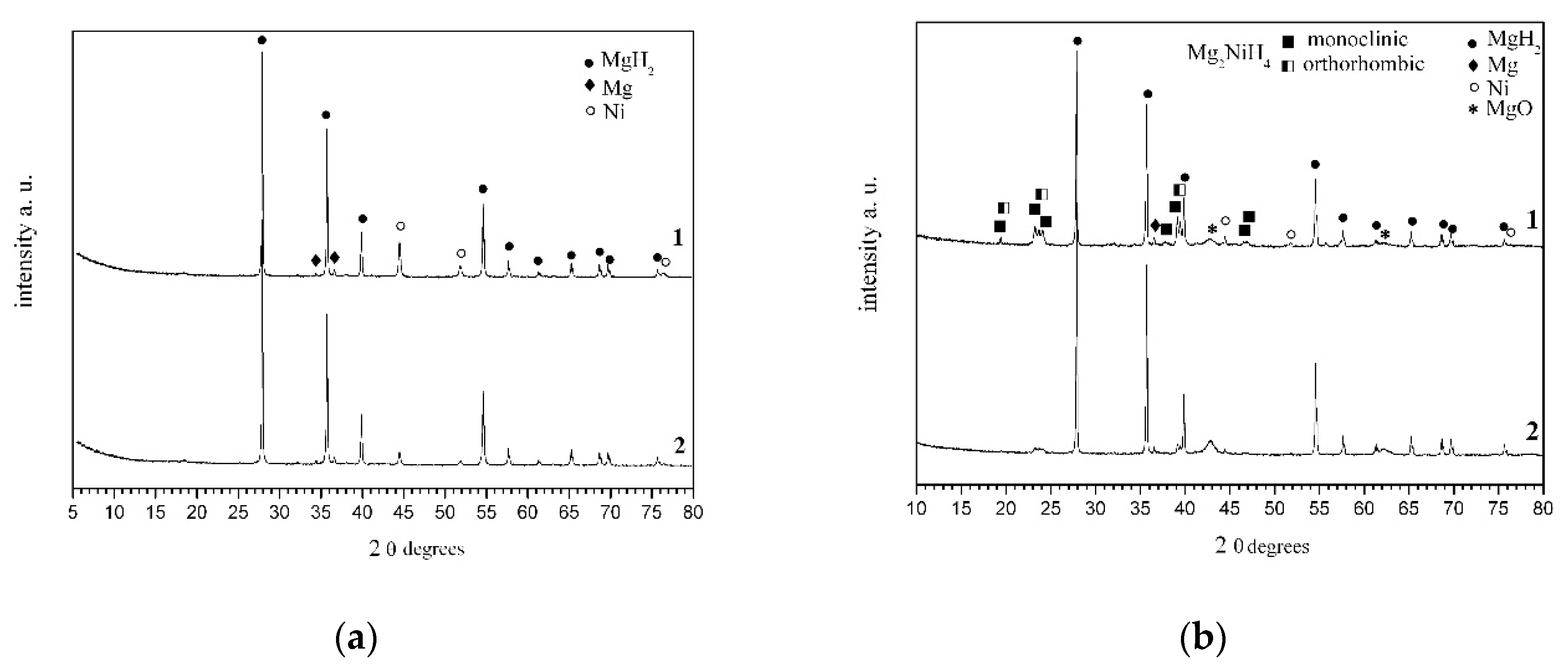
2.1. X-ray Powder Diffraction of Ball Milled 80 wt % MgH2-15 wt % Ni-5 wt % POW and 90 wt % MgH2-5 wt % Ni-5 wt % POW
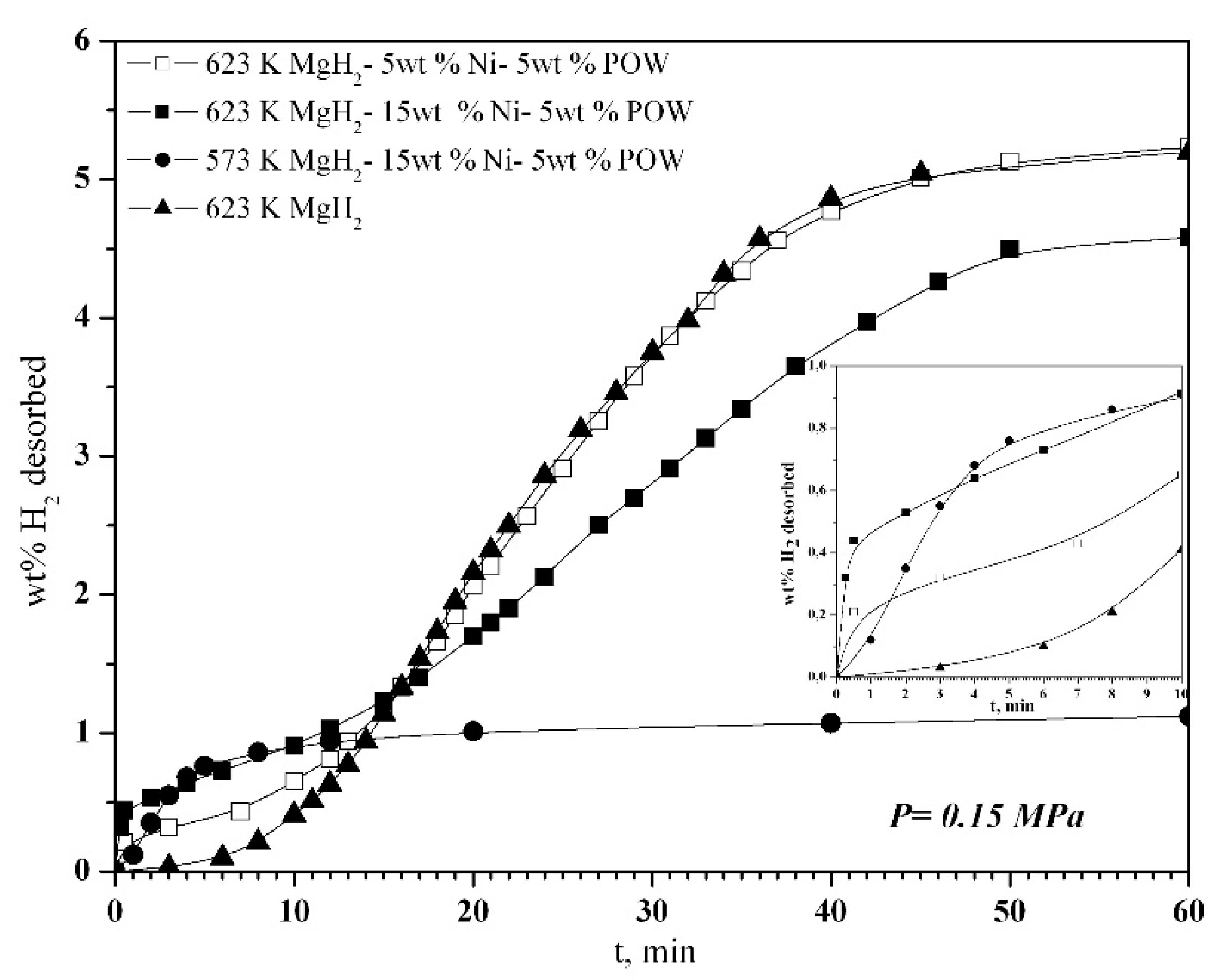
2.2. Hydrogen Absorption and Desorption Characteristics of 80 wt % MgH2-15 wt % Ni-5 wt % POW and 90 wt % MgH2-5 wt % Ni-5 wt % POW
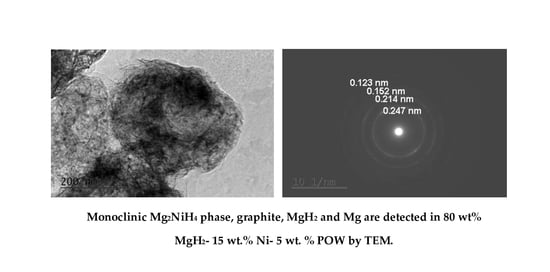
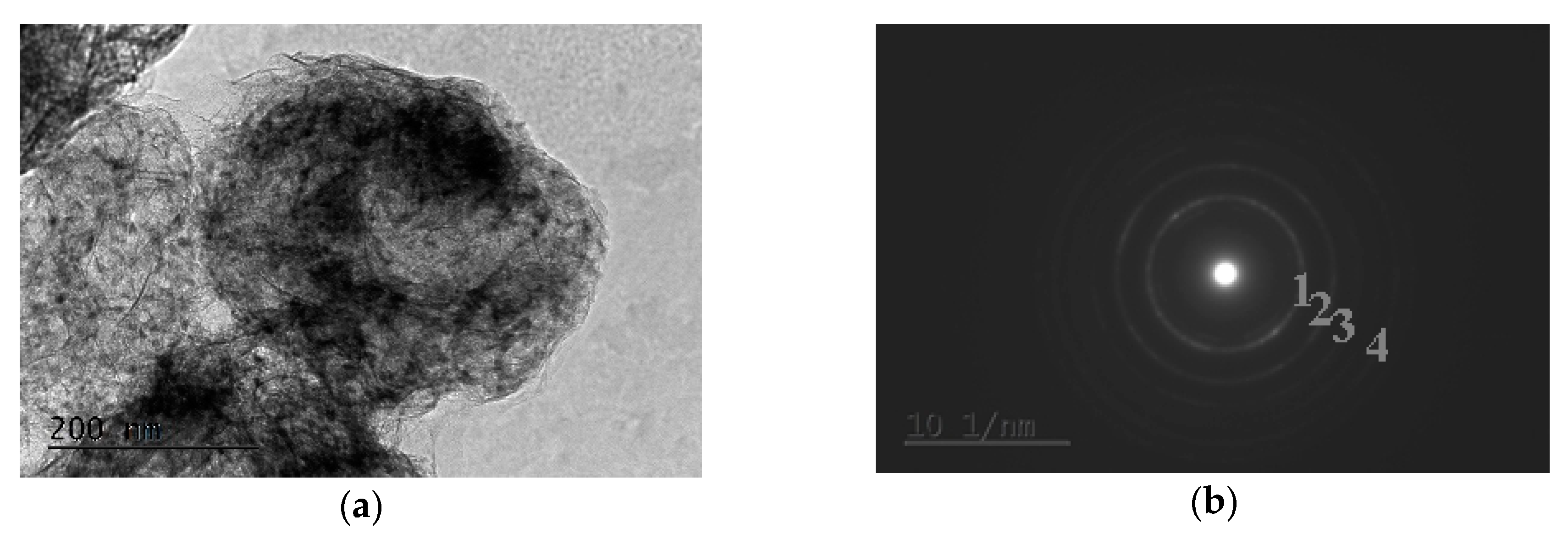
2.3. Transmission Electron Microscopy Characterization
2.4. X-ray Powder Diffraction of 80 wt % MgH2-15 wt % Ni-5 wt % POW and 90 wt % MgH2-5 wt % Ni-5 wt % POW after Hydrogenation at a Temperature of 573 K and Pressure of 1 MPa H2
3. Discussion
4. Materials and Methods
4.1. Ball Milling in a Planetary Mill
4.2. Hydrogen Sorption Measurements
4.3. X-ray Diffraction and TEM Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Progress Nat. Sci. Mater. Int. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Aguey-Zinsou, K.-F. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2018, 10, 168–198. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; Bellost von Colbe, J.M.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Wu, C.Z.; Wang, P.; Yao, X.; Liu, C.; Chen, D.M.; Lu, G.Q.; Cheng, H.M. Effect of carbon/noncarbon addition on hydrogen storage behaviors of magnesium hydride. J. Alloys Compd. 2006, 414, 259–264. [Google Scholar] [CrossRef]
- Fuster, V.; Castro, F.J.; Troiani, H.; Urretavizcaya, G. Characterization of graphite catalytic effect in reactively ball milled MgH2-C and Mg-C composites. Int. J. Hydrogen Energy 2011, 36, 9051–9061. [Google Scholar] [CrossRef]
- Gattia, D.M.; Montone, A.; Pasquini, L. Microstructure and morphology changes in MgH2/expanded natural graphite pellets upon hydrogen cycling. Int. J. Hydrogen Energy 2013, 38, 1918–1924. [Google Scholar] [CrossRef]
- Awad, A.S.; Tayeh, T.; Nakhl, M.; Zakhour, M.; Ourane, B.; Le Troëdec, M.; Bobet, J.-L. Effect of carbon type (graphite, CFs and diamond) on the hydrogen desorption of Mg-C powder mixtures under microwave irradiation. J. Alloys Compd. 2014, 607, 223–229. [Google Scholar] [CrossRef]
- Alsabawi, K.; Webb, T.A.; Gray, E.M.; Webb, C.J. The effect of C60 additive on magnesium hydride for hydrogen storage. Int. J. Hydrogen Energy 2015, 40, 10508–10515. [Google Scholar] [CrossRef]
- Grigorova, E.; Khristov, M.; Stoycheva, I.; Tsyntsarski, B. Effect of activated carbon from polyolefin wax on the hydrogen sorption properties of magnesium. Int. J. Hydrogen Energy 2017, 42, 26872–26876. [Google Scholar] [CrossRef]
- Thongtan, P.; Dansirima, P.; Thiangviriya, S.; Thaweelap, N.; Suthummapiwat, A.; Plerdsranoy, P.; Utke, R. Reversible hydrogen sorption and kinetics of hydrogen storage tank based on MgH2 modified by TiF4 and activated carbon. Int. J. Hydrogen Energy 2018, 43, 12260–12270. [Google Scholar] [CrossRef]
- Grigorova, E.; Khristov, M.; Stoycheva, I.; Tsyntsarski, B.; Nihtianova, D.; Markov, P. Effect of activated carbons derived from apricot stones or polyolefin wax on hydrogen sorption properties of MgH2. Bulg. Chem. Commun. 2017, 49, 109–114. [Google Scholar]
- Cermak, J.; David, B. Catalytic effect of Ni, Mg2Ni and Mg2NiH4 upon hydrogen desorption from MgH2. Int. J. Hydrogen Energy 2011, 36, 13614–13620. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T. Overview of processing of nanocrystalline hydrogen storage intermetallics by mechanical alloying/milling. Mater. Manuf. Processes 2002, 17, 129–156. [Google Scholar] [CrossRef]
- Polanski, M.; Nielsen, T.K.; Kunce, I.; Norek, M.; Płociński, T.; Jaroszewicz, L.R.; Gundlach, C.; Jensen, T.R.; Bystrzycki, J. Mg2NiH4 synthesis and decomposition reactions. Int. J. Hydrogen Energy 2013, 38, 4003–4010. [Google Scholar] [CrossRef]
- Martínez-Coronado, R.; Retuerto, M.; Torres, B.; Martínez-Lope, M.J.; Fernández-Díaz, M.T.; Alonso, J.A. High-pressure synthesis, crystal structure and cyclability of the Mg2NiH4 hydride. Int. J. Hydrogen Energy 2013, 38, 5738–574538. [Google Scholar] [CrossRef]
- Yahya, M.S.; Ismail, M. Synergistic catalytic effect of SrTiO3 and Ni on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2018, 43, 6244–6255. [Google Scholar] [CrossRef]
- House, S.D.; Vajo, J.J.; Ren, C.; Rockett, A.A.; Robertson, I.M. Effect of ball-milling duration and dehydrogenation on the morphology, microstructure and catalyst dispersion in Ni-catalyzed MgH2 hydrogen storage materials. Acta Mater. 2015, 86, 55–68. [Google Scholar] [CrossRef]
- Gkanas, E.I.; Damian, A.; Ioannidou, A.; Stoian, G.; Lupu, N.; Gjoka, M.; Makridis, S.S. Synthesis, characterisation and hydrogen sorption properties of mechanically alloyed Mg(Ni1−xMnx)2. Mater. Today Energy 2019, 13, 186–194. [Google Scholar] [CrossRef]
- Song, M.Y.; Baek, S.H.; Bobet, J.-L.; Kwon, S.N.; Hong, S.-H. Hydrogen-storage performance of an Mg-Ni-Fe alloy prepared by reactive mechanical grinding. J. Mater. Sci. 2009, 44, 4827–4833. [Google Scholar] [CrossRef]
- Grigorova, E.; Nihtianova, D.; Tsyntsarski, B.; Markov, P.; Stoycheva, I. Characterization and investigation of hydrogen storage properties of 80 wt % MgH2-15 wt %Ni-5 wt % POW. Int. Multidiscip. Sci. GeoConf. Surv. Geol. Min. Ecol. Manag. SGEM 2019, 19, 75–81. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorova, E.; Nihtianova, D.; Tsyntsarski, B.; Stoycheva, I. Investigation of Hydrogen Storage Characteristics of MgH2 Based Materials with Addition of Ni and Activated Carbon. Inorganics 2020, 8, 12. https://doi.org/10.3390/inorganics8020012
Grigorova E, Nihtianova D, Tsyntsarski B, Stoycheva I. Investigation of Hydrogen Storage Characteristics of MgH2 Based Materials with Addition of Ni and Activated Carbon. Inorganics. 2020; 8(2):12. https://doi.org/10.3390/inorganics8020012
Chicago/Turabian StyleGrigorova, Eli, Diana Nihtianova, Boyko Tsyntsarski, and Ivanka Stoycheva. 2020. "Investigation of Hydrogen Storage Characteristics of MgH2 Based Materials with Addition of Ni and Activated Carbon" Inorganics 8, no. 2: 12. https://doi.org/10.3390/inorganics8020012
APA StyleGrigorova, E., Nihtianova, D., Tsyntsarski, B., & Stoycheva, I. (2020). Investigation of Hydrogen Storage Characteristics of MgH2 Based Materials with Addition of Ni and Activated Carbon. Inorganics, 8(2), 12. https://doi.org/10.3390/inorganics8020012