Redox-Induced Aromatic C–H Bond Functionalization in Metal Complex Catalysis from the Electrochemical Point of View
Abstract
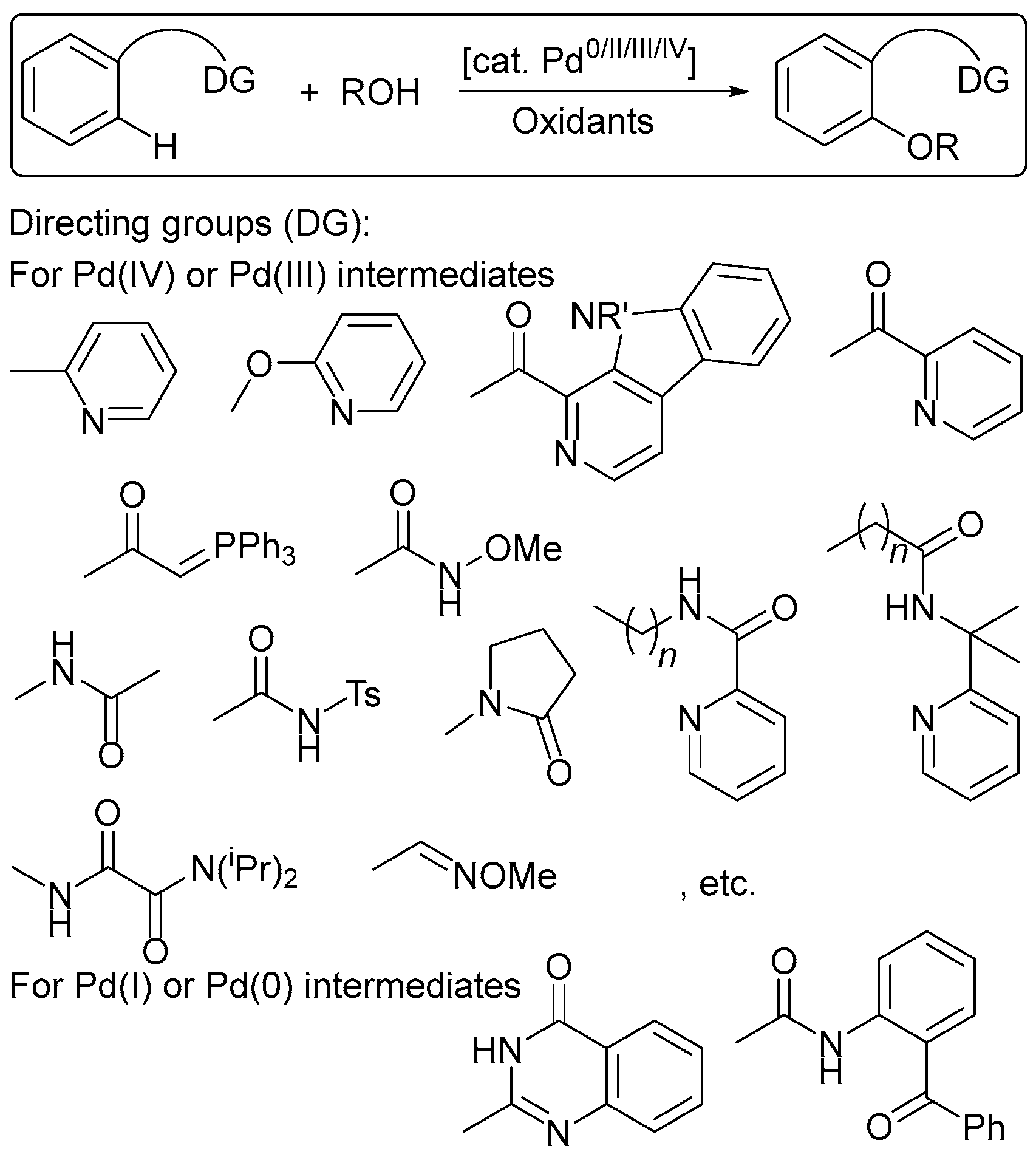
1. Introduction
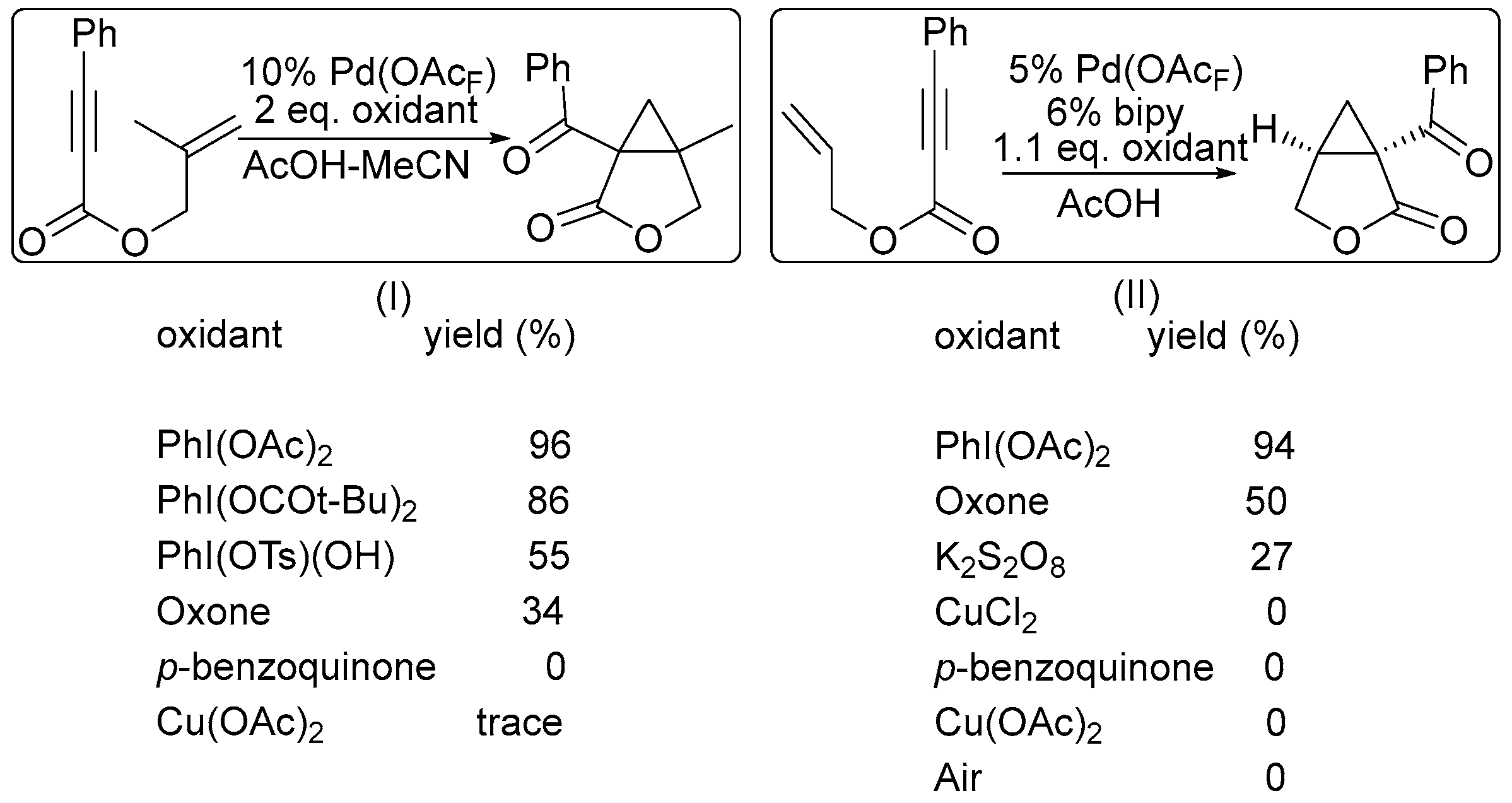
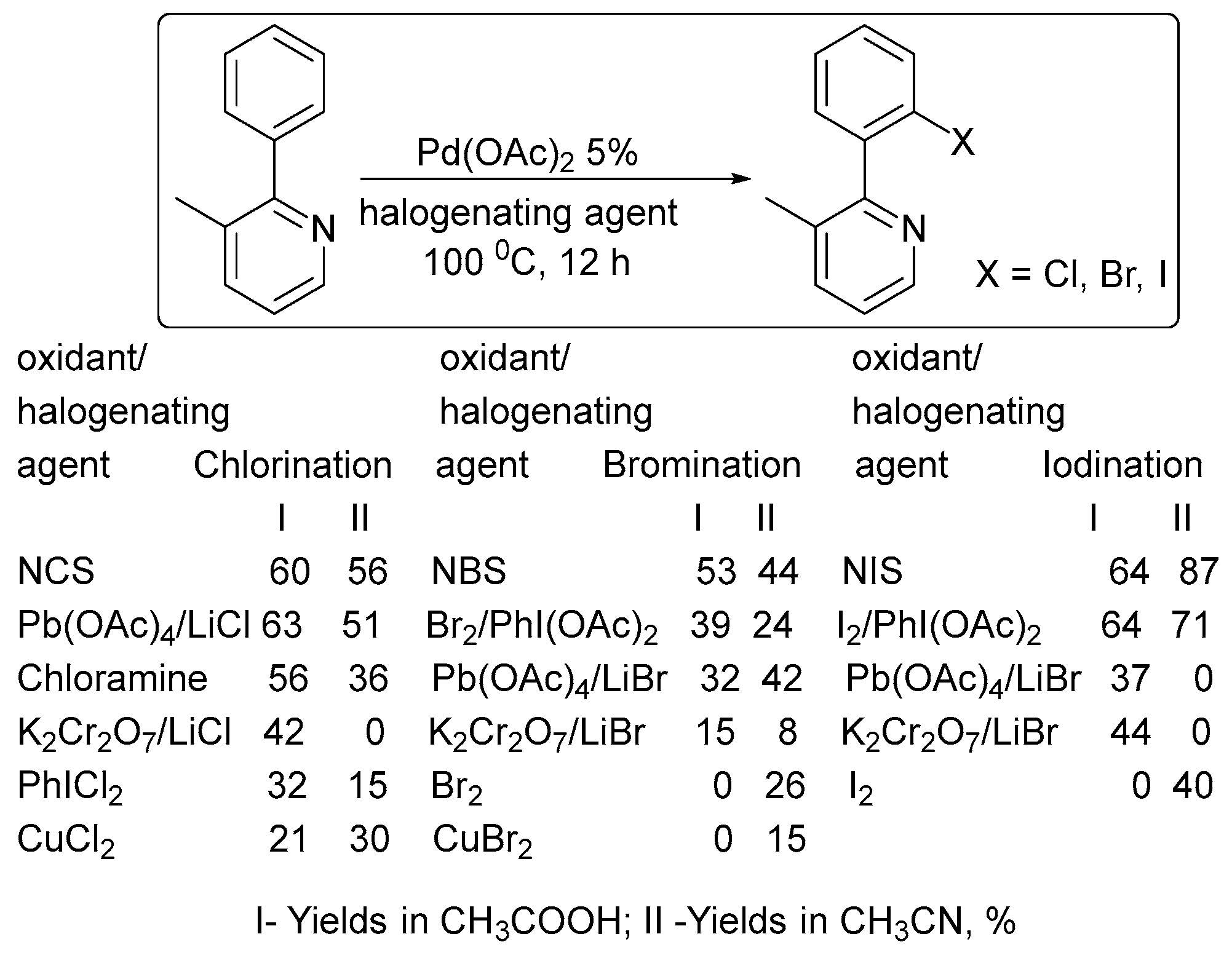
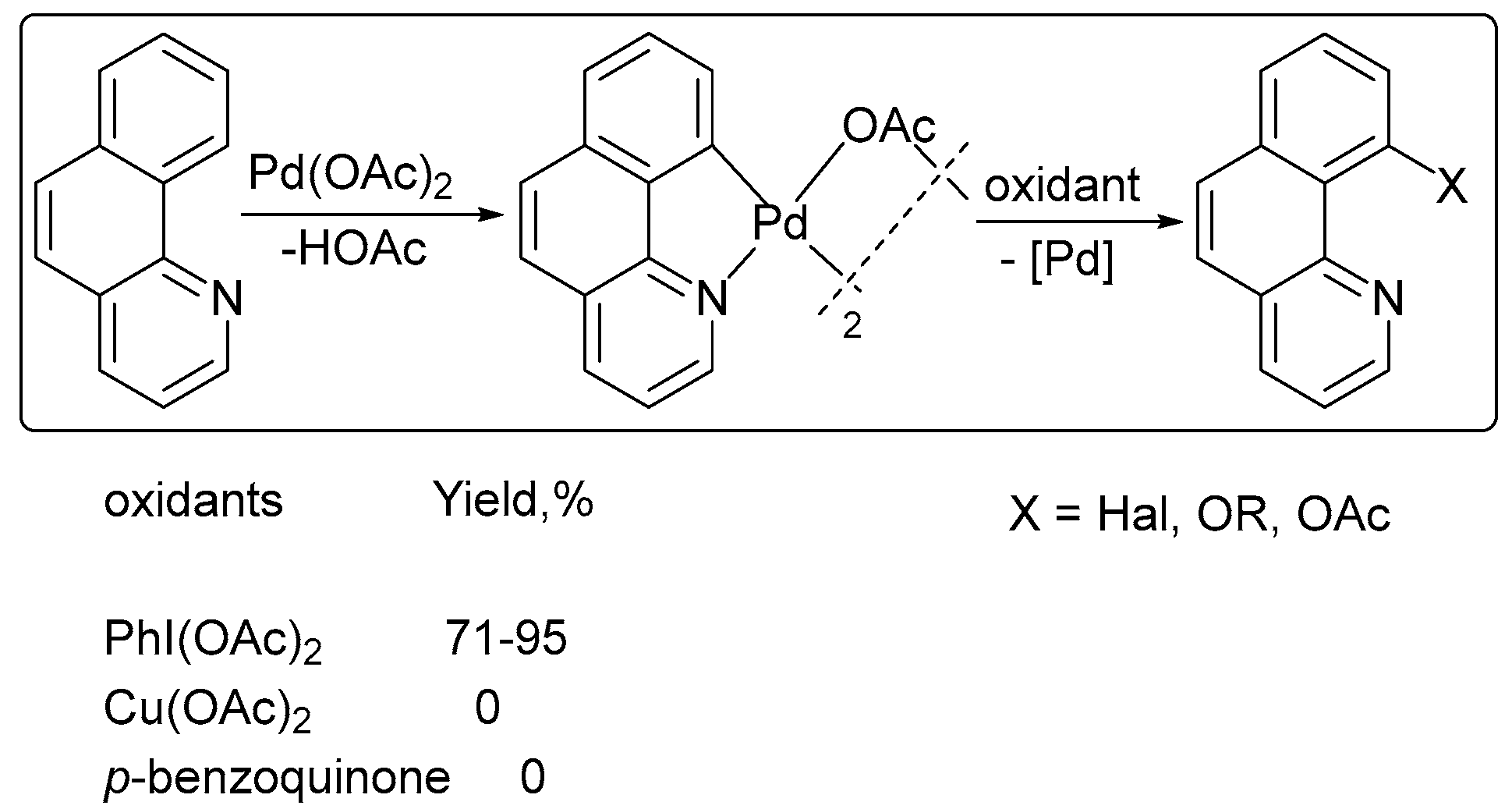
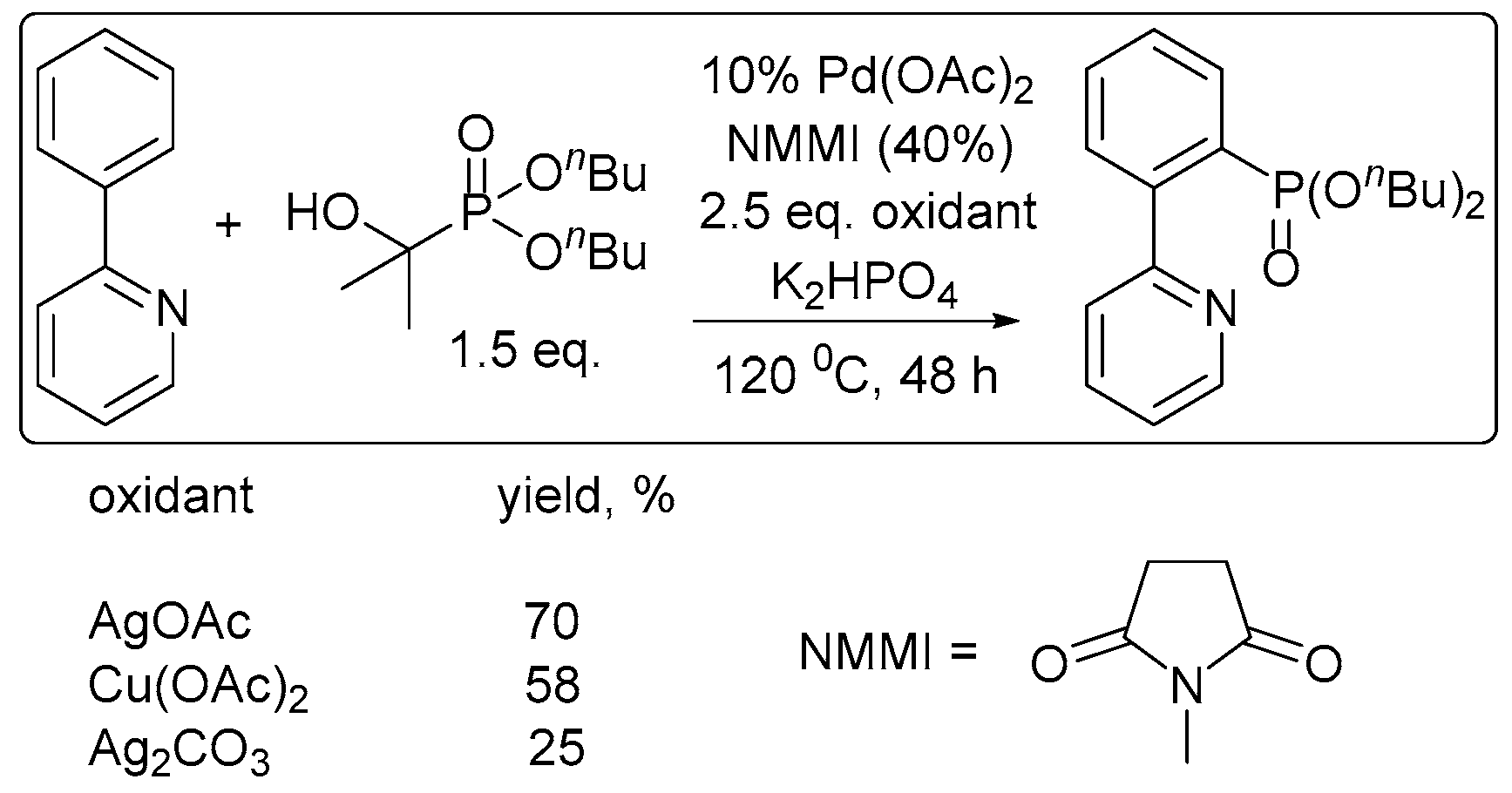
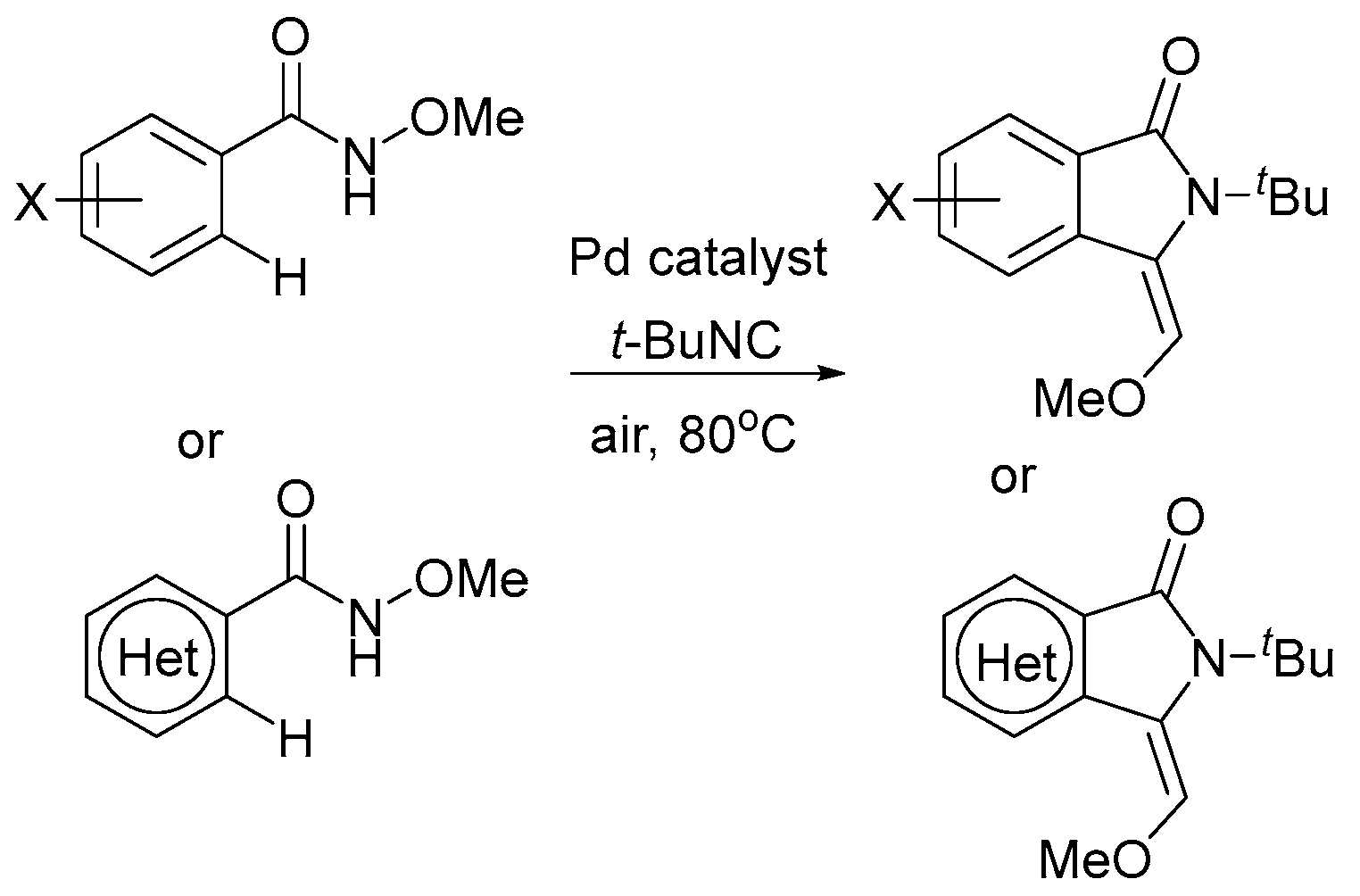
2. Oxidant Screening in Palladium Catalysis
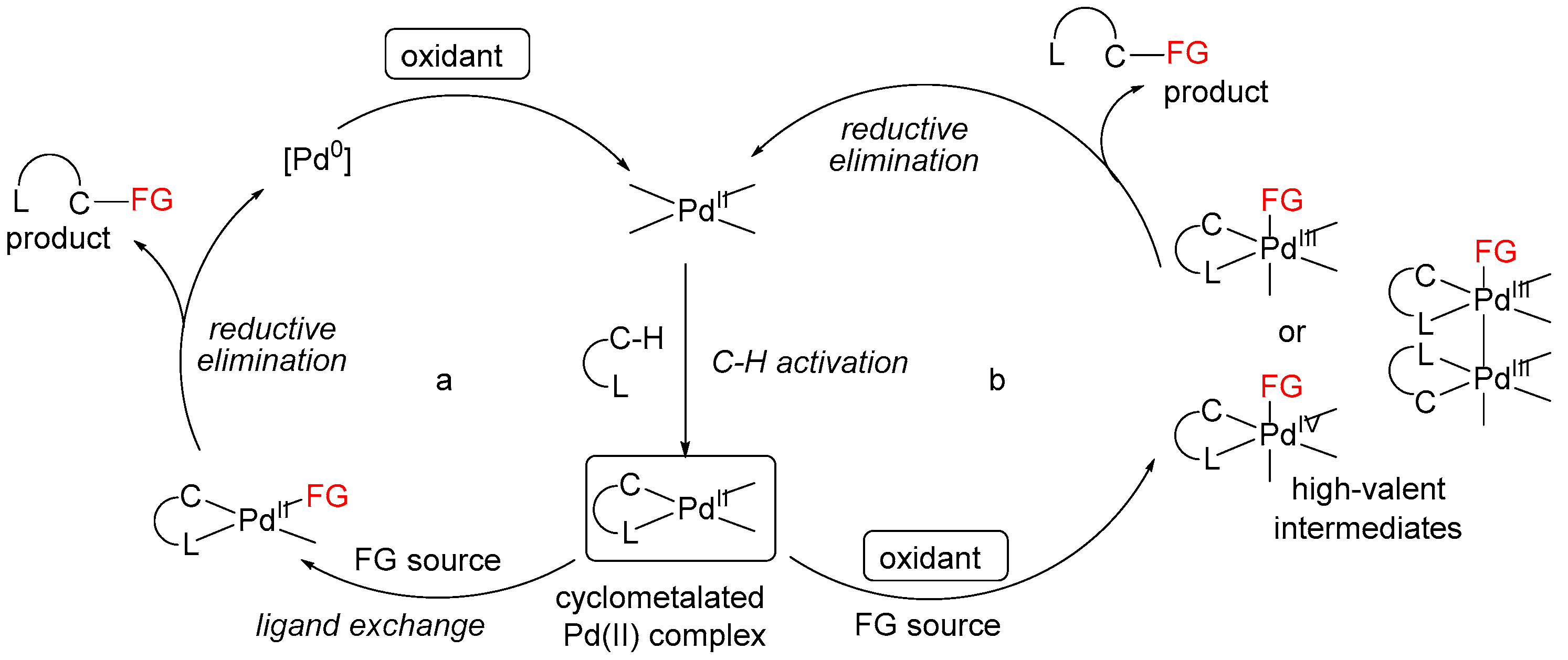
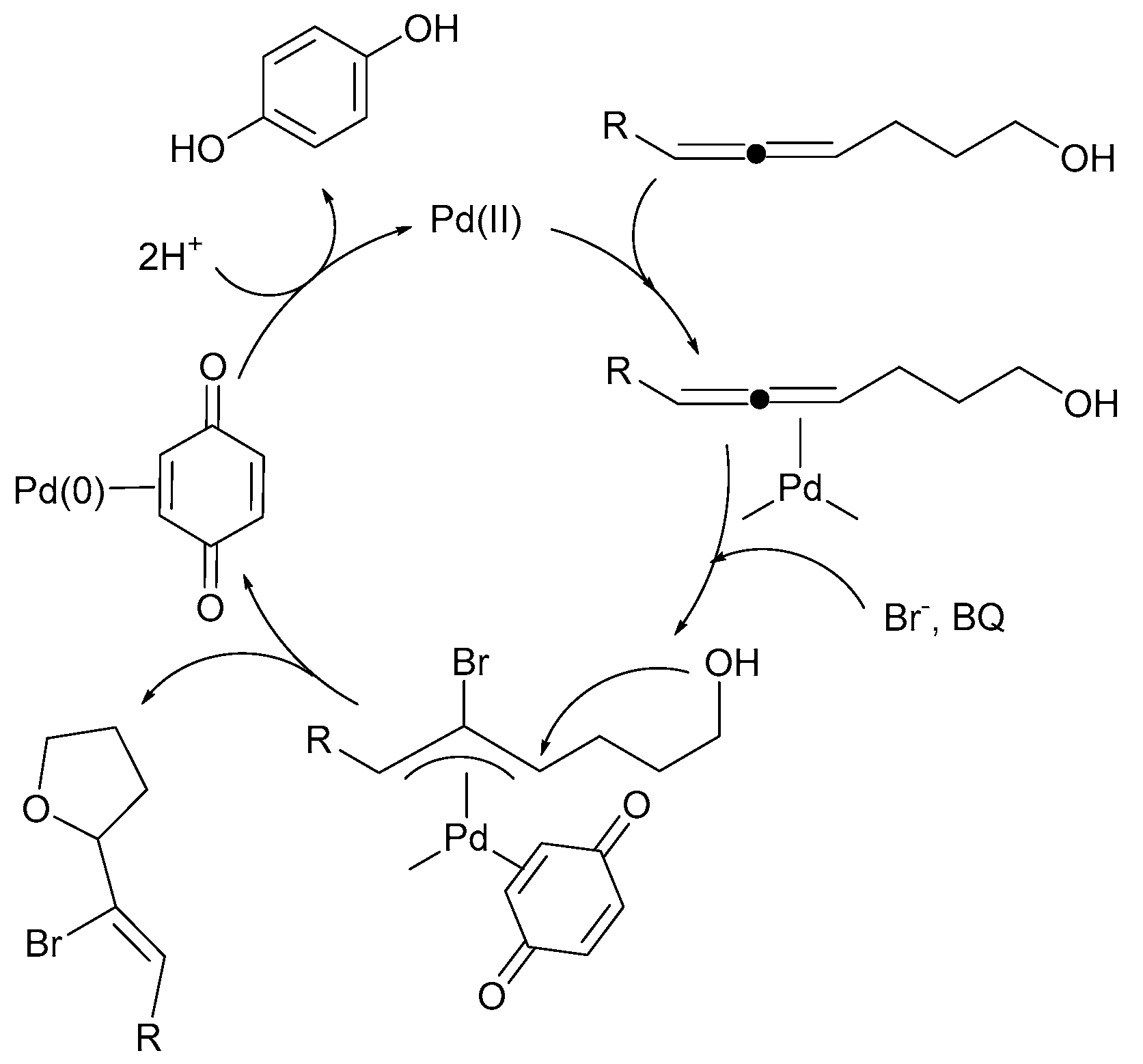
3. Mechanistic Considerations
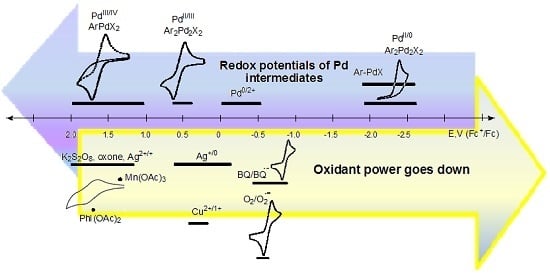
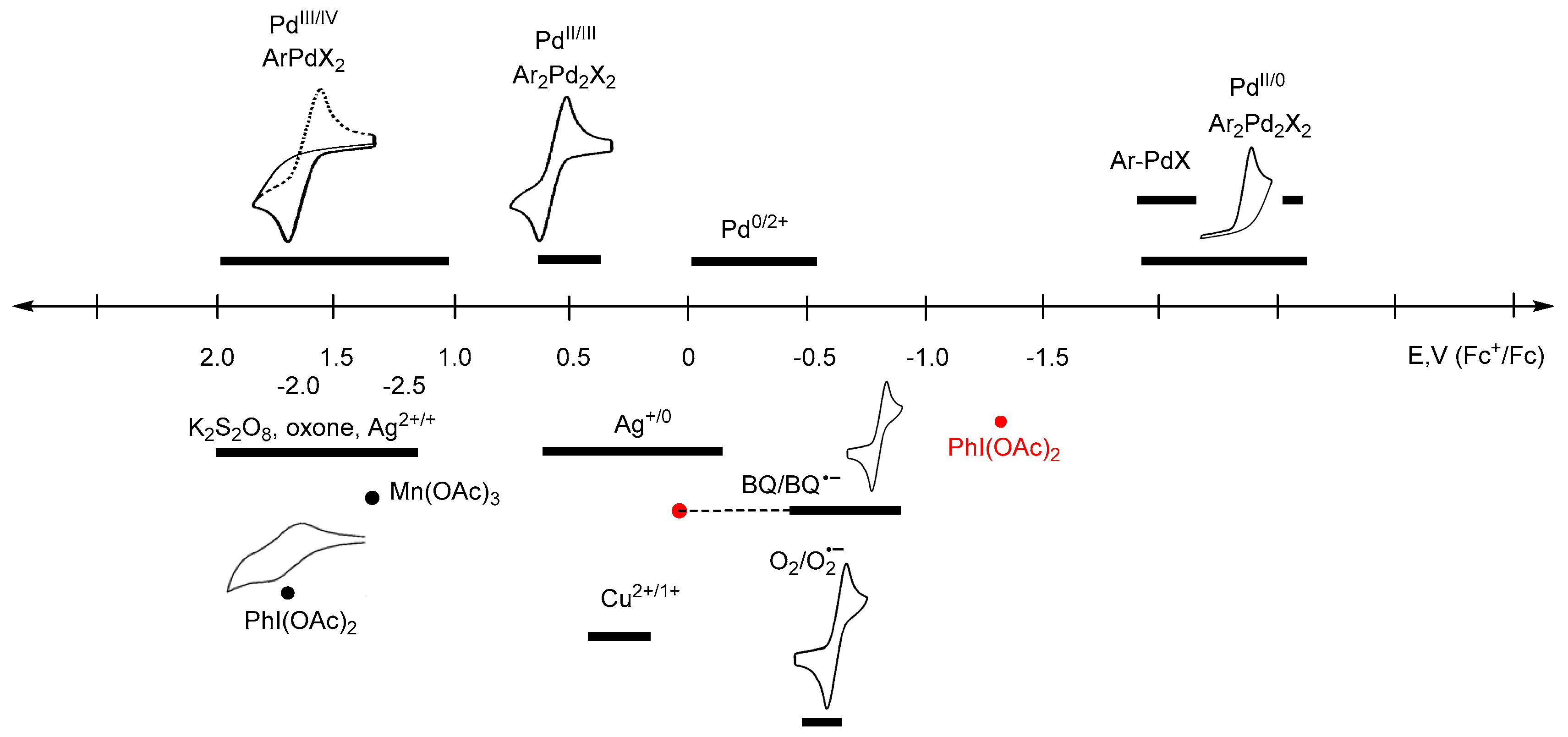
4. Electrode Potentials as the Measure of Oxidant/Reductant Strength
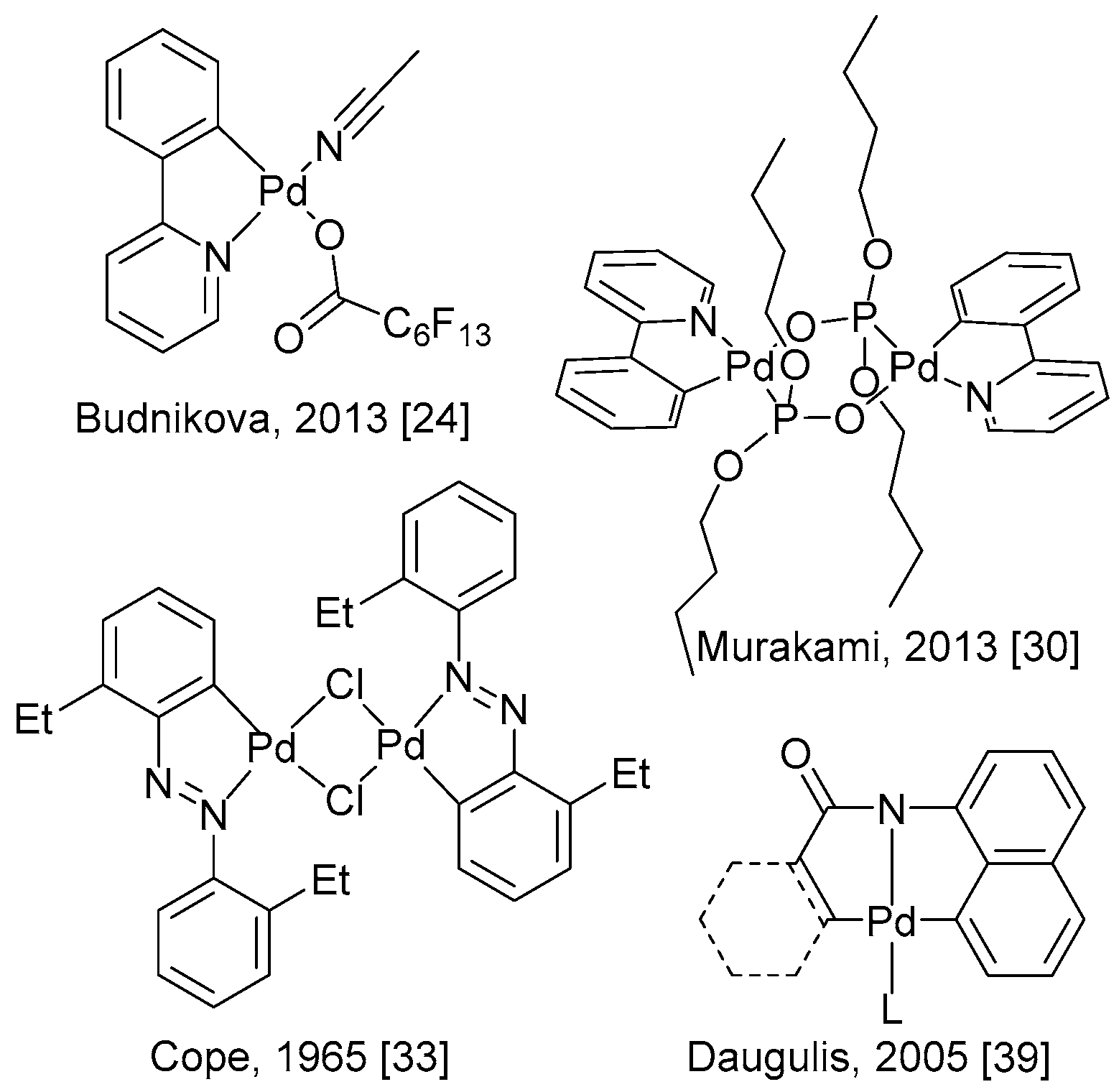
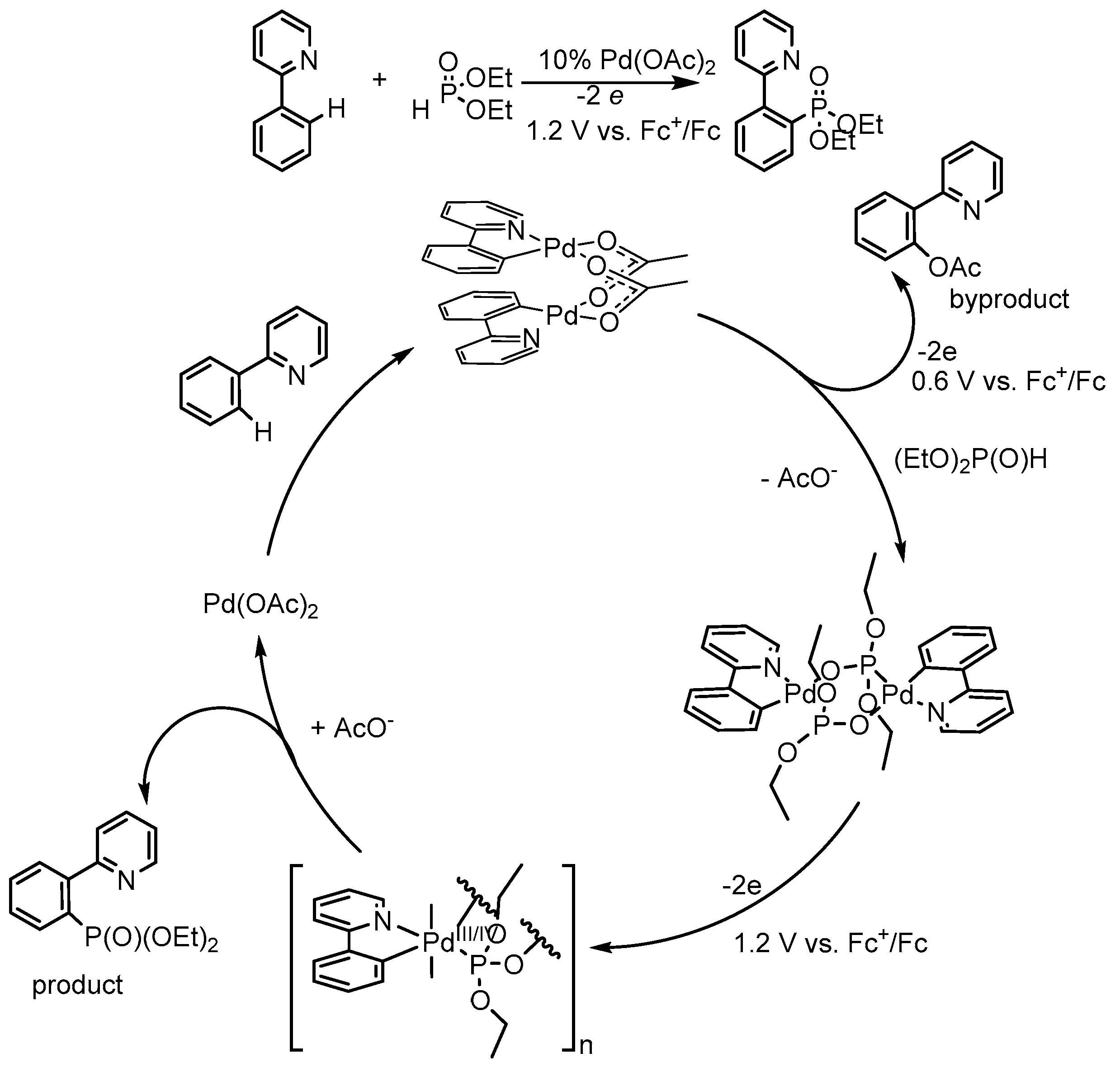
5. Electrocatalytic Ligand-Directed Substitution of C(sp2)–H Bonds
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gensch, T.; Hopkinson, M.N.; Glorius, F.; Wencel-Delord, J. Mild metal-catalyzed C–H activation: Examples and concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Shi, Z.; Yuan, Y. Transition-metal-catalyzed Chelation-assisted C–H Functionalization of Aromatic Substrates. Chem. Rec. 2016, 16, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C−H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed]
- Wencel-Delord, J.; Kim, J.G.; Carr, K.J.T.; Bellina, F.; Soulé, J.-F.; Wang, G.-W.; Bruneau, C.; Dana, S.; Li, J.; Ackermann, L.; et al. C–H Bond Activation and Catalytic Functionalization I; Dixneuf, P.H., Doucet, H., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Ilies, L.; Nakamura, E.; Chatani, N.; Hirano, K.; Jones, W.; Sustac Roman, D.; Zhou, T.; Dailler, D.; Samantaray, M.K.; Bézier, D.; et al. C–H Bond Activation and Catalytic Functionalization II; Dixneuf, P.H., Doucet, H., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, W.; Shi, R.; Lei, A. Oxidative Coupling between Two Hydrocarbons: An Update of Recent C–H Functionalizations. Chem. Rev. 2015, 115, 12138–12204. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-M.; Li, B.-J.; Yang, Z.; Shi, Z.-J. Organopalladium(IV) chemistry. Chem. Soc. Rev. 2010, 39, 712–733. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, P.; Taylor, R.J.K.; Fairlamb, I.J.S. Emergence of Palladium(IV) Chemistry in Synthesis and Catalysis. Chem. Rev. 2010, 110, 824–889. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.M.; Mei, T.-S.; Wang, X.; Yu, J.-Q. Bystanding F+ Oxidants Enable Selective Reductive Elimination from High-Valent Metal Centers in Catalysis. Angew. Chem. Int. Ed. 2011, 50, 1478–1491. [Google Scholar] [CrossRef] [PubMed]
- Hickman, A.J.; Sanford, M.S. High-valent organometallic copper and palladium in catalysis. Nature 2012, 484, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Powers, D.C.; Geibel, M.A.L.; Klein, J.E.M.N.; Ritter, T. Bimetallic Palladium Catalysis: Direct Observation of Pd(III)−Pd(III) Intermediates. J. Am. Chem. Soc. 2009, 131, 17050–17051. [Google Scholar] [CrossRef] [PubMed]
- Deprez, N.R.; Sanford, M.S. Synthetic and Mechanistic Studies of Pd-Catalyzed C−H Arylation with Diaryliodonium Salts: Evidence for a Bimetallic High Oxidation State Pd Intermediate. J. Am. Chem. Soc. 2009, 131, 11234–11241. [Google Scholar] [CrossRef] [PubMed]
- Budnikova, Y.H.; Gryaznova, T.V.; Grinenko, V.V.; Dudkina, Y.B.; Khrizanforov, M.N. Eco-efficient electrocatalytic C–P bond formation. Pure Appl. Chem. 2017, 89, 311–330. [Google Scholar] [CrossRef]
- Grayaznova, T.V.; Dudkina, Y.B.; Islamov, D.R.; Kataeva, O.N.; Sinyashin, O.G.; Vicic, D.A.; Budnikova, Y.H. Pyridine-directed palladium-catalyzed electrochemical phosphonation of C(sp2)–H bond. J. Organomet. Chem. 2015, 785, 68–71. [Google Scholar] [CrossRef]
- Gryaznova, T.; Dudkina, Y.; Khrizanforov, M.; Sinyashin, O.; Kataeva, O.; Budnikova, Y. Electrochemical properties of diphosphonate-bridged palladacycles and their reactivity in arene phosphonation. J. Solid State Electrochem. 2015, 19, 2665–2672. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Strekalova, S.O.; Kholin, K.V.; Khrizanforova, V.V.; Kadirov, M.K.; Gryaznova, T.V.; Budnikova, Y.H. Novel approach to metal-induced oxidative phosphorylation of aromatic compounds. Catal. Today 2017, 279, 133–141. [Google Scholar] [CrossRef]
- Grinenko, V.V.; Khrizanforov, M.N.; Strekalova, S.O.; Khrizanforova, V.V.; Kholin, K.V.; Gryaznova, T.V.; Budnikova, Y.H. Electrooxidative phosphorylation of coumarins by bimetallic catalytic systems Ni(II)/Mn(II) or Co(II)/Mn(II). Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1660–1661. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Strekalova, S.O.; Grinenko, V.V.; Khrizanforova, V.V.; Gryaznova, T.V.; Budnikova, Y.H. Various ways of C–P bonds formation via selective electrochemical phosphorylation of aromatic C–H bonds. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1491–1493. [Google Scholar] [CrossRef]
- Gryaznova, T.V.; Khrizanforov, M.N.; Strekalova, S.O.; Budnikova, Y.H.; Sinyashin, O.G. Electrochemical oxidative phosphonation of azoles. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1658–1659. [Google Scholar] [CrossRef]
- Strekalova, S.O.; Khrizanforov, M.N.; Shamsieva, A.V.; Grinenko, V.V.; Gryaznova, T.V.; Musina, E.I.; Karasik, A.A.; Budnikova, Y.H. Direct phosphorylation of pyridine in the presence of Ni(BF4)2bpy and CoCl2bpy metal complexes. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1545–1546. [Google Scholar] [CrossRef]
- Khrizanforova, V.V.; Khrizanforov, M.N.; Gryaznova, T.V.; Budnikova, Y.H. Electrochemical pathway to CH/PH functionalization of diphenylphosphine oxide. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1602–1603. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Strekalova, S.O.; Kholin, K.V.; Khrizanforova, V.V.; Grinenko, V.V.; Gryaznova, T.V.; Budnikova, Y.H. One-stage Synthesis of FcP(O)(OC2H5)2 from Ferrocene and α-Hydroxyethylphosphonate. RSC Adv. 2016, 6, 42701–42707. [Google Scholar] [CrossRef]
- Dudkina, Y.B.; Mikhaylov, D.Y.; Gryaznova, T.V.; Sinyashin, O.G.; Vicic, D.A.; Budnikova, Y.H. MII/MIII-Catalyzed ortho-Fluoroalkylation of 2-Phenylpyridine. Eur. J. Org. Chem. 2012, 2114–2117. [Google Scholar] [CrossRef]
- Dudkina, Y.B.; Mikhaylov, D.Y.; Gryaznova, T.V.; Tufatullin, A.I.; Kataeva, O.N.; Vicic, D.A.; Budnikova, Y.H. Electrochemical Ortho Functionalization of 2-Phenylpyridine with Perfluorocarboxylic Acids Catalyzed by Palladium in Higher Oxidation States. Organometallics 2013, 32, 4785–4792. [Google Scholar] [CrossRef]
- Dudkina, Y.B.; Kholin, K.V.; Gryaznova, T.V.; Islamov, D.R.; Kataeva, O.N.; Rizvanov, I.K.; Levitskaya, A.I.; Fominykh, O.D.; Balakina, M.Y.; Sinyashin, O.G.; et al. Redox Trends in Cyclometalated Palladium(II) Complexes. Dalton Trans. 2017, 46, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, D.; Dick, A.R.; Anani, W.Q.; Sanford, M.S. Scope and selectivity in palladium-catalyzed directed C–H bond halogenation reactions. Tetrahedron 2006, 62, 11483–11498. [Google Scholar] [CrossRef]
- Dick, A.R.; Hull, K.L.; Sanford, M.S. A Highly Selective Catalytic Method for the Oxidative Functionalization of C–H Bonds. J. Am. Chem. Soc. 2004, 126, 2300–2301. [Google Scholar] [CrossRef] [PubMed]
- Tsujihara, T.; Takenaka, K.; Onitsuka, K.; Hatanaka, M.; Sasai, H. PdII/PdIV Catalytic Enantioselective Synthesis of Bicyclo[3.1.0]hexanes via Oxidative Cyclization of Enynes. J. Am. Chem. Soc. 2009, 131, 3452–3453. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.W.; Sanford, M.S. Palladium(II/IV) catalyzed cyclopropanation reactions: Scope and mechanism. Tetrahedron 2009, 65, 3211–3221. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yano, T.; Ishida, N.; Murakami, M. Pyridine-directed palladium-catalyzed phosphonation of C(sp2)–H bonds. Angew. Chem. Int. Ed. 2013, 52, 9801–9804. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, J.; Muzart, J. Dehydrogenative (Hetero)arene Alkoxylations Triggered by PdII-Catalyzed C(sp2)–H Activation and Coordinating Substituent: PdII,III or PdIV Complex as Key Intermediate? Eur. J. Org. Chem. 2017, 3528–3548. [Google Scholar] [CrossRef]
- Hartwell, G.E.; Lawrence, R.V.; Smas, M.J. The formation of palladium(II)– and platinum(II)–carbon bonds by proton abstraction from benzo[h]quinoline and 8-Methylquinoline. J. Chem. Soc. Chem. Commun. 1970, 912. [Google Scholar] [CrossRef]
- Cope, A.C.; Siekman, R.W. Formation of Covalent Bonds from Platinum or Palladium to Carbon by Direct Substitution. J. Am. Chem. Soc. 1965, 87, 3272–3273. [Google Scholar] [CrossRef]
- Cope, A.C.; Friedrich, E.C. Electrophilic aromatttic substitution reactions by platinum(II) and palladium(II) chlorides on N,N-dimethylbenzylamines. J. Am. Chem. Soc. 1968, 90, 909–913. [Google Scholar] [CrossRef]
- Constable, A.G.; McDonald, W.G.; Sawkins, L.C.; Shaw, B.L. Palladation of dimethylhydrazones, oximes, and oxime O-allyl ethers: Crystal structure of [Pd3(ON=CPriPh)6]. J. Chem. Soc. Chem. Commun. 1978, 1061–1062. [Google Scholar] [CrossRef]
- Fuchita, Y.; Hiraki, K.; Uchiyama, T. Metallation of aliphatic carbon atoms. Part 1. Synthesis and characterization of the cyclopalladated complexes of 2-neopentylpyridine. J. Chem. Soc. Dalton Trans. 1983, 897–899. [Google Scholar] [CrossRef]
- Alsters, P.L.; Engel, P.F.; Hogerheide, M.P.; Copijn, M.; Spek, A.L.; van Koten, G. Rigid five- and six-membered C,N,N’-bound aryl-, benzyl-, and alkylorganopalladium complexes: sp2 vs. sp3 carbon-hydrogen activation during cyclopalladation and palladium(IV) intermediates in oxidative addition reactions with dihalogens and alkyl halides. Organometallics 1993, 12, 1831–1844. [Google Scholar] [CrossRef]
- Rouquet, G.; Chatani, N. Catalytic Functionalization of C(sp2)–H and C(sp3)–H Bonds by Using Bidentate Directing Groups. Angew. Chem. Int. Ed. 2013, 52, 11726–11743. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.G.; Shabashov, D.; Daugulis, O. Highly Regioselective Arylation of sp3 C−H Bonds Catalyzed by Palladium Acetate. J. Am. Chem. Soc. 2005, 127, 13154–13155. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.M.; Mei, T.-S.; Wasa, M.; Yu, J.-Q. Weak Coordination as a Powerful Means for Developing Broadly Useful C–H Functionalization Reactions. Acc. Chem. Res. 2012, 45, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Powers, D.C.; Raynaud, J.; Graham, M.J.; Xie, P.; Lee, E.; Ritter, T. Synthesis and structure of solution-stable one-dimensional palladium wires. Nat. Chem. 2011, 3, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Bercaw, J.E.; Durrell, A.C.; Gray, H.B.; Green, J.C.; Hazari, N.; Labinger, J.A.; Winkler, J.R. Electronic Structures of PdII Dimers. Inorg. Chem. 2010, 49, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.N.; Adrio, L.A.; Albrecht, T.; White, A.J.P.; Newton, M.A.; Nachtegaal, M.; Figueroa, S.J.A.; Hii, K.K.M. Electronic structures of cyclometalated palladium complexes in the higher oxidation states. Dalton Trans. 2015, 44, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Fabre, I.; von Wolff, N.; Le Duc, G.; Flegeau, E.F.; Bruneau, C.; Dixneuf, P.H.; Jutand, A. Autocatalytic Intermolecular versus Intramolecular Deprotonation in C–H Bond Activation of Functionalized Arenes by Ruthenium(II) or Palladium(II) Complexes. Chem. Eur. J. 2013, 19, 7595–7604. [Google Scholar] [CrossRef] [PubMed]
- Connelly, N.G.; Geiger, W.E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Grübel, K.; Cěrník, M. Simple spectrophotometric determination of monopersulfate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Block, P.A.; Brown, R.A.; Robinson, D. Novel Activation Technologies for Sodium Persulfate in Situ Chemical Oxidation. In Proceedings of the Fourth International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, 24–27 May 2004. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Wulfsberg, G. Inorganic Chemistry; University Science Books: Sausalito, CA, USA, 2000. [Google Scholar]
- Nayak, B.; Dash, U.N. The silver/silver acetate electrode Part I. Standard potential in formamide at 25 °C. J. Electroanal. Chem. 1973, 41, 323–328. [Google Scholar] [CrossRef]
- Felton, G.A.N.; Mebi, C.A.; Petro, B.J.; Vannucci, A.K.; Evans, D.H.; Glass, R.S.; Lichtenberger, D.L. Review of electrochemical studies of complexes containing the Fe2S2 core characteristic of [FeFe]-hydrogenases including catalysis by these complexes of the reduction of acids to form dihydrogen. J. Organomet. Chem. 2009, 694, 2681–2699. [Google Scholar] [CrossRef]
- Bour, J.R.; Camasso, N.M.; Meucci, E.A.; Kampf, J.W.; Canty, A.J.; Sanford, M.S. Carbon–carbon bond-forming reductive elimination from isolated Nickel(III) complexes. J. Am. Chem. Soc. 2016, 138, 16105–16111. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Prieto-Simón, B.; Fàbregas, E. Comparative study of electron mediators used in the electrochemical oxidation of NADH. Biosens. Bioelectron. 2004, 19, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kitagawa, T. Solvent effects of 1,4-benzoquinone and its anion radicals probed by resonance Raman and absorption spectra and their correlation with redox potentials. J. Raman Spectrosc. 1998, 29, 773–780. [Google Scholar] [CrossRef]
- Jin, B.-K.; Li, L.; Huang, J.-L.; Zhang, S.-Y.; Tian, Y.-P.; Yang, J.-X. IR Spectroelectrochemical Cyclic Voltabsorptometry and Derivative Cyclic Voltabsorptometry. Anal. Chem. 2009, 81, 4476–4481. [Google Scholar] [CrossRef] [PubMed]
- Steckhan, E. Indirect Electroorganic Syntheses—A Modern Chapter of Organic Electrochemistry. Angew. Chem. Int. Ed. 1986, 28, 683–701. [Google Scholar] [CrossRef]
- Kokkinidis, G.; Papadopoulou, M.; Varvoglis, A. Electrochemical reduction of [bis(acyloxy)iodo]arenes. Electrochim. Acta 1989, 34, 133–139. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J. Electrocatalytic Oxygen Reduction Reaction. In PEM Fuel Cell Electrocatalysts and Catalyst Layers. Fundamentals and Applications; Zhang, J., Ed.; Springer-Verlag: London, UK, 2008. [Google Scholar]
- Jeena, V.; Robinson, R.S. Convenient photooxidation of alcohols using dye sensitised zinc oxide in combination with silver nitrate and TEMPO. Chem. Commun. 2012, 48, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Rychnosky, S.D.; Vaidyanathan, R.; Beauchamp, T.; Lin, R.; Farmer, P.J. AM1-SM2 Calculations Model the Redox Potential of Nitroxyl Radicals Such as TEMPO. J. Org. Chem. 1999, 64, 6745–6749. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Arkhipova, D.M.; Shekurov, R.P.; Gerasimova, T.P.; Ermolaev, V.V.; Islamov, D.R.; Miluykov, V.A.; Kataeva, O.N.; Khrizanforova, V.V.; Sinyashin, O.G.; et al. Novel paste electrodes based on phosphonium salt room temperature ionic liquids for studying the redox properties of insoluble compounds. J. Sol. State Electrochem. 2015, 19, 2883–2890. [Google Scholar] [CrossRef]
- Favier, I.; Duñach, E. New protic salts of aprotic polar solvents. Tetrahedron Lett. 2004, 45, 3393–3395. [Google Scholar] [CrossRef]
- Amatore, C.; Jutand, A.; Lemaître, F.; Ricard, J.L.; Kozuch, S.; Shaik, S. Formation of anionic palladium(0) complexes ligated by the trifluoroacetate ion and their reactivity in oxidative addition. J. Organomet. Chem. 2004, 689, 3728–3734. [Google Scholar] [CrossRef]
- Amatore, C.; Jutand, A.; Khalil, F. Neutral palladium(0) complexes from Pd(OAc)2 and tri-2-furylphosphine and their reactivity in oxidative addition of iodobenzene. ARKIVOC 2006, 38–48. [Google Scholar] [CrossRef]
- Diculescu, V.C.; Chiorcea-Paquim, A.-M.; Corduneanu, O.; Oliveira-Brett, A.M. Palladium nanoparticles and nanowires deposited electrochemically: AFM and electrochemical characterization. J. Solid State Electrochem. 2007, 11, 887–898. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Stahl, S.S.; Alsters, P.L. Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications and Academic Perspectives; Wiley-VCH: WeinHeim, Germany, 2016. [Google Scholar]
- Jutand, A. Contribution of Electrochemistry to Organometallic Catalysis. Chem. Rev. 2008, 108, 2300–2347. [Google Scholar] [CrossRef] [PubMed]
- Jutand, A.; Mosleh, A. Nickel- and Palladium-Catalyzed Homocoupling of Aryl Triflates. Scope, Limitation, and Mechanistic Aspects. J. Org. Chem. 1997, 62, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Lal, G.S.; Pez, G.P.; Syvret, R.G. Electrophilic NF Fluorinating Agents. Chem. Rev. 1996, 96, 1737–1755. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.D.; Kitamura, T. Alternative, Easy Preparation of (Diacetoxyiodo)arenes from Iodoarenes Using Potassium Peroxodisulfate as the Oxidant. Synthesis 2005, 12, 1932–1934. [Google Scholar] [CrossRef]
- Varvoglis, A. Aryliodine(III) dicarboxylates. Chem. Soc. Rev. 1981, 10, 377–407. [Google Scholar] [CrossRef]
- Reddy, B.V.S.; Revathi, G.; Reddy, A.S.; Yadav, J.S. Regioselective ortho-acetoxylation/methoxylation of N-(2-benzoylphenyl)benzamides via substrate directed C–H activation. Tetrahedron Lett. 2011, 52, 5926–5929. [Google Scholar] [CrossRef]
- Reddy, B.V.S.; Narasimhulu, G.; Umadevi, N.; Yadav, J.S. Quinazolinone-Directed C–H Activation: A Novel Strategy for the Acetoxylation–Methoxylation of the Arenes. Synlett 2012, 23, 1364–1370. [Google Scholar] [CrossRef]
- Reddy, B.V.S.; Umadevi, N.; Narasimhulu, G.; Yadav, J.S. Oxidative C–H functionalization: A novel strategy for the acetoxylation/alkoxylation of arenes tethered to 3,4-dihydroisoquinolines. Tetrahedron Lett. 2012, 53, 6091–6094. [Google Scholar] [CrossRef]
- Wang, G.-W.; Yuan, T.-T. Palladium-Catalyzed Alkoxylation of N-Methoxybenzamides via Direct sp2 C−H Bond Activation. J. Org. Chem. 2010, 75, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cui, X.; Yang, F.; Zhang, Q.; Zhu, Y.; Wu, Y. Palladium-catalyzed direct ortho C–O bond construction of azoxybenzenes with carboxylic acids and alcohols. Org. Chem. Front. 2015, 2, 951–955. [Google Scholar] [CrossRef]
- Piancatelli, G.; Leonelli, F. Oxidation of nerol to neral with iodobenzene diacetate and tempo. Org. Synth. 2006, 18–23. [Google Scholar] [CrossRef]
- Jonasson, C.; Horvath, A.; Backvall, J.E. Intramolecular Palladium(II)-Catalyzed 1,2-Addition to Allenes. J. Am. Chem. Soc. 2000, 122, 9600–9609. [Google Scholar] [CrossRef]
- Giri, R.; Lam, J.K.; Yu, J.-Q. Synthetic Applications of Pd(II)-Catalyzed C−H Carboxylation and Mechanistic Insights: Expedient Routes to Anthranilic Acids, Oxazolinones, and Quinazolinones. J. Am. Chem. Soc. 2010, 132, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Kuang, C. Palladium-Catalyzed Ortho-Alkoxylation of 2-Aryl-1,2,3-triazoles. J. Org. Chem. 2014, 79, 6105–6112. [Google Scholar] [CrossRef] [PubMed]
- Hull, K.L.; Lanni, E.L.; Sanford, M.S. Highly Regioselective Catalytic Oxidative Coupling Reactions: Synthetic and Mechanistic Investigations. J. Am. Chem. Soc. 2006, 128, 14047–14049. [Google Scholar] [CrossRef] [PubMed]
- Topczewski, J.J.; Sanford, M.S. Carbon–hydrogen (C–H) bond activation at PdIV: A Frontier in C–H functionalization catalysis. Chem. Sci. 2015, 6, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Han, J.; Chen, C.P.; Shi, D.Q.; Zhao, Y.S. Palladium-catalyzed oxygenation of C(sp2)–H and C(sp3)–H bonds under the assistance of oxalyl amide. RSC Adv. 2015, 5, 28430–28434. [Google Scholar] [CrossRef]
- Li, W.; Sun, P. Pd(OAc)2-Catalyzed Alkoxylation of Arylnitriles via sp2 C–H Bond Activation Using Cyano as the Directing Group. J. Org. Chem. 2012, 77, 8362–8366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, P. Palladium-Catalyzed Direct C(sp2)–H Alkoxylation of 2-Aryloxypyridines Using 2-Pyridyloxyl as the Directing Group. J. Org. Chem. 2014, 79, 8457–8461. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kim, M.; Park, J.; Kim, M.; Kwak, J.H.; Jung, Y.H.; Oh, J.S.; Lee, Y.; Kim, I.S. Palladium-Catalyzed Direct Acylation of Ketoximes and Aldoximes from the Alcohol Oxidation Level through C–H Bond Activation. Eur. J. Org. Chem. 2013, 6656–6665. [Google Scholar] [CrossRef]
- Gandeepan, P.; Cheng, C.-H. Allylic Carbon–Carbon Double Bond Directed Pd-Catalyzed Oxidative ortho-Olefination of Arenes. J. Am. Chem. Soc. 2012, 134, 5738–5741. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.M.; Wang, D.-H.; Yu, J.-Q. Ligand-Accelerated C–H Activation Reactions: Evidence for a Switch of Mechanism. J. Am. Chem. Soc. 2010, 132, 14137–14151. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.D.; Sale, D.; Engle, K.M.; Yu, J.-Q.; Blackmond, D.G. Mechanistic Rationalization of Unusual Kinetics in Pd-Catalyzed C–H Olefination. J. Am. Chem. Soc. 2012, 134, 4600–4606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Xu, H.; Kong, W.-J.; Shang, M.; Dai, H.-X.; Yu, J.-Q. Overcoming the limitations of directed C–H functionalizations of heterocycles. Nature 2014, 515, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.N.; Stahl, S.S. Overcoming the “Oxidant Problem”: Strategies to Use O2 as the Oxidant in Organometallic C–H Oxidation Reactions Catalyzed by Pd (and Cu). Acc. Chem. Res. 2012, 45, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jiang, H. Palladium-Catalyzed Oxidation of Unsaturated Hydrocarbons Using Molecular Oxygen. Acc. Chem. Res. 2012, 45, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, N.; Schweitzer-Chaput, B.; Klussmann, M. Oxidative coupling reactions for the functionalisation of C–H bonds using oxygen. Catal. Sci. Technol. 2014, 4, 2778–2796. [Google Scholar] [CrossRef]
- Baslé, O. Cross-Dehydrogenative-Coupling Reactions with Molecular Oxygen as the Terminal Oxidant. In From C–H to C–C Bonds: Cross-Dehydrogenative-Coupling; Li, C.-J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 197–218. ISBN 978-1-84973-797-5. [Google Scholar]
- Wencel-Delord, J.; Colobert, F. A remarkable solvent effect of fluorinated alcohols on transition metal catalysed C–H functionalizations. Org. Chem. Front. 2016, 3, 394–400. [Google Scholar] [CrossRef]
- Dherbassy, Q.; Schwertz, G.; Chessé, M.; Hazra, C.K.; Wencel-Delord, J.; Colobert, F. 1,1,1,3,3,3-Hexafluoroisopropanol as a Remarkable Medium for Atroposelective Sulfoxide-Directed Fujiwara–Moritani Reaction with Acrylates and Styrenes. Chem. Eur. J. 2016, 22, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Wesch, T.; Leroux, F.R.; Colobert, F. Atropodiastereoselective C–H Olefination of Biphenyl p-Tolyl Sulfoxides with Acrylates. Adv. Synth. Catal. 2013, 355, 2139–2144. [Google Scholar] [CrossRef]
- Villuendas, P.; Serrano, E.; Urriolabeitia, E.P. Pd-catalysed ortho-alkoxylation of benzamides N-protected with an iminophosphorane functionality. New J. Chem. 2015, 39, 3077–3083. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Tian, M.; Lu, C.; Li, S.; Du, H. Purinyl N3-Directed Palladium-Catalyzed C–H Alkoxylation of N9-Arylpurines: A Late-Stage Strategy to Synthesize N9-(ortho-Alkoxyl)arylpurines. J. Org. Chem. 2016, 81, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Dudkina, Y.B.; Khrizanforov, M.N.; Gryaznova, T.V.; Budnikova, Y.H. Prospects of Synthetic Electrochemistry in the Development of New Methods of Electrocatalytic Fluoroalkylation. J. Organomet. Chem. 2014, 751, 301–305. [Google Scholar] [CrossRef]
- Budnikova, Y.H. Metal complex catalysis in organic electrosynthesis. Russ. Chem. Rev. 2002, 71, 111–139. [Google Scholar] [CrossRef]
- Mikhaylov, D.Y.; Gryaznova, T.V.; Dudkina, Y.B.; Khrizanphorov, M.N.; Latypov, S.K.; Kataeva, O.N.; Vicic, D.A.; Sinyashin, O.G.; Budnikova, Y.G. Electrochemical nickel-induced fluoroalkylation: Synthetic, structural and mechanistic study. Dalton Trans. 2012, 41, 165–172. [Google Scholar] [CrossRef] [PubMed]
| Strength | Oxidants | Reductants |
|---|---|---|
| Very strong | >0.8 | <−2.5 |
| Strong | 0.8–0.2 | −1.5 to −2.5 |
| Mild | 0.2 to −0.5 | −0.5 to −1.5 |
| Weak | <−0.5 | >−0.5 |
| Oxidant | Solvent | E° (V vs. Fc+/Fc) | Reference |
|---|---|---|---|
| Oxone | H2O | 1.98 | [46] |
| K2S2O8 | H2O | 1.48 | [47] |
| H2O | 1.39 | [48] | |
| H2O + [H+] | 1.50 | [48] | |
| CPE, solid | 1.58 | a | |
| [N(C6H2Br3-2,4,6)3]+ | CH3CN | 1.36 | [45] |
| Ag2+ | H2O | 1.36 | [49] |
| CPE, solid | 1.16 | a | |
| Mn(OAc)3 | CPE, solid | 1.32 | a |
| [N(C6H3Br2-2,4)3]+ | CH3CN | 1.14 | [45] |
| H2O2 | H2O | 1.18 | [47] |
| [NO]+ | CH2Cl2 | 1.00 | [45] |
| [Ru(phen)3]3+ | CH3CN | 0.87 | [45] |
| [NO]+ | CH3CN | 0.87 | [45] |
| [N(C6H4Br-4)3]+ | CH2Cl2 | 0.70 | [45] |
| CH3CN | 0.67 | [45] | |
| Ag+ | CH2Cl2 | 0.65 | [45] |
| THF | 0.41 | [45] | |
| acetone | 0.18 | [45] | |
| AgOAc | CPE, solid | 1.16 | a |
| AgOAc | formamide | −0.198 | [50] |
| AgNO3 | CH3CN | −0.08 | [51] |
| AgBF4 | CH3CN | −0.04 | [52] |
| Ag2O | CPE, solid | −1.26 | a |
| [Fe(η-C5H4COMe)2]+ | CH2Cl2 | 0.49 | [45] |
| [CuTf2] | CH3CN | 0.40 | [45] |
| Cu(OAc)2·H2O | CPE, solid | 0.33 | a |
| [Ni(tfd)2] | CH2Cl2 | 0.33 | [45] |
| [PtCl6]2− | H2O | 0.31 | [45] |
| Cl2 | CH3CN | 0.18 | [45] |
| DDQ | CH3CN | 0.13 | [45] |
| 1,4-BQ | H2O | 0.16 | [53,54] |
| H2O | −0.535 | [55] | |
| H2O | −0.526 | b | |
| CH3CN | −0.73 | [56] | |
| CH3CN | −0.86 | [55] | |
| CH3CN | −0.88 | c | |
| CH3CN + [H+] | d | ||
| CH2Cl2 | −0.805 | [55] | |
| Acetone | −0.875 | [55] | |
| Br2 | CH3CN | 0.07 | [45] |
| (FcBF4) [FeCp2]+ | 0 | [45] | |
| I2 | CH3CN | −0.14 | [45] |
| 0.0 | [57] | ||
| I+ | 0.33 | [56] | |
| TCNE | CH3CN | −0.27 | [45] |
| TCNQ | CH3CN | −0.30 | [45] |
| [FeCp*2]+ | CH3CN | −0.59 | [45] |
| CH2Cl2 | −0.48 | [45] | |
| PhI(OAc)2 | CH3CN | −1.293 | [43,58] |
| CH3CN | 1.70 | c | |
| CPE, solid | 1.70 | a | |
| O2 | H2O | −0.78 | [59] |
| H2O | −0.81 | b | |
| DMSO | −1.16 | [59] | |
| DMF | −1.24 | [59] | |
| Py | −1.24 | [59] | |
| MeCN | −1.25 | [59] | |
| Quinoline | −1.25 | [59] | |
| EMIBF4 | −1.23 | [59] | |
| PMIBF4 | −1.20 | [59] | |
| BMIBF4 | −1.24 | [59] | |
| [bmim]HFP | −1.26 | [59] | |
| TEMPO | CH2Cl2 | 0.014 | [60,61] |
| Palladium Complex | Solvent | E° (V vs. Fc+/Fc) | Reference |
|---|---|---|---|
| Pd2+/0 | DMF | −0.38 | [64] a |
| DMF | −0.02 | [65] b | |
| 0.1 M phosphate buffer | −0.64 | [66] | |
| −0.29 | [67] | ||
| H2O | −0.02 | [68] c | |
| PhPdIL | DMF | −0.88 reduction | [69] d |
| PdCl2(PPh3)2 | DMF | −1.29 reduction | [70] |
| Pd(OAc)2(TFP)2 | −1.34 reduction | [65] | |
| ArPdCl(PPh3) | DMF | >−2.03 reduction | [70] |
| [Pd(C^N)(OR)]2 | ACN | −2.03 to −2.37 reduction | [25] |
| 0.44–0.58 oxidation | [25] | ||
| DCM | −1.81 to −2.47 reduction | [25] | |
| 0.4 to 0.75 oxidation | [25] | ||
| [Pd(C^N)X]2 | ACN | −1.85 to −2.35 reduction | [25] |
| 0.63 to 1.00 oxidation | [25] | ||
| DCM | −2.29 to −2.43 reduction | [25] | |
| 0.73 to 0.74 oxidation | [25] | ||
| Pd(C^N)(CH3CN)ORF | ACN | −1.61 to −1.71 reduction | [25] |
| 1.19 to 1.32 oxidation | [25] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budnikova, Y.H.; Dudkina, Y.B.; Khrizanforov, M.N. Redox-Induced Aromatic C–H Bond Functionalization in Metal Complex Catalysis from the Electrochemical Point of View. Inorganics 2017, 5, 70. https://doi.org/10.3390/inorganics5040070
Budnikova YH, Dudkina YB, Khrizanforov MN. Redox-Induced Aromatic C–H Bond Functionalization in Metal Complex Catalysis from the Electrochemical Point of View. Inorganics. 2017; 5(4):70. https://doi.org/10.3390/inorganics5040070
Chicago/Turabian StyleBudnikova, Yulia H., Yulia B. Dudkina, and Mikhail N. Khrizanforov. 2017. "Redox-Induced Aromatic C–H Bond Functionalization in Metal Complex Catalysis from the Electrochemical Point of View" Inorganics 5, no. 4: 70. https://doi.org/10.3390/inorganics5040070
APA StyleBudnikova, Y. H., Dudkina, Y. B., & Khrizanforov, M. N. (2017). Redox-Induced Aromatic C–H Bond Functionalization in Metal Complex Catalysis from the Electrochemical Point of View. Inorganics, 5(4), 70. https://doi.org/10.3390/inorganics5040070