Abstract
The investigation of the reactivity of six membered N-heterocyclic carbene 1,3-bis(2,4,6-trimethylphenyl)-3,4,5,6-tetrahydropyrimidin-1-ylidene (6-Mes) towards dialkylgallium and dialkylindium alkoxides/aryloxides has shown that both steric hindrances and donor properties of 6-Mes significantly influence the strength of M–C6-Mes bond, as well as the formation, structure and reactivity of Me2MOR(6-Mes) (M = Ga, In) complexes. While the reactions of simple dimethylgallium alkoxides with 6-Mes lead to the formation of stable monomeric Me2Ga(OCH2CH2OMe)(6-Mes) (1) and Me2GaOMe(6-Mes) complexes, the analogous Me2InOR(6-Mes) are unstable and disproportionate to methylindium alkoxides and Me3In(6-Mes) (2). The use of bulky alkoxide ligand—OCPh2Me or aryloxide ligand—OC6H4OMe allowed for the synthesis of stable Me2M(OCPh2Me)(6-Mes) (M = Ga (3) and In (4)) as well as Me2M(OC6H4OMe)(6-Mes) (M = Ga (5) and In (6)). The structures of 1–6 have been determined using both spectroscopic methods in solution and X-ray diffraction studies, which confirmed the effect of both steric hindrances and donor properties of 6-Mes on their structure and catalytic properties in the ring-opening polymerization (ROP) of rac-lactide.
1. Introduction
Over recent years, we have reported Me2MOR(NHC) (M = Ga [1,2], In [3]; NHC = N-heterocyclic carbene) complexes, which constitute the first examples of gallium and indium, as well as group 13 metal alkoxides, with NHCs [4]. Moreover, they represent isoselective catalysts for the ring-opening polymerization (ROP) of rac-lactide (rac-LA), which are additionally highly active at low temperatures. Therefore, they still constitute rare examples of isoselective catalysts operating at mild conditions, both among group 13 metal complexes [5,6,7,8,9], and beyond [10,11,12,13]. Importantly, both the structure and reactivity of Me2MOR(NHC) (M = Ga, In; NHC = SIMes, IMes) were found to be strongly dependent on the M–CNHC bond. While in the case of corresponding gallium complexes, a relatively strong Ga–CNHC bond led to stable complexes [1,2], in the case of mostly unstable dialkylindium alkoxides with NHCs, a significantly weaker In–CNHC bond was observed [3]. Consequently, in the ring-opening polymerization with Me2GaOR(NHC), Ga–CNHC bond remained intact, resulting in the exclusive insertion of rac-LA into Ga–Oalkoxide bond, similar to Zn [14,15,16], Al [17], as well as Ti and Zr [18,19,20], metal alkoxides with NHCs, which have been reported to polymerize rac-LA due to the insertion into M–Oalkoxide bond. On the other hand, rac-LA could insert, even at low temperature, into In–CNHC bond of Me2GaOR(NHC), considerably weaker in comparison with Ga–CNHC [3]. Analogously, the insertion of lactide into the M–CNHC bond of Mg alkoxides with alkoxide ligands possessing NHC termini [16], Me3Al(NHC) adducts [21], or yttrium and titanium complexes [22], have been reported so far.
Similarly to the observed effect of a metal on the corresponding M–CNHC bond, as well as the structure and ROP activity of discussed main group metal alkoxides with NHCs, we should expect a considerable effect of steric and electronic properties of NHC on both the strength and reactivity of the M–CNHC bond. Our initial studies revealed the adverse effect of steric hindrances of NHC on the synthesis of Me2GaOR(SIPr) as well as the formation and strength of Ga–CSIPr bond [2], which was additionally supported by the structure of Me3M(NHC) (M = Al, Ga, In) adducts [3,21,23]. However, the more detailed effect of both steric and electronic effect of NHC on the M–CNHC bond has not been addressed so far for main group metal alkoxides with NHCs, including Me2MOR(NHC) (M = Ga, In) complexes. In order to probe the effect of NHC on the latter, we chose 6-Mes—an N-heterocyclic carbene of stronger donor properties, along with the increased steric demand, in comparison with SIMes or IMes (Figure 1) [24,25]. While the increased steric hindrances of 6-Mes result from wider N–C–N angle, this should be responsible for the hybridization at carbene carbon and the higher energy of HOMO orbital of 6-Mes occupied by a lone pair of electrons, and therefore its donating properties [26].
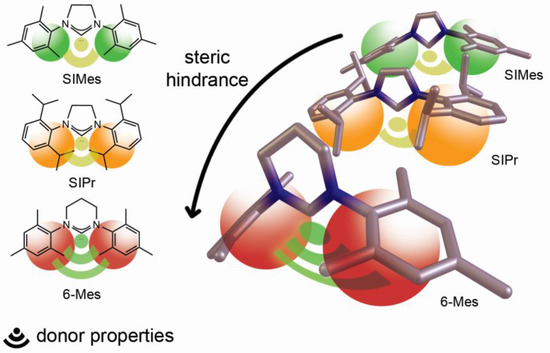
Figure 1.
Relationship between steric and electronic properties of 6-Mes and SIMes/SIPr —previously used for the synthesis of Me2MOR(NHC) (M = Ga, In) complexes [1,2,3].
Our studies, reported hereby, have allowed for the determination of the considerable effect of steric and electronic properties of 6-Mes, as well as the relative importance of electronic and steric properties of 6-Mes, on the synthesis, structure and stability of a series of Me2MOR(NHC) (M = Ga, In) complexes, as well as their catalytic activity in the ROP of rac-LA.
2. Results and Discussion
In order to observe the influence of steric and electronic properties NHC on the synthesis, structure and reactivity of Me2MOR(NHC) (M = Ga, In) complexes, we investigated the reactivity of dimethylgallium and dimethylindium alkoxides and aryloxides towards 1,3-bis(2,4,6-trimethylphenyl)-3,4,5,6-tetrahydropyrimidin-1-ylidene (6-Mes). The synthesis, structure of resulting Me2MOR(6-Mes) (M = Ga, In) complexes, and their catalytic activity in the ROP of rac-LA was compared to the analogous dialkylgallium and dialkylindium derivatives stabilized with 1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene (SIMes) or 1,3-bis(2,4,6-trimethylphenyl) imidazolin-2-ylidene (IMes) [1,2,3].
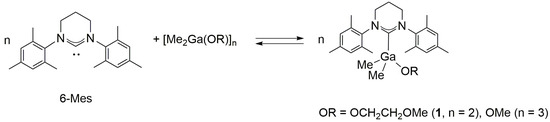
2.1. Reactivity of [Me2M(μ-OR)]n (M= Ga, In ; OR = OCH2CH2OMe, OMe ; n = 2, 3) towards 6-Mes
The reaction between dimeric gallium complex [Me2Ga(μ-OCH2CH2OMe)]2 and 6-Mes (Ga:6-Mes = 1:1, Scheme 1) led to the essentially instant formation of gallium complex Me2Ga(OCH2CH2OMe)(6-Mes) (1). Compound 1 was isolated as an off-white solid in high yield. Unfortunately, the crystals of 1 decomposed during an X-ray diffraction experiment, which precluded the determination of its X-ray structure. Notably, the increased reactivity of 1 towards grease during X-ray experiment, in comparison with Me2GaOR(NHC) (NHC = SIMes, IMes), should not be surprising in the light of its structure and reactivity in solution.
Scheme 1.
Synthesis of Me2GaOR(6-Mes) complexes.
In solution, both 1H NMR, and 13C NMR were indicative of the formation of 1. Although two sets of signals were revealed by 1H NMR of 1 in toluene-d8, the main set of signals, including a singlet corresponding to Ga–Me protons (−1.06 ppm), which was significantly shifted to higher field, similarly to Me2Ga(OCH2CH2OMe)(SIMes) [2], was indicative of the formation of Me2Ga(OCH2CH2OMe)(6-Mes). The additional minor set of signals corresponded to [Me2Ga(OCH2CH2OMe)]2 (16%), which suggested the presence of an equilibrium, presented in Scheme 1. Such an equilibrium was further evidenced by a 2D ROESY (rotating frame Overhauser effect spectroscopy) experiment (Figure S3). Moreover, essentially the same ratio of Me2Ga(OCH2CH2OMe)(6-Mes): [Me2Ga(OCH2CH2OMe)]2, for 1, and the reaction mixture of [Me2Ga(μ-OCH2CH2OMe)]2 and 6-Mes (Ga:6-Mes = 1:1), was in line with the presence of indicated equilibrium. Most probably, the latter was also responsible for the instant reaction of 1 with CD2Cl2 resulting in the formation of [Me2Ga(μ-OCH2CH2OMe)]2 and [6-Mes-D]+, which were evidenced by NMR spectroscopy (see the Supplementary Materials). Interestingly, similar reactivity was observed for the reaction between free SIMes and methylene chloride. On the contrary, more acidic chloroform was required in order to react instantly with N-heterocyclic carbene of Me2GaOR(SIMes), while the latter remained essentially stable in CD2Cl2 during the NMR experiment [1]. While the presence of the equilibrium presented in Scheme 1 is not surprising in the light of similar equilibrium observed in the case of Me2In(OCH(Me)CO2Me)(NHC) (NHC = SIMes, IMes) [3], the one observed for 1 represents the first such equilibrium for Me2GaOR(NHC) complexes, which is in line with the weaker Ga–C6-Mes bond in comparison with Me2GaOR(NHC) (NHC = SIMes, IMes). On the contrary, the shift between free 6-Mes (Δ = 244.9 ppm) and coordinated 6-Mes in the case of 1 (197.1 ppm, Δ = −47.8 ppm) was considerably larger in comparison with Me2MOR(NHC) (NHC = SIMes (Δ = −43.5–(−45.2) ppm), IMes (Δ = −43.1–(−43.6) ppm) [1,2]. Although the latter could indicate the presence of stronger Ga–C6-Mes bond in solution [25], it rather reflects the stronger donor properties of 6-Mes vs SIMes/IMes in the light of both the structure of 1, as well as the weaker Ga–C6-Mes evidenced by the X-ray analysis of Me2Ga(OCPh2Me)(6-Mes) (3) (see below). While the carbene carbon signal in 13C NMR could not be observed for Me2GaOMe(6-Mes), which was synthesized analogously to 1, the 1H NMR was in line with the presence of the equilibrium presented in Scheme 1 (see the Supplementary Materials), and therefore, similarly to 1, indicated weaker Ga–C6-Mes bond in comparison with Ga–CNHC of Me2GaOMe(NHC) (NHC = SIMes, IMes).
Despite the adverse effect of steric hindrances of 6-Mes on the strength of Ga–C6-Mes bond, the stronger donor properties of 6-Mes in comparison with SIMes/IMes, prompted us to investigate its effect on the synthesis and structure of Me2InOR(NHC) complexes. The main question was, whether the larger radius of indium, in comparison with gallium, could allow for the formation of a stronger In–C6-Mes bond and the stabilization of Me2InOR(NHC), which had been previously shown by us to be unstable and prone to ligand disproportionation [3]. Although the reaction between [Me2In(μ-OCH2CH2OMe)]2 and 6-Mes led to the formation of Me2In(OCH2CH2OMe)(6-Mes), the latter complex disproportioned readily to Me3In(6-Mes) and Mitsubishi type complex In{Me2In(μ-OCH2CH2OMe)}3 (Scheme 2) (see the Supplementary Materials), analogously to Me2In(OCH2CH2OMe)(NHC) (NHC = SIMes, IMes) [3]. The formation of both Me3In(6-Mes) and In{Me2In(μ-OCH2CH2OMe)}3 was evidenced by NMR spectroscopy, while, in contrast to our previous studies, In{Me2In(μ-OCH2CH2OMe)}3 could be isolated as a white crystalline solid, similar to the only two other examples of Mitsubishi type indium In{Me2In(µ-OR)}3 complexes [27,28]. Unfortunately, we did not succeed in obtaining crystals of In{Me2In(μ-OCH2CH2OMe)2}3 suitable for X-ray analysis, and its structure has been confirmed using 1H and 13C NMR spectroscopy (see the Supplementary Materials).
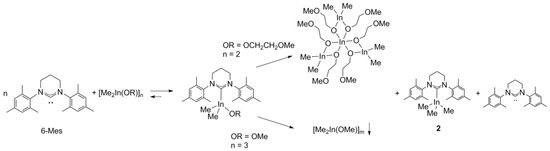
Scheme 2.
Synthesis and ligand disproportionation of Me2InOR(6-Mes).
Although the reaction of 6-Mes with [Me2In(μ-OMe)]3 led to the formation of Me2InOMe(6-Mes) complex (see the Supplementary Materials), which was more stable in comparison with Me2In(OCH2CH2OMe)(6-Mes), it seemed to disproportionate more readily in comparison with Me2InOMe(NHC) (NHC = SIMes, IMes), and as result could not be isolated in contrast to the latter [3]. While we could not estimate the strength of In-C6-Mes in the case of Me2InOR(6-Mes), due to the tendency of the latter to disproportionate , it could be analyzed for the Me3In(6-Mes) (2). The crystals of 2 suitable for X-ray analysis could be obtained either by the decomposition of Me2In(OCH2CH2OMe)(6-Mes) or quantitative reaction between 6-Mes and Me3In. Compound 2 was synthesized and isolated in bulk using only the second method, and was used to confirm the formation of 2 in solution due to the decomposition of Me2In(OCH2CH2OMe)(6-Mes). X-ray diffraction analysis of 2 revealed, similarly to Me3In(NHC) (NHC = SIMes, IMes) monomeric structure, with the coordination sphere adopting a distorted-tetrahedral geometry (Figure 2). The In–C6-Mes bond (2.367(2) Å, 0.51 valence units (vu) [29]) in 2 turned out to be noticeably longer, and therefore weaker, than In–CSIMes (2.334(6) Å, 0.55 vu [3]; 2.316(8) Å [23]), In–CIMes (2.307(2) Å, 0.60 vu [3]; 2.304(7) Å [23], 2.292(6) Å [21]), In–CSIPr (2.342(2) Å) [23] and In–CIPr (2.309(2) Å) [23] in Me3In(SIMes), Me3In(IMes), Me3In(SIPr) and Me3In(IPr) adducts, respectively. It showed a more significant effect of steric hindrances of 6-Mes, in comparison with SIMes, IMes, and even SIPr and IPr, on the strength of In–C6-Mes bond. Interestingly, the latter was in agreement with smaller steric hindrances of SIMes and IMes, and in contrast with larger steric demand of SIPr and IPr, in comparison with 6-Mes, as represented by the buried volume of discussed NHCs [25]. The analysis of the strength of In–C6-Mes bond of 2 in solution, in comparison with In–CNHC bonds of already characterized Me3In(NHC) adducts [3,21,23], was in sharp contrast with the solid state result. In solution, the shift between free and coordinated NHCs in 13C NMR, indicated stronger In–C6-Mes bond (δ = 207.5 ppm, Δ = −37.4 ppm) in comparison with In-CSIMes (Δ = −35.2 ppm) and In–CIMes (Δ = −35.7 ppm) of Me3In(SIMes) and Me3In(IMes) respectively. While the relationship between the distance of essentially the same bonds and their strength should be considered a tenet in the case of interpretations of molecular structures [30], the analysis of the strength of a M–CNHC bond using the 13C NMR spectroscopy could be influenced by other factors affecting the distribution of electron density at carbene carbon. However, it must be noticed that the shift between free and coordinated NHCs in solution, using 13C NMR spectroscopy, reflected stronger donor properties of 6-Mes in comparison with SIMes and IMes. Notably, analogous results concerning the strength of M–CNHC bonds were obtained for alkoxide derivatives Me2M(OCPh2Me)(6-Mes) (M = Ga, In).
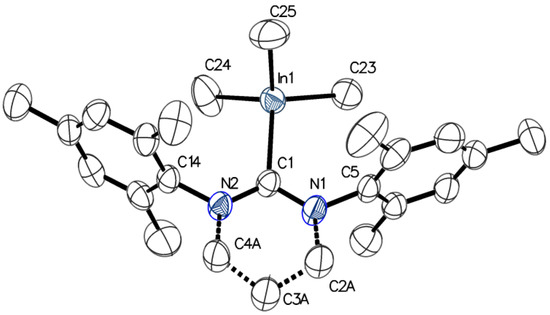
Figure 2.
Molecular structure of 2 with thermal ellipsoids at 50% probability level. Selected bond lengths [Å] and angles [°]: In(1)–C(1) 2.367(2), In(1)–C(25) 2.185(3), In(1)–C(24) 2.179(3), In(1)–C(23) 2.198(3), C(23)–In(1)–C(1) 112.04(9), C(24)–In(1)–C(1) 110.26(10), C(25)–In(1)–C(1) 104.99(11), C(24)–In(1)–C(23) 107.31(12), C(24)–In(1)–C(25) 112.31(15), C(25)–In(1)–C(23) 110.00(14), N(1)–C(1)–N(2) 116.75(19), NHC tilt 7.0(1), 5.6(1) (The tilt is quantified by the offset angle of the M–C bond to the C2 axis of the NHC (defined by C(1) and the centroid of C(2) and C(4)), which is split into pitch and yaw angles [31,32]).
2.2. Reactivity of [Me2M(μ-OCPh2Me)]2 (M = Ga, In) towards 6-Mes
While the reaction between [Me2Ga(μ-OCPh2Me)]2 and 6-Mes (Ga:6-Mes = 1:1, Scheme 3) resulted in the formation of Me2Ga(OCPh2Me)(6-Mes) (3), the prolonged time of 24 h was required to reach c.a. 87% of conversion. Monitoring of the reaction progress using 1H NMR revealed significantly slower reaction in comparison with the essentially instant reaction of [Me2Ga(μ-OCPh2Me)]2 with less sterically demanding carbenes (i.e. SIMes, IMes) [3], therefore indicating the strong adverse effects of 6-Mes steric hindrances on its reactivity towards dimeric [Me2Ga(μ-OCPh2Me)]2 and formation of monomeric Me2Ga(OCPh2Me)(6-Mes) (3) (Scheme 3). The analogous effect was not observed in the case of the reaction of [Me2In(μ-OCPh2Me)]2 with 6-Mes (In:6-Mes = 1:1, Scheme 3), leading to the formation of the indium complex Me2In(OCPh2Me)(6-Mes) (4), which can be explained by the significantly larger radius of indium in comparison with gallium. Complexes 3 and 4 were isolated in high yields as colorless crystals.
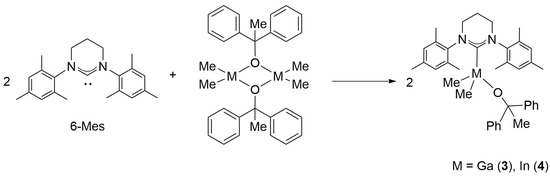
Scheme 3.
Synthesis of Me2M(OCPh2Me)(6-Mes) complexes (M = Ga, In).
For complexes 3 and 4 X-ray diffraction analysis revealed the presence of four-coordinated gallium/indium species with the coordination sphere adopting a distorted-tetrahedral geometry. Interestingly, in contrast to essentially symmetrical gallium complex 3 (Figure 3), due to the orientation of the 6-Mes ligand with respect to In–CMe bonds, the presence of essentially asymmetrical indium complex 4 (Figure 4) was observed. These observations, reflected by O(1)–Ga(1)–C(1)–N(1) (–81.98(15)°) (Figure 3) and O(1)–In(1)–C(1)–N(1) (–139.0(8)°) (Figure 4) torsion angles, were in line with the previously characterized essentially symmetric Me2Ga(OCPh2Me)(IMes) and asymmetric Me2In(OCPh2Me)(IMes) complexes [3]. While for 3 and 4 the symmetrical/asymmetrical orientation of NHC in the solid state, with respect to M–CMe (M = Ga, In) bonds, could be tentatively explained by the presence of different weak CH···π interactions, leading to the formation of chains in the solid state (see the Supplementary Materials), the essentially analogous CH···π interactions had been previously observed for Me2Me(OCPh2Me)(IMes) (M = Ga, In) [3]. Although the symmetrical/asymmetrical orientation of NHC with respect to M–CMe bonds could influence the catalytic properties, including stereoselectivity, of Me2MOR(NHC), and is of our current interest, the factors controlling the arrangement of NHC in the case of 3 and 4 could not be unequivocally indicated. However, it was the effect of M–C6-Mes bond on the structure and reactivity of Me2MeOR(6-Mes), which focused our attention. In the case of complex 3, the Ga–C6-Mes bond (2.139(2) Å, 0.57 vu) was significantly weaker in comparison with the Ga–CIMes bond of Me2Ga(OCPh2Me)IMes (2.096(4) Å, 0.64 vu) [3], although similarly small pitch angles (out of NHC plane tilting) and yaw angles (in plane tilting) were observed for both gallium complexes. Moreover, the longest Ga–C6-Mes bond of 3, among other crystallographically characterized Me2GaOR(NHC) complexes [1,2,3], should be expected to result from steric hindrances of 6-Mes. Although the In–C6-Mes bond of 4 (2.329(10) Å, 0.56 vu) was also weaker in comparison with the In–CIMes bond of Me2In(OCPh2Me)IMes (2.301(8) Å, 0.61 vu), the smaller effect of the steric hindrances of 6-Mes on the In–C6-Mes bond should be explained by a larger radius of indium in comparison with gallium. Interestingly, the latter resulted in only slightly weaker In–C6-Mes bond of 4 (2.329(10) Å, 0.56 vu) in comparison with Ga–C6-Mes bond (2.139(2) Å, 0.57 vu) of 3.
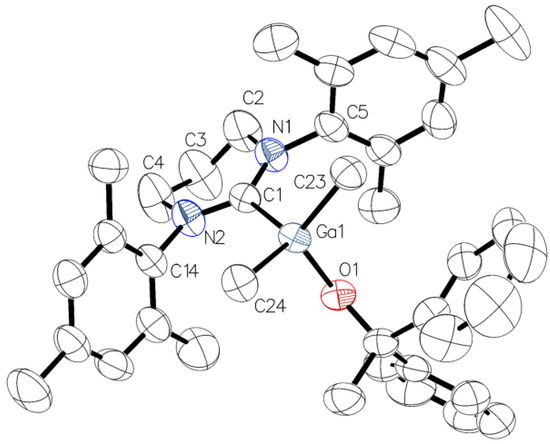
Figure 3.
Molecular structure of 3 with thermal ellipsoids at 50% probability level. Selected bond lengths [Å] and angles [°]: Ga(1)–O(1) 1.8849(12), Ga(1)–C(1) 2.1386(19), Ga(1)–C(23) 1.9879(19), Ga(1)–C(24) 1.982(2), O(1)–Ga(1)–C(1) 92.73(6), O(1)–Ga(1)–C(24) 111.74(8), O(1)–Ga(1)–C(23) 116.40(8), C(24)–Ga(1)–C(23) 107.87(9), C(24)–Ga(1)–C(1) 115.30(8), C(23)–Ga(1)–C(1) 112.48(8), N(2)–C(1)–N(1) 116.58(17), O(1)–Ga(1)–C(1)–N(1) −81.98(15), NHC tilt 4.42(9) (The tilt is quantified by the offset angle of the M–C bond to the C2 axis of the NHC (The tilt is quantified by the offset angle of the M–C bond to the C2 axis of the NHC (defined by C(1) and the centroid of C(2) and C(4)), which is split into pitch and yaw angles [31,32]).
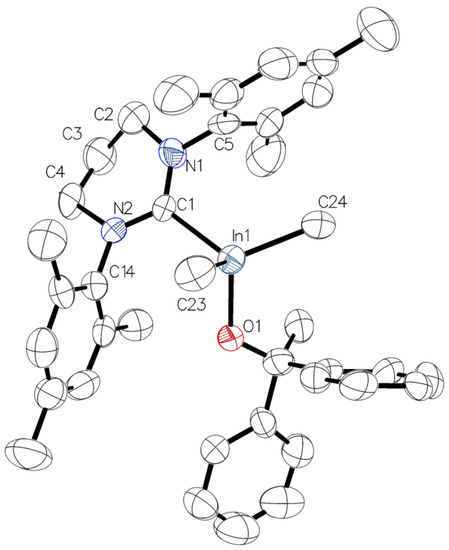
Figure 4.
Molecular structure of 4 with thermal ellipsoids at 50% probability level. Selected bond lengths [Å] and angles [°]: In(1)–O(1) 2.081(8), In(1)–C(1) 2.331(10), In(1)–C(23) 2.180(13), In(1)–C(24) 2.154(13), O(1)–In(1)–C(1) 100.9(3), O(1)–In(1)–C(24) 109.0(4), O(1)–In(1)–C(23) 108.3(4), C(24)–In(1)–C(23) 114.4(6), C(24)–In(1)–C(1) 115.3(5), C(23)–In(1)–C(1) 107.9(5), N(1)–C(1)–N(2) 115.6(8). O(1)–In(1)–C(1)–N(1) −139.0(8), NHC tilt 1.0(5)(The tilt is quantified by the offset angle of the M–C bond to the C2 axis of the NHC (The tilt is quantified by the offset angle of the M–C bond to the C2 axis of the NHC (defined by C(1) and the centroid of C(2) and C(4)), which is split into pitch and yaw angles [31,32]).
While the major set of signals revealed by the 1H NMR of 3 and 4 was in line with the formation of Me2M(OCPh2Me)(6-Mes) (M = Ga, In), the presence of minor signal could be ascribed to the [Me2Ga(μ-OCPh2Me)]2, therefore suggesting the presence of equilibrium, analogously to 1. However, due to relatively slow reaction rate between [Me2M(μ-OCPh2Me)]2 and 6-Mes (see above), the presence of minor set of signals could not be unequivocally associated with the equilibrium. The different ratios of Me2M(OCPh2Me)(6-Mes):[Me2Ga(μ-OCPh2Me)]2 both in the reaction mixture, as well as in the spectrum of 3 or 4 were not clearly indicative of the presence of such equilibrium. In the 13C NMR of both 3 and 4, significant shifts of carbene carbon signals between free and coordinated 6-Mes were observed. In the case of gallium complex 3, the observed shift (Δ = −47.5 ppm) was comparable with Me2Ga(OCH2CH2OMe)(6-Mes) (Δ = −47.8 ppm), and slightly smaller than for Me2Ga((S)-OCH(Me)CO2Me)(6-Mes) (Δ = −48.4 ppm) (see below), but was larger in comparison with Me2GaOR(NHC) (NHC = SIMes, IMes) complexes (Δmax = −45.2 ppm) [2]. Similarly, for 4 the observed shift (Δ = −41.7 ppm) was larger in comparison with Me2InOR(NHC) complexes (NHC = SIMes, IMes) (Δmax = −38.9 ppm) [3]. However, in the light of X-ray analysis of 3 and 4, which revealed weaker M–C6-Mes bonds (M = Ga, In) in comparison with M–CNHC of Me2M(OCPh2Me)(NHC) (M = Ga, In; NHC = SIMes, IMes), the shift of carbene carbon observed in 13C NMR was indicative, similarly to 1 and 2, of the stronger donor properties of 6-Mes in comparison with SIMes or IMes, rather than stronger M–C6-Mes bonds in comparison with M–CNHC (NHC = SIMes, IMes). While the detailed discussion on the factors affecting the carbene carbon shift in 13C NMR, and therefore the analysis of the strength of the M–CNHC bond using the 13C NMR spectroscopy, cannot be done at the current stage, our present research in this area focuses on the effect of the character of the M–CNHC bond, rather than only its strength, on the synthesis, structure and reactivity of group 13 metal Me2MOR(NHC) complexes.
2.3. Reactivity of [Me2M(μ-OC6H4OMe)]2 (M = Ga, In) towards 6-Mes
Although the investigation of the synthesis and structure of Me2M(OC6H4OMe)(6-Mes) (M = Ga (5), In (6)) (Scheme 4) was important prior to the studies on their catalytic activity in the ring-opening polymerization (ROP) of rac-lactide (rac-LA) (see below), they were also interesting with regard to our studies on Me2Ga(O,CNHC) complexes possessing chelate alkoxide and aryloxide ligands with NHC functionality [33]. The latter showed that aryloxide (OAr,CNHC) ligands, in comparison with alkoxide chelate (O,CNHC) ligands, resulted in stronger Ga–CNHC bond, which could be crucial for the synthesis and structure of 5 and 6 in comparison with their alkoxide analogues Me2MOR(6-Mes) (M = Ga, In). The reactions of 6-Mes with [Me2M(μ-OC6H4OMe)]2 (M:6-Mes = 1:1, M = Ga, In) led to the instant formation of 5 and 6, which were isolated in high yields as white crystalline solids. However, the crystals suitable for X-ray analysis could not be isolated. Therefore, the structure of 5 and 6 was determined in solution using NMR spectroscopy.
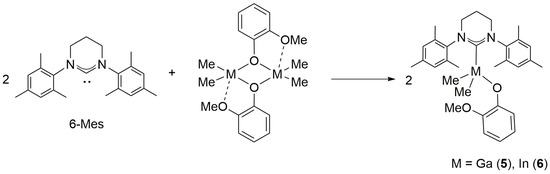
Scheme 4.
Synthesis of Me2M(OC6H4OMe)(6-Mes) complexes (M = Ga, In).
Importantly, 1H NMR spectra of 5 and 6, which were essentially identical to the corresponding reaction mixtures of [Me2M(μ-OC6H4OMe)]2 (M = Ga, In) and 2 equivalents of 6-Mes, revealed only one set of signals of Me2M(OC6H4OMe)(6-Mes). The lack of any signals corresponding to [Me2M(μ-OC6H4OMe)]2, indicating the presence of the equilibrium analogues to that of 1, could be associated in this case both with the significantly weaker M2O2 central bridges of dialkylgallium and dialkylindium aryloxides, in comparison with respective alkoxide derivatives, and stronger M–C6-Mes bonds in the case of aryloxide complexes 5 and 6. These observations were in line with the shift between free and coordinated carbene carbon signal in 13C NMR for 5 (196.1 ppm, Δ = –48.8 pm), which was only slightly different from 1 (Δ = −47.8 ppm) and 3 (Δ = −47.5 ppm), and considerably larger in comparison with Me2GaOR(NHC) (NHC = SIMes, IMes; Δmax = −44.8 ppm) complexes, as well as Me2Ga(OAr)(SIMes) (Δ = −45.2 ppm) [34]. In the case of 6, the carbene carbon of 6-Mes in 13C NMR (204.3 ppm, −40.6 ppm) was only slightly shifted to the higher field in comparison with Me2InOR(NHC) (NHC = SIMes, IMes; Δmax = −38.9 ppm), and even slightly shifted to the lower field in contrast to 4 (Δ = −41.7 ppm). However, the observed carbene carbon shifts could not be directly related to the stronger M–C6-Mes bond in 5 and 6, in comparison with alkoxide-Me2M(OR)(NHC), and aryloxide-Me2Ga(OAr)(NHC) complexes [1,2,3], in the light of the structure of 1–4.
2.4. Reactivity of [Me2M(μ-(S)-OCH(Me)CO2Me)]2 (M = Ga, In) towards 6-Mes
The synthesis and structure of Me2M((S)-OCH(Me)CO2Me)(6-Mes) (Me2Ga((S)-melac)(6-Mes)) (M = Ga, In), in which the methyl lactate (melac) ligand mimics a growing polylactide (PLA) chain [1,2,35,36,37], were investigated prior to the studies on the activity of Me2MOR(6-Mes) in the ROP of rac-LA (see below). Although both gallium and indium complexes could not be isolated in this case, among others due to the reactivity of 6-Mes with (S)-melac ligand, they were indicative of the reactivity of sterically hindered NHCs towards methyl lactate ligands, as well as indicative of the structure and reactivity of Me2M(O(PLA))(NHC) propagating species in the ROP of rac-LA. The reaction of [Me2Ga(μ-(S)-melac)]2 with 6-Mes (Ga:6-Mes = 1:1) resulted in the formation of Me2Ga((S)-melac)(6-Mes), which was evidenced both by 1H NMR and 13C NMR spectroscopy (see the Supplementary Materials). The presence of two singlets at −1.10 and −1.14 ppm, considerably shifted to higher field in comparison with [Me2Ga(μ-(S)-melac)]2, could be unequivocally ascribed to Ga–Me protons of Me2Ga((S)-melac)(6-Mes) on the basis of our earlier studies on Me2Ga((S)-melac)(NHC) (NHC = SIMes, IMes) [1,2]. Additionally, the considerable shift of carbene carbon signal in the 13C NMR spectra (196.5 ppm, Δ = −48.4 ppm) was in line with the formation of Ga–C6-Mes bond. However, the presence of additional signals in the 1H NMR spectra, including broad signal at −0.03 ppm indicated the presence of other alkylgallium species, most probably without 6-Mes coordinated to gallium. The presence of the latter signal could be associated, similarly to the synthesis of 3, with Me2Ga protons of unreacted [Me2Ga(μ-(S)-melac)]2. However, further monitoring of the reaction in time, which revealed an exclusive presence of a signal at −0.02 ppm after 4 days, suggested that initially observed broad signal at −0.03 ppm could rather result from the transformation of (Me2Ga((S)-melac)(6-Mes)) to new alkylgallium species. While the instability of (Me2Ga((S)-melac)(6-Mes)) was in contrast with the strong Ga–C6-Mes bond, the reactivity of 6-Mes towards methyl lactate ligand could be expected, based on the reactivity of SIPr towards (S)-melac ligand of [Me2Ga(μ-(S)-melac)]2, which led to the formation of among others [Me2Ga(OCH(Me)CO2)][SIPr-H] [2]. However, the possible equilibrium between Me2M(O-(S)-CH(Me)CO2Me)(6-Mes) and [Me2Ga(μ-(S)-melac)]2/6-Mes mixture, as revealed by the structure of 1, could result in the complete reaction of 6-Mes with (S)-melac, leading to the formation of a complex mixture of products, as shown in Scheme 5. Although no alkylgallium species could be isolated from the reaction mixture of [Me2Ga(μ-(S)-melac)]2 with 6-Mes (Ga:6-Mes = 1:1) the obtained results were in line with the reactivity of 6-Mes towards (S)-melac) of Me2M((S)-melac)(6-Mes) presented in Scheme 5, which was suggested on the basis of the reaction of [Me2Ga(μ-(S)-melac)]2 with SIPr (Ga:SIPr = 1:1) [2], as well as the reactivity of [MeIn(μ-(S)-melac)]2 with 6-Mes (In:6-Mes = 1:1). Contrary to the reactivity of [MeGa(μ-(S)-melac)]2 with 6-Mes, the formation of monomeric Me2In((S)-melac)(6-Mes) was not even indicated by 1H NMR in the analogous reaction between [MeIn(μ-(S)-melac)]2 and 6-Mes (In:6-Mes = 1:1) (see the Supplementary Materials). Moreover, in contrast to the formation of unstable Me2In((S)-melac)(NHC) (NHC = SIMes, IMes), which showed the tendency for the ligand disproportionation and the formation of Me3In(NHC) and alkylindium alkoxides [3], the reactivity of 6-Mes towards (S)-melac) was confirmed by the isolation of (6-Mes)=CH2, which was evidenced by X-ray analysis (Figure 5). Interestingly, the formation of the latter confirmed the possibility of the abstraction of Me+ from (S)-melac ligand with NHC, which was only suggested in the case of our previous studies by the formation of [Me2Ga(OCH(Me)CO2)]−. Moreover, the isolation of (6-Mes)=CH2 strongly indicated the conversion of [(6-Mes)-Me]+ to (6-Mes)=CH2 with the evolution of H+. The latter could be responsible for the formation of [(6-Mes)-H]+, leading to the complex mixture of products. Moreover, it could also explain the formation of SIPr-H+ in our previous reaction of [Me2Ga(μ-(S)-melac)]2 with SIPr (Ga:SIPr = 1:1) (Scheme 5), instead of the possible abstraction of H+ directly from (S)-melac ligand, which was tentatively suggested in the latter case [2].
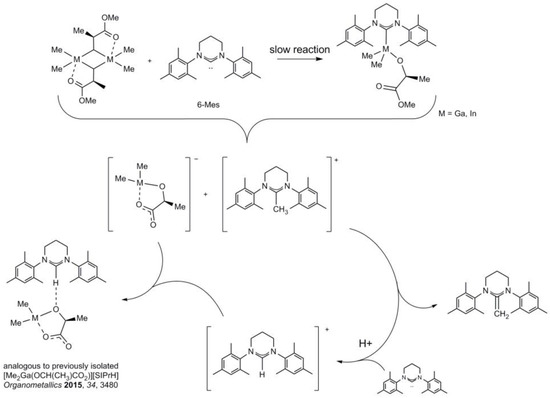
Scheme 5.
Reactivity of [Me2M((S)-OCH(Me)CO2Me)]2 towards 6-Mes.
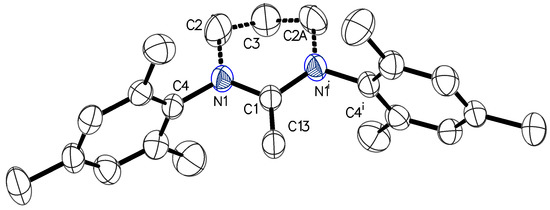
Figure 5.
Molecular structure of (6-Mes)=CH2 with thermal ellipsoids at 50% probability level. Selected bond lengths [Å] and angles [°]: C(1)–N(1) 1.3875(18), C(1)–C(13) 1.336(3), C(1)–N(1)–C(13) 122.43(10).
2.5. Activity of Me2Ga(OCPh2Me)(6-Mes) (3 and 4), Me2M(OC6H4OMe)(6-Mes) (5 and 6) (M = Ga, In) and Me2Ga(OCH2CH2OMe)(6-Mes) (1) in the ROP of rac-Lactide
In order to investigate the effect of 6-Mes on the catalytic properties of Me2MOR(NHC), we examined the activity of 1, 3, 4, 5 and 6 complexes in the ring-opening polymerization (ROP) of rac-LA. With regard to the latter, we focused on the effect of M–C6-Mes bond on the catalytic properties of selected complexes. While the M–C6-Mes bond affects the structure of all selected complexes, it should be expected to influence their reactivity, as well the structure and reactivity of Me2MO(PLA)(6-Mes), where O(PLA) represents growing polylactide chain, and therefore the microstructure of resulting polylactide (PLA). While our previous results showed that both gallium and indium Me2MOR(NHC) complexes were highly active already at −20 °C [1,2,3], in order to compare our results with the latter, we investigated the ROP of rac-LA with 1, 3, 4, 5 and 6 at identical conditions. Gallium complexes: alkoxide derivative Me2M(OCPh2Me)(6-Mes) (3) and aryloxide derivative Me2Ga(OC6H4OMe)(6-Mes) (5), were inactive in the polymerization of rac-LA at −20 °C. While in the case of complexes 3 and 5 the bulky alkoxide or aryloxide group did not allow for the insertion of rac-LA into Ga–O bond, most importantly, the strong Ga–C6-Mes bond precluded, the initiation of rac-LA polymerization by N-heterocyclic carbene, similarily to Me2Ga(OCPh2Me)(NHC) (NHC = SIMes, IMes) [3]. Contrary to the latter, a much weaker In–CNHC bond in Me2In(OCPh2Me)(NHC) (NHC = SIMes, IMes) resulted in the initiation of rac-LA by N-heterocyclic carbenes [3], and similar reactivity was observed in the case of Me2In(OCPh2Me)(6-Mes) (4) and Me2In(OC6H4OMe)(6-Mes) (6), which led to the formation of cyclic PLA (see the Supplementary Materials). However, the observed reactivity of 6-Mes of indium complexes 4 and 6 towards lactide, as well as the lack of activity of gallium derivatives 3 and 5, may also indicate the considerable effect of a character of the M–CNHC bond on the reactivity of investigated complexes. As we were unable to isolate any stable alkoxide Me2InOR(6-Mes) complex, we focused on the activity of 1 in the ROP of rac-LA. Due to the reactivity of 1 towards CH2Cl2, which led to the almost quantitative and instant formation of [Me2Ga(μ-OCH2CH2OMe)]2 [9,35], no activity of 1 in the ROP of rac-LA in CH2Cl2, at −20 °C was observed. On the other hand, the polymerization of rac-LA with 1 (25:1) in toluene led to essentially full conversion after 12 h (−20 °C) or 10 min (room temperature). For the PLA obtained at both temperatures the MALDI-TOF analysis revealed the presence of an end group of 76 Da, which was in agreement with the insertion of rac-LA into Ga–OCH2CH2OMe bond (see the Supplementary Materials). Additionally, for PLA obtained at both −20 °C and r.t. the extensive intermolecular transesterification was evidenced, while minor distributions referring to cyclic PLA could be assigned to the presence of an intermolecular transesterification or additional initiation of rac-LA ROP by 6-Mes. Although the initiation of the lactide polymerization by 6-Mes of 1, especially at −20 °C, was unlikely in the light of the lack of activity of 3 and 5, it could be assumed for Me2GaO(PLA)(6-Mes) propagating species. However, as we were not able to synthesize Me2Ga((S)-melac)(6-Mes) (see above), which mimics propagating species Me2GaO(PLA)(6-Mes), we synthesized the latter in the reaction between 1 and 10 equiv of rac-LA both at −20 °C and r.t.. Although the 1H NMR spectra after 36 h, both at −20 °C and r.t. were essentially the same, as the spectrum registered after mixing of 1 and 10 equiv of rac-LA at room temperature, they were inconclusive for the presence of Me2GaO(PLA)(6-Mes) with 6-Mes coordinated to gallium. In this case, the presence of complex Ga–Me signals in 1H NMR in the range between −0.31 and 0.02 was in contrast to Me2Ga((S)-melac)(SIMes) (−0.80, −0.82 ppm) or Me2Ga(OCH(Me)C(O))2O(CH2)2OMe(IMes) (−0.79, −0.87 ppm) [2], and could not strongly support the presence of Me2GaO(PLA)(6-Mes). Moreover, the lack of signal corresponding to coordinated/uncoordinated carbene carbon in 13C NMR was also inconclusive for the formation of the latter. Therefore, we could not unequivocally conclude whether the initiation of the ROP of rac-LA by 6-Mes was possible, or the presence of discussed transesterification reactions simply resulted from the catalytic properties of Me2GaO(PLA)(6-Mes) propagating species. However, irrespective of the origin, the presence of transesterification in the case of the ROP of rac-LA with 1 at –20 °C, which is in contrast with the essentially no transesterification observed for the ROP of rac-LA with Me2GaOR(NHC) (NHC = SIMes, IMes) [1,2], led to a considerable decrease of isoselectivity (Pm (probability of meso linkages in PLA) = 0.66–0.69 at −20 °C, Pm = 0.63–0.66 at r.t.) in comparison with the ROP of rac-LA with Me2GaOR(NHC) (Pm = 0.76–0.78 at −20 °C, Pm = 0.65–0.68 at r.t.). Importantly, the ROP of rac-LA with 1 showed the considerable effect of the structure of NHC on the catalytic properties of Me2GaOR(NHC), which should be of importance and is of our current interest as Me2GaOR(NHC) are still rare examples of highly active metal alkoxides for the isoselective ROP of rac-LA [5,6,7,8,9,10,11,12,13].
3. Materials and Methods
3.1. General Procedures
All operations were carried out under dry argon using standard Schlenk techniques. Solvents and reagents were purified and dried prior to use. Solvents were purified using MBRAUN Solvent Purification Systems (MB-SPS-800) (MBRAUN, Garching, Germany) and stored over molecular sieves. rac-Lactide was purchased from Aldrich (Poland) and further purified by crystallization from anhydrous toluene and then sublimated. (S)-Methyl lactate, 2-methoxyethanol, and methanol were purchased from Aldrich, dried over molecular sieves, and distilled under argon. 1,1-diphenylethanol was purchased from Aldrich and used as received. Me3In and Me3Ga were purchased from STREM Chemicals, Inc. (Bischheim, France) and used as received. 6-Mes was synthesized according to the literature [38]. Indium and gallium complexes: [Me2M(μ-OCH2CH2OMe)]2, [Me2M(μ-OMe)]3, [Me2M(μ-OCH(CH3)COOMe)]2, (Me2M(μ-OCPh2Me)]2, Me2M(OC6H4OMe)]2 were synthesized as described by us previously [1,2,3]. 1H and 13C NMR spectra were recorded on an Agilent 400-MR DD2 400 MHz spectrometer (Agilent, Palo Alto, CA, USA) with shifts given in ppm according to the deuterated solvent shift. MALDI-TOF spectra were recorded on a Bruker Model ultrafleXtreme instrument (Bruker Daltonics, Bremen, Germany). Elemental analysis was performed on a Vario EL III instrument (ELEMENTAR Analysensysteme, Hanau, Germany).
3.2. Synthesis of Indium and Gallium Complexes
Synthesis of 1. To a solution of [Me2Ga(μ-OCH2CH2OMe)]2 (76.4 mg, 0.44 mmol) in toluene (2 mL) was added 2 mL of a toluene solution of 6-Mes (140.0 mg, 0.44 mmol) at room temperature, and the resulting solution was stirred for 2 h. Then toluene was removed under vacuum to give a faint-yellow oil, which turned to an off-white solid after 45 min of drying. The solid was subsequently crystallized from toluene (0.3 mL), Et2O (4 mL) and hexane (3 mL) at −40°C to give off-white crystals, which were dried under vacuum (1, 158 mg, 73%). Anal. Calcd. for C27H41GaN2O2: C, 65.47; H, 8.34; N, 5.66. Found: C, 65.32; H, 8.34; N, 5.60. 1H NMR (toluene-d8, 400 MHz): −1.08 (s, 6H, GaCH3), 1.49 (q, 2H, 3J (H,H) = 5.9 Hz, CH2CH2CH2), 2.11 (s, 6H, CH3), 2.29 (s, 12H, CH3), 2.60 (t, 4H, 3J (H,H) = 5.9 Hz, CH2CH2CH2), 3.27 (s, 3H, OCH3), 3.45 (t, 2H, 3J (H,H) = 6.5 Hz, CH2CH2), 3.69 (t, 2H, 3J (H,H) = 6.5 Hz, CH2CH2), 6.75 (s, 4H, CHAr). 13C{1H} NMR (toluene-d8, 100 MHz): −5.6 (GaMe2), 18.2, 20.9, 21.0, 42.0, 47.2, 58.5, 64.0, 77.8, 129.3, 129.8, 135.0, 135.6, 137.7, 141.8, 197.1 (carbene).
Synthesis of 2. A stirred solution of Me3In (116 mg, 0.73 mmol) in toluene (5 mL) was cooled to −20 °C, and a toluene solution (2 mL) of 6-Mes (232 mg, 0.73 mmol) was added dropwise. After addition the reaction mixture was warmed to room temperature and stirred for 2 h. Solvent and volatile residues were then removed under vacuum to give a white solid. The solid was subsequently recrystallized from a toluene/hexane solution (2/6 mL) at −20 °C to give white crystals, which were washed twice with 3 mL of hexane and dried under vacuum to give Me3In(6-Mes) (nr; 311 mg, 92%). Anal. Calcd. for C25H37InN2: C, 62.50; H, 7.76; N, 5.83. Found: C, 62.36; H, 7.88; N, 5.76. 1H NMR (toluene-d8, 400 MHz): −0.88 (s, 9H, InCH3), 1.50 (m, 2H, CH2), 2.11 (s, 6H, CH3), 2.19 (s, 12H, CH3), 2.58 (m, 4H, CH2CH2), 6.77 (s, 4H, CHAr). 13C{1H} NMR (toluene-d8, 100 MHz): −8.0 (InMe3), 18.1, 20.9, 46.3, 130.1, 135.1, 137.4, 137.7, 142.1, 207.5 (carbene).
Synthesis of 3 and 4. To a solution of [Me2In(μ-OCPh2Me)]2 (110 mg, 0.32 mmol) or [Me2Ga(μ-OCPh2Me)]2 (96 mg, 0.32 mmol) in toluene (6 mL) was added 2 mL of a toluene solution of 6-Mes (103 mg, 0.32 mmol) at room temperature, and the resulting solution was stirred for 24 h. Then, toluene was removed under vacuum to give a light yellow solid. The solid was subsequently crystallized from toluene (2 mL) after addition of hexane (6 mL) at −20 °C to give white crystals, which were dried under vacuum (nr In, 187 mg, 88%; nr Ga, 158 mg, 80%). Data for 4 are as follows. Anal. Calcd. for C38H47InN2O: C, 68.88; H, 7.15; N, 4.23. Found: C, 66.92; H, 7.25; N, 4.17. 1H NMR (toluene-d8, 400 MHz): −1.30 (s, 6H, InCH3), 1.42 (q, 2H, 3J (H,H) = 5.9 Hz, CH2CH2CH2), 1.78 (s, 3H, CCH3), 2.14 (s, 6H, CH3), 2.26 (s, 12H, CH3), 2.54 (t, 4H, 3J (H,H) = 5.9 Hz, CH2CH2CH2), 6.77 (br s, 4H, CHAr), 7.03–7.07 (m, 2H, CHAr), 7.16–7.21 (m, 4H, CHAr), 7.51 (m, 4H, CHAr). 13C{1H} NMR (toluene-d8, 100 MHz): −5.0 (InMe2), 14.3, 18.4, 21.0, 23.1, 32.0, 33.7, 46.4, 77.6, 124.9, 126.9, 127.2, 127.6, 128.4, 129.2, 130.2, 135.6, 138.0, 141.9, 155.7, 203.2 (carbene). Data for 3 are as follows. Anal. Calcd. for C38H47GaN2O·C6H8(toluene): C, 76.16; H, 7.81; N, 3.95. Found: C, 76.30; H, 8.14; N, 3.80. 1H NMR (toluene-d8, 400 MHz): −1.36 (s, 6H, GaCH3), 1.45 (q, 2H, 3J (H,H) = 5.9 Hz, CH2CH2CH2), 1.78 (s, 3H, CCH3), 2.14 (s, 6H, CH3), 2.25 (s, 12H, CH3), 2.58 (t, 4H, 3J (H,H) = 5.9 Hz, CH2CH2CH2), 6.74 (s, 4H, CHAr), 7.04 (m, 2H, CHAr), 7.17 (m, 4H, CHAr), 7.46 (m, 4H, CHAr); 13C{1H} NMR (toluene-d8, 100 MHz): −1.2 (GaMe2), 17.3, 18.5, 20.9, 21.0, 21.3, 32.6, 47.5, 77.7, 125.0, 125.6, 127.1, 127.8, 128.4, 129.2, 129.9, 135.5, 137.7, 142.0, 154.8, 156.3, 197.4 (carbene).
Synthesis of 5 and 6. To a solution of [Me2In(μ-OC6H4OMe)]2 (143 mg, 0.53 mmol) or [Me2Ga(μ-OC6H4OMe)]2 (118 mg, 0.53 mmol) in toluene (6 mL) was added 2 mL of a toluene solution of 6-Mes (171 mg, 0.53 mmol) at room temperature, and the resulting solution was stirred for 2 h. Then toluene was removed under vacuum to give a pale white solid. The solid was subsequently crystallized from toluene (1.5 mL) after addition of hexane (5 mL) at −20 °C to give white crystals, which were dried under vacuum (nr In, 283 mg, 90%; nr Ga, 249 mg, 86%). Data for 6 are as follows. Anal. Calcd. for C31H41InN2O2: C, 63.27; H, 7.02; N, 4.76. Found: C, 63.09; H, 7.20; N, 4.80. 1H NMR (toluene-d8, 400 MHz): −1.00 (s, InCH3), 1.56 (q, 2H, 3J (H,H) = 5.8 Hz, CH2CH2CH2), 2.07 (s, 6H, CH3), 2.31 (s, 12H, CH3), 2.63 (t, 4H, 3J (H,H) = 5.8 Hz, CH2CH2CH2), 3.29 (s, 3H, OCH3), 6.49 (m, 2H, CHAr), 6.67 (br s, 4H, CHAr), 6.86 (m, 1H, CHAr), 6.96 (m, 1H, CHAr); 13C{1H} NMR (toluene-d8, 100 MHz): −6.7 (InMe2), 18.0, 20.9, 46.5, 54.1, 110.9, 112.7, 118.9, 121.8, 130.2, 135.6, 137.9, 141.8, 150.8, 157.3, 204.3 (carbene). Data for 5 are as follows. Anal. Calcd. for C31H41GaN2O2: C, 68.52; H, 7.61; N, 5.16. Found: C, 68.22; H, 7.56; N, 5.16. 1H NMR (toluene-d8, 400 MHz): −1.00 (s, GaCH3), 1.55 (q, 2H, 3J (H,H) = 5.9 Hz, CH2CH2CH2), 2.06 (s, 6H, CH3), 2.29 (s, 12H, CH3), 2.65 (t, 4H, 3J (H,H) = 5.9 Hz, CH2CH2CH2), 3.55 (s, 3H, OCH3), 6.54 (m, 1H, CHAr), 6.62 (m, 1H, CHAr), 6.67 (br s, 4H, CHAr), 6.70 (m, 1H, CHAr), 6.80 (m, 1H, CHAr); 13C{1H} NMR (toluene-d8, 100 MHz): −3.9 (GaMe2), 18.1, 20.9, 47.4, 55.7, 113.3, 114.4, 119.8, 121.7, 129.9, 135.6, 137.8, 141.5, 152.1, 155.9, 196.1 (carbene).
3.3. General Procedure for the ROP of rac-LA with 1 and 3–6
To a rac-LA (334.8 mg, 2.32 mmol, 25 eq) suspension in toluene (10 mL) cooled to −20 °C was added a toluene solution (1 mL) of the catalyst (0.09 mmol, 1 eq), and the reaction was stirred overnight at −20 °C until the rac-LA fully dissolved. In the case of polymerization at room temperature, rac-LA dissolved fully after 10 min. Each polymerization was quenched by the addition of a HCl solution (5%, 50 mL). The organic phase was separated, washed twice with water (50 mL), dried over anhydrous MgSO4 and dried under vacuum to give PLA as a white solid. 1H NMR (CDCl3, 400 MHz): (a) PLA signals, 1.46–1.55 (m, 3H, CHCH3), 5.10–5.23 (m, 1H, CHCH3); (b) end groups for PLA obtained with 1 3.36 (s, 3H, OCH3), 3.57 (s, 2H, CH2), 4.26 (s, 2H, CH2). PLA (~0.2 g) was also precipitated from methylene chloride solution (0.5 mL) with 20 mL of MeOH, filtered off, and dried under vacuum to yield PLA with approximately 70% yield. The precipitated PLA was further analyzed by 1H NMR, homodec NMR and 13C NMR (see Supplementary Materials).
3.4. X-Ray Structure Determination
Single crystals of 2, 3, 4 and (6-Mes)=CH2 were grown from toluene/hexane solutions. Suitable single crystals were selected under a polarizing microscope and glued to a glass capillary. The diffraction data were collected on an Oxford Diffraction Gemini A ultra diffractometer at room temperature. Data collection and reduction were performed using the CrysAlisPRO software developed by Rigaku Oxford Diffraction [39]. The structures were solved with the ShelXT structure solution program using Intrinsic Phasing and refined with the ShelXL refinement package using Least Squares minimization [40]. These programs were invoked from within Olex2 suite which was also used for the production of Figure 2, Figure 3, Figure 4 and Figure 5 [41].
Crystal Data for 2, C25H37InN2 (M = 480.38 g/mol): monoclinic, space group P21/n (no. 14), a = 9.5680(3) Å, b = 16.9878(4) Å, c = 16.0384(5) Å, β = 106.868(3)°, V = 2494.73(13) Å3, Z = 4, T = 293(2) K, μ(MoKα) = 0.959 mm−1, Dcalc = 1.279 g/cm3, 22528 reflections measured (6.5° ≤ 2Θ ≤ 52.0°), 4884 unique (Rint = 0.0219, Rsigma = 0.0162) which were used in all calculations. The final R1 was 0.0245 (I > 2σ(I)) and wR2 was 0.0646 (all data).
Crystal Data for 3, C38H47GaN2O (M = 617.49 g/mol): monoclinic, space group P21/c (no. 14), a = 11.5312(3) Å, b = 18.7807(5) Å, c = 19.0639(5) Å, β = 100.916(3)°, V = 4053.87(19) Å3, Z = 4, T = 293(2) K, μ(MoKα) = 0.704 mm−1, Dcalc = 1.012 g/cm3, 65065 reflections measured (6.7° ≤ 2Θ ≤ 53.0°), 8392 unique (Rint = 0.0504, Rsigma = 0.0305) which were used in all calculations. The final R1 was 0.0373 (I > 2σ(I)) and wR2 was 0.0947 (all data).
Crystal Data for 4, C38H47InN2O (M =662.59 g/mol): tetragonal, space group I41cd (no. 110), a = 28.4159(12) Å, c = 19.2051(10) Å, V = 15507.4(15) Å3, Z = 16, T = 293(2) K, μ(MoKα) = 0.636 mm–1, Dcalc = 1.135 g/cm3, 21599 reflections measured (7.1° ≤ 2Θ ≤ 50.0°), 6490 unique (Rint = 0.0763, Rsigma = 0.0914) which were used in all calculations. The final R1 was 0.0505 (I > 2σ(I)) and wR2 was 0.1532 (all data).
Crystal Data for (6-Mes)=CH2, C23H30N2 (M =334.49 g/mol): orthorhombic, space group Pbcn (no. 60), a = 15.7258(15) Å, b = 7.9668(8) Å, c = 16.0813(14) Å, V = 2014.7(3) Å3, Z = 4, T = 293(2) K, μ(MoKα) = 0.064 mm−1, Dcalc = 1.103 g/cm3, 7777 reflections measured (7.2° ≤ 2Θ ≤ 53.0°), 2080 unique (Rint = 0.0267, Rsigma = 0.0224) which were used in all calculations. The final R1 was 0.0552 (I > 2σ(I)) and wR2 was 0.1570 (all data).
4. Conclusions
In summary, we have investigated the influence of steric and electronic properties of 1,3-bis(2,4,6-trimethylphenyl)-3,4,5,6-tetrahydropyrimidin-1-ylidene (6-Mes) on the synthesis, structure and reactivity of Me2MOR(NHC). Particularly, we have focused on the M–C6-Mes bond in Me2MOR(6-Mes), how it is influenced by the structure of 6-Mes in comparison with other NHCs, and how it can influence the structure of Me2MOR(6-Mes) as well as their catalytic activity in the ring-opening polymerization (ROP) of racemic lactide (rac-LA). The considerable effect of steric hindrances of 6-Mes was reflected both by significantly longer, and therefore weaker M–C6-Mes in the solid state in comparison to M–CSIMes and M–CIMes, which was revealed by X-ray analysis and the presence of equilibrium between Me2MOR(NHC) and [Me2M(µ-OR)]2/NHC in solution. The stronger donor properties of 6-Mes, in comparison with other NHCs, were revealed in solution by significant shift of carbene carbon in 13C NMR upon coordination of 6-Mes to Ga and In of Me2MOR(6-Mes) (M = Ga, In) and Me3In(6-Mes). However, in light of other results, including X-ray analysis, they could not indicate the presence of a stronger M–C6-Mes bond for investigated complexes in comparison with M–CSIMes, M–CIMes, and M–CSIPr of their Me2MOR(NHC) (M = Ga, In; NHC = SIMes, IMes) and Me3In(NHC) (NHC = SIMes, IMes, SIPr) analogues. The formation of In–C6-Mes bond in the case of dimethylindiumalkoxides was not sufficient in order to stabilize Me2InOR(NHC) complexes. Finally, the investigation of the reactivity of Me2M(OCH(Me)CO2Me) towards 6-Mes, with regard to the structure of propagating Me2M(OPLA)(6-Mes) species in the ROP of rac-LA, allowed to clarify the mechanism of the reaction of Me2InOR(NHC) towards sterically hindered NHCs. With regard to the catalytic activity of Me2MOR(NHC), the effect of Ga–C6-Mes and a resulting structure of Me2GaOR(NHC) influenced mostly stereoselectivity, as well as the extent of transesterification reactions in the polymerization of rac-LA, which demonstrated the crucial role of NHC on the catalytic properties of Me2GaOR(NHC). The mechanism of the ROP of rac-LA was in line with the insertion of rac-LA into Ga–Oalkoxide bond, although the insertion into Ga–C6-Mes could not be excluded. As a result of even weaker In–C6-Mes bond in comparison to other In–CNHCs in Me2InOR(NHC) the insertion of rac-LA into In–C6-Mes occurred and was not surprising in the light of the polymerization of rac-LA with previously described by us Me2InOR(NHC) (NHC = SIMes, IMes). Importantly, the reported studies, concerning both the structure and catalytic activity of Me2MOR(6-Mes), revealed the effect of the character of M–CNHC, rather than only its strength, which is the subject of our current research.
Supplementary Materials
The following are available online at http://www.mdpi.com/2304-6740/6/1/28/s1. Figures S1–S58 (including mainly NMR spectra of gallium and indium complexes, NMR data of PLA obtained with selected indium and gallium complexes, NMR spectra of experiments of 1 with 10 eq rac-LA, MALDI-TOF of PLA obtained with 1 and weak interactions within crystal structures of 3 and 4), cif and cif-checked files.
Supplementary File 1Acknowledgments
This work was financially supported by the National Science Centre of Poland (SONATA BIS2 Programme, Grant No. DEC-2012/07/E/ST5/02860) and partially supported by Warsaw University of Technology. The authors thank Dr. Ireneusz Wielgus from Warsaw University of Technology for MALDI-TOF measurements, Dr. Łukasz Dobrzycki from University of Warsaw for help with X-ray measurements and Prof. Janusz Zachara from Warsaw University of Technology for helpful discussions.
Author Contributions
Martyna Cybularczyk-Cecotka and Paweł Horeglad conceived and designed the experiments; Martyna Cybularczyk-Cecotka and Anna Maria Dąbrowska performed the experiments; Piotr Guńka was responsible for X-ray measurements; Martyna Cybularczyk-Cecotka, Anna Maria Dąbrowska and Paweł Horeglad analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Horeglad, P.; Szczepaniak, G.; Dranka, M.; Zachara, J. The first facile stereoselectivity switch in the polymerization of rac-lactide—From heteroselective to isoselective dialkylgallium alkoxides with the help of N-heterocyclic carbenes. Chem. Commun. 2012, 48, 1171–1173. [Google Scholar] [CrossRef] [PubMed]
- Horeglad, P.; Cybularczyk, M.; Trzaskowski, B.; Żukowska, G.Z.; Dranka, M.; Zachara, J. Dialkylgallium Alkoxides Stabilized with N-Heterocyclic Carbenes: Opportunities and Limitations for the Controlled and Stereoselective Polymerization of rac-Lactide. Organometallics 2015, 34, 3480–3496. [Google Scholar] [CrossRef]
- Cybularczyk, M.; Dranka, M.; Zachara, J.; Horeglad, P. Effect of In–CNHC Bonds on the Synthesis, Structure, and Reactivity of Dialkylindium Alkoxides: How Indium Compares to Gallium. Organometallics 2016, 35, 3311–3322. [Google Scholar] [CrossRef]
- Fliedel, C.; Schnee, G.; Avilés, T.; Dagorne, S. Group 13 metal (Al, Ga, In, Tl) complexes supported by heteroatom-bonded carbene ligands. Coord. Chem. Rev. 2014, 275, 63–86. [Google Scholar] [CrossRef]
- Osten, K.M.; Mehrkhodavandi, P. Indium Catalysts for Ring Opening Polymerization: Exploring the Importance of Catalyst Aggregation. Acc. Chem. Res. 2017, 50, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Myers, D.; White, A.J.P.; Forsyth, C.M.; Williams, C.K. Phosphasalen Indium Complexes Showing High Rates and Isoselectivities in rac-Lactide Polymerizations. Angew. Chem. Int. Ed. 2017, 56, 5277–5282. [Google Scholar] [CrossRef] [PubMed]
- Aluthge, D.C.; Ahn, J.M.; Mehrkhodavandi, P. Overcoming aggregation in indium salen catalysts for isoselective lactide polymerization. Chem. Sci. 2015, 6, 5284–5292. [Google Scholar] [CrossRef]
- Normand, M.; Dorcet, V.; Kirillov, E.; Carpentier, J.-F. {Phenoxy-imine}aluminum versus -indium Complexes for the Immortal ROP of Lactide: Different Stereocontrol, Different Mechanisms. Organometallics 2013, 32, 1694–1709. [Google Scholar] [CrossRef]
- Horeglad, P.; Litwińska, A.; Żukowska, G.Z.; Kubicki, D.; Szczepaniak, G.; Dranka, M.; Zachara, J. The influence of organosuperbases on the structure and activity of dialkylgallium alkoxides in the polymerization of rac-lactide: The road to stereo diblock PLA copolymers. Appl. Organometal. Chem. 2013, 27, 328–336. [Google Scholar] [CrossRef]
- Dai, Z.; Sun, Y.; Xiong, J.; Pan, X.; Tang, N.; Wu, J. Simple sodium and potassium phenolates as catalysts for highly isoselective polymerization of rac-lactide. Catal. Sci. Technol. 2016, 6, 515–520. [Google Scholar] [CrossRef]
- Bakewell, C.; White, A.J.P.; Long, N.J.; Williams, C.K. Scandium and Yttrium Phosphasalen Complexes as Initiators for Ring-Opening Polymerization of Cyclic Esters. Inorg. Chem. 2015, 54, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Abbina, S.; Du, G. Zinc-Catalyzed Highly Isoselective Ring Opening Polymerization of rac-Lactide. ACS Macro Lett. 2014, 3, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Buffet, J.-C.; Blaudeck, R.P.; Sujecki, S.; Blake, A.J.; Wilson, C. C3-Symmetric Lanthanide Tris(alkoxide) Complexes Formed by Preferential Complexation and Their Stereoselective Polymerization of rac-Lactide. Angew. Chem., Int. Ed. 2008, 47, 6033–6036. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.R.; Breyfogle, L.E.; Hillmyer, M.A.; Tolman, W.B. Stereoelective polymerization of D,L-lactide using N-heterocyclic carbene based compounds. Chem. Commun. 2004, 2504–2505. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.R.; Schaller, C.P.; Hillmyer, M.A.; Tolman, W.B. Zinc N-heterocyclic carbene complexes and their polymerization of D,L-lactide. J. Organomet. Chem. 2005, 690, 5881–5891. [Google Scholar] [CrossRef]
- Arnold, P.L.; Casely, I.J.; Turner, Z.R.; Bellabarba, R.; Tooze, R.B. Magnesium and zinc complexes of functionalised, saturated N-heterocyclic carbene ligands: Carbene lability and functionalisation, and lactide polymerisation catalysis. Dalton Trans. 2009, 7236–7247. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Fliedel, C.; Bellemin-Laponnaz, S.; Dagorne, S. NHC Bis-Phenolate Aluminum Chelates: Synthesis, Structure, and Use in Lactide and Trimethylene Carbonate Polymerization. Organometallics 2014, 33, 5730–5739. [Google Scholar] [CrossRef]
- Zhao, N.; Hou, G.; Deng, X.; Zi, G.; Walter, M.D. Group 4 metal complexes with new chiral pincer NHC-ligands: Synthesis, structureand catalytic activity. Dalton Trans. 2014, 43, 8261–8272. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Heinrich, B.; Bellemin-Laponnaz, S.; Dagorne, S. A robust zirconium N-heterocyclic carbene complex for the living and highly stereoselective ring-opening polymerization of rac-lactide. Chem. Commun. 2012, 48, 2213–2215. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Brelot, L.; Bellemin-Laponnaz, S.; Dagorne, S. Synthesis and Structural Characterization of a Novel Family of Titanium Complexes Bearing a Tridentate Bis-phenolate-N-heterocyclic Carbene Dianionic Ligand and Their Use in the Controlled ROP of rac-Lactide. Organometallics 2010, 29, 1191–1198. [Google Scholar] [CrossRef]
- Schnee, G.; Bolley, A.; Hild, F.; Specklin, D.; Dagorne, S. Group 13 metal (Al, Ga, In) alkyls supported by N-heterocyclic carbenes for use in lactide ring-opening polymerization catalysis. Catal. Today 2017, 289, 204–210. [Google Scholar] [CrossRef]
- Patel, D.; Liddle, S.T.; Mungur, S.A.; Rodden, M.; Blake, A.J.; Arnold, P. Bifunctional yttrium(III) and titanium(IV) NHC catalysts for lactide polymerisation. Chem. Commun. 2006, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Gill, A.M.; Yunpeng, L.; Yongxin, L.; Ganguly, R.; Falivene, L.; Garcia, F. Aryl-NHC-group 13 trimethyl complexes: Structural, stability and bonding insights. Dalton Trans. 2017, 46, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.; Beetstra, D.J.; Knight, J.C.; Ooi, L.-L.; Stasch, A.; Coles, S.; Male, L.; Hursthouse, M.B.; Cavell, K.J.; Dervisi, A.; Fallis, I.A. Novel Expanded Ring N-Heterocyclic Carbenes: Free Carbenes, Silver Complexes, And Structures. Organometallics 2008, 27, 3279–3289. [Google Scholar] [CrossRef]
- Dröge, T.; Glorius, F. The Measure of All Rings—N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2010, 49, 6940–6953. [Google Scholar] [CrossRef] [PubMed]
- Bourissou, D.; Guerret, O.; Gabbaï, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–91. [Google Scholar] [CrossRef] [PubMed]
- Chitsaz, S.; Neumüller, B.Z. Compounds with Organometallic Alkoxo–Indium Cages. Anorg. Allg. Chem. 2001, 627, 2451–2459. [Google Scholar] [CrossRef]
- Chitsaz, S.; Iravani, E.; Neumüller, B.Z. Sesquialkoxides of Gallium and Indium. Anorg. Allg. Chem. 2002, 628, 2279–2285. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Kaupp, M.; Metz, B.; Stoll, H. Breakdown of Bond Length-Bond Strength Correlation: A Case Study. Angew. Chem. Int. Ed. 2000, 39, 4607–4609. [Google Scholar] [CrossRef]
- Higelin, A.; Keller, S.; Göhringer, C.; Jones, C.; Krossing, I. Unusual Tilted Carbene Coordination in Carbene Complexes of Gallium(I) and Indium(I). Angew. Chem. Int. Ed. 2013, 52, 4941–4944. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Liddle, S.T. f-block N-heterocyclic carbene complexes. Chem. Commun. 2006, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- Horeglad, P.; Ablialimov, O.; Szczepaniak, G.; Dąbrowska, A.M.; Dranka, M.; Zachara, J. Dialkylgallium Complexes with Alkoxide and Aryloxide Ligands Possessing N-Heterocyclic Carbene Functionalities: Synthesis and Structure. Organometallics 2014, 33, 100–111. [Google Scholar] [CrossRef]
- Dąbrowska, A.M.; Hurko, A.; Dranka, M.; Varga, V.; Urbańczyk, M.; Horeglad, P. Towards NHC stabilized alkylgallium alkoxide/aryloxide cations—The advances, the limitations and the challenges. J. Organomet. Chem. 2017, 840, 63–69. [Google Scholar] [CrossRef]
- Horeglad, P.; Kruk, P.; Pécaut, J. Heteroselective Polymerization of rac-Lactide in the Presenceof Dialkylgallium Alkoxides: The Effect of Lewis Base on Polymerization Stereoselectivity. Organometallics 2010, 29, 3729–3734. [Google Scholar] [CrossRef]
- Lewiński, J.; Horeglad, P.; Wójcik, K.; Justyniak, I. Chelation Effect in Polymerization of Cyclic Esters by Metal Alkoxides: Structure Characterization of the Intermediate Formed by Primary Insertion of Lactide into the Al–OR Bond of an Organometallic Initiator. Organometallics 2005, 24, 4588–4593. [Google Scholar] [CrossRef]
- Dagorne, S.; Le Bideau, F.; Welter, R.; Bellemin-Laponnaz, S.; Maisse-François, A. Well-Defined Cationic Alkyl- and Alkoxide-Aluminum Complexes and Their Reactivity with ε-Caprolactone and Lactides. Chem. Eur. J. 2007, 13, 3202–3217. [Google Scholar] [CrossRef] [PubMed]
- Kolychev, E.L.; Asachenko, A.F.; Dzhevakov, P.B.; Bush, A.A.; Shuntikov, V.V.; Khrustalev, V.N.; Nechaev, M.S. Expanded ring diaminocarbene palladium complexes: Synthesis, structure, and Suzuki–Miyaura cross-coupling of heteroaryl chlorides in water. Dalton Trans. 2013, 42, 6859–6866. [Google Scholar] [CrossRef] [PubMed]
- CRYSALISPRO Software System, version 1.171.38.43 (1–3 and (6-Mes)=CH2) and 1.171.38.46 (4); Agilent Technologies: Oxford, UK, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).