Adsorption of Water on Two-Dimensional Crystals: Water/Graphene and Water/Silicatene
Abstract
:1. Introduction and Literature Survey
1.1. Why Graphene for Heterogeneous Catalysis?
1.2. Functionalized Graphene and Water Adsorption
1.3. Inorganic 2D Crystals and Water Adsorption
2. Results and Discussion of Our Own Work
2.1. Methodology
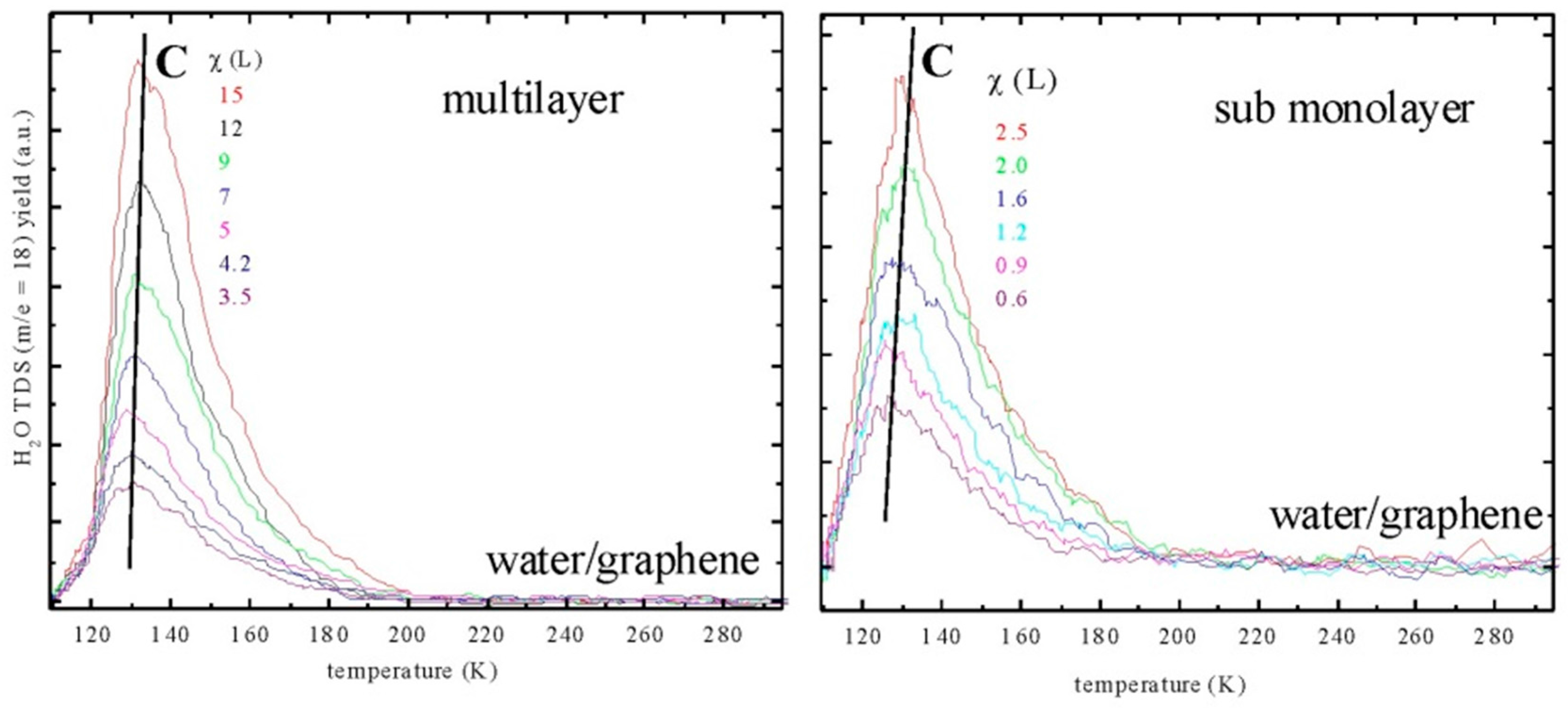
2.2. Water Adsorption on Graphene
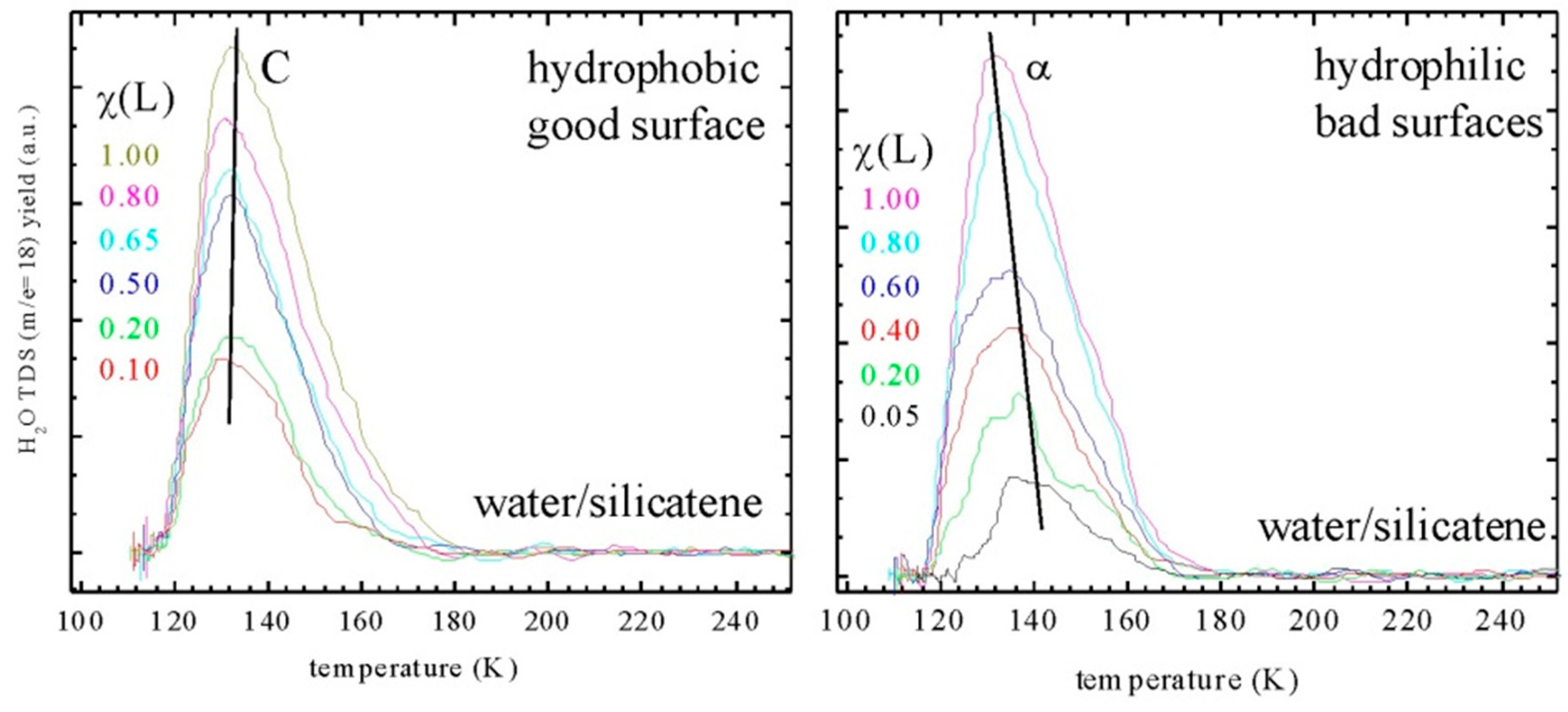
2.3. Water Adsorption on Silicatene
3. Experimental Section
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hu, S.; Lozada-Hidalgo, M.; Wang, F.C.; Mishchenko, A.; Schedin, F.; Nair, R.R.; Hill, E.W.; Boukhvalov, D.W.; Katsnelson, M.I.; Dryfe, R.A.W.; et al. Proton transport through one-atom-thick crystals. Nature 2014, 516, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Guo, Z.; Huang, X. Graphene-based hybrids for chemiresistive gas sensors. Anal. Chem. 2015, 68, 37–44. [Google Scholar] [CrossRef]
- Yavari, F.; Koratkar, N. Graphene-based chemical sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Maroo, S.C.; Wang, E.N. Wettability of graphene. Nano Lett. 2013, 13, 1509–1503. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.J.; Wang, Q.H.; Lin, S.; Park, K.C.; Jin, Z.; Strano, M.S.; Blankschtein, D. Breakdown in the wetting transparency of graphene. Phys. Rev. Lett. 2012, 109, 176101–176109. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, J.; Mi, X.; Gullapalli, H.; Thomas, A.V.; Yavari, F.; Shi, Y.; Ajayan, P.M.; Koratkar, N.A. Wetting transparency of graphene. Nat. Mater. 2012, 11, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Kozbial, A.; Shenoy, G.; Zhou, F.; McGinley, R.; Ireland, P.; Morganstein, B.; Kunkel, A.; Surwade, S.P.; et al. Graphene wetting. Nat. Mater. 2013, 12, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Aria, A.I.; Kidambi, P.R.; Weatherup, R.S.; Xiao, L.; Williams, J.A.; Hofmann, S. Time evolution of the wettability of supported graphene under ambient air exposure. J. Phys. Chem. C 2016, 120, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Chakarov, D.V.; Osterlund, L.; Kasemo, B. Water adsorption on graphite(0001). Vacuum 1995, 46, 1109–1116. [Google Scholar] [CrossRef]
- Chakarov, D.V.; Osterlund, L.; Kasemo, B. Water adsorption and coadsorption with potassium on graphite(0001). Langmuir 1995, 11, 1201–1209. [Google Scholar] [CrossRef]
- Chakradhar, A.; Burghaus, U. Adsorption of water on graphene/Ru(0001)—An experimental ultra-high vacuum study. Chem. Commun. 2014, 50, 7698–7702. [Google Scholar] [CrossRef] [PubMed]
- Chakradhar, A.; Sivapragasam, N.; Nayakasinghe, M.T.; Burghaus, U. Support effects in the adsorption of water on CVD graphene: An ultra-high vacuum adsorption study. Chem. Commun. 2015, 51, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Chakradhar, A.; Sivapragasam, N.; Nayakasinghe, M.T.; Burghaus, U. Adsorption kinetics of benzene on CVD graphene: An ultra-high vacuum study. J. Vac. Sci. Technol. A 2016, 34, 021402–021411. [Google Scholar] [CrossRef]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Icephobicity. Nat. Rev. Mater. 2016, 1, 1–23. [Google Scholar]
- You, Y.; Sahajwalla, V.; Yoshimurab, M.; Joshi, R.K. Graphene and graphene oxide for desalination. Nanoscale 2016, 8, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Wei, T.; Stultz, J.; Goodman, D.W. Dissociation of water on a flat, ordered silica surface. Langmuir 2003, 19, 1140–1150. [Google Scholar] [CrossRef]
- Wendt, S.; Frerichs, M.; Wei, T.; Chen, M.S.; Kempter, V.; Goodman, D.W. The interaction of water with silica thin films grown on Mo(112). Surf. Sci. 2004, 565, 107–113. [Google Scholar] [CrossRef]
- Kaya, S.; Weissenrieder, J.; Stacchiola, D.; Shaikutdinov, S.; Freund, H.J. Formation of an ordered ice layer on a thin silica film. J. Phys. Chem. C 2007, 111, 759–765. [Google Scholar] [CrossRef]
- Morais, A.; Longo, C.; Araujo, J.R.; Barroso, M.; Durrant, J.R.; Nogueira, A.F. Nanocrystalline anatase TiO2/reduced graphene oxide composite films as photoanodes for photoelectrochemical water splitting studies: The role of reduced graphene oxide. Phys. Chem. Chem. Phys. 2016, 18, 2608–2618. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, M.; Gupta, U.; Yadgarov, L.; Rosentsveig, R.; Tenne, R.; Rao, C.N.R. Beneficial effect of Re doping on the electrochemical HER activity of MoS2 fullerenes. Dalton Trans. 2015, 44, 16399–16407. [Google Scholar] [CrossRef] [PubMed]
- Bakaev, V.A.; Steele, W.A. On the computer simulation of a hydrophobic vitreous silica surface. J. Chem. Phys. 1999, 111, 9803–9809. [Google Scholar] [CrossRef]
- Weissenrieder, J.; Kaya, S.; Lu, J.L.; Gao, H.J.; Shaikhutdinov, S.; Freund, H.J.; Sierka, M.; Todorova, T.K.; Sauer, J. Atomic structure of a thin silica film on a Mo(112) substrate: A two-dimensional network of SiO4 tetrahedra. Phys. Rev. Lett. 2005, 95, 076103–076110. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Baron, M.; Stacchiola, D.; Weissenrieder, J.; Shaikhutdinov, S.; Todorova, T.K.; Sierka, M.; Sauer, J.; Freund, H.J. Review: On the geometrical and electronic structure of an ultra-thin crystalline silica film grown on Mo(112). Surf. Sci. 2007, 601, 4849–4854. [Google Scholar] [CrossRef]
- Nayakasinghe, M.T.; Chakradhar, A.; Sivapragasam, N.; Burghaus, U. Water adsorption on two-dimensional silica films. Appl. Surf. Sci. 2016, 364, 822–829. [Google Scholar] [CrossRef]
- Schmitz, P.J.; Polta, J.A.; Chang, S.L.; Thiel, P.A. Isotope effect in water desorption from Ru(001). Surf. Sci. 1987, 186, 219–226. [Google Scholar] [CrossRef]
- Parobek, D.; Liu, H. Wettability of graphene. 2D Mater. 2015, 2, 032001–032016. [Google Scholar] [CrossRef]
- Chakradhar, A.; Trettel, K.M.; Burghaus, U. Benzene adsorption on Ru(0001) and graphene/Ru(0001)—How to synthesize epitaxial graphene without STM or LEED? Chem. Phys. Lett. 2013, 590, 146–150. [Google Scholar] [CrossRef]
- Batzill, M. The surface science of graphene: Metal interfaces, CVD synthesis, nanoribbons, chemical modifications, and defects. Surf. Sci. Rep. 2012, 67, 83–147. [Google Scholar] [CrossRef]
- Hossain, Z.; Johns, J.E.; Bevan, K.H.; Karmel, H.J.; Liang, Y.T.; Yoshimoto, S.; Mukai, K.; Koitaya, T.; Yoshinobu, J.; Kawai, M.; et al. Chemically homogeneous and thermally reversible oxidation of epitaxial graphene. Nat. Chem. 2012, 4, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, Z.Q.; Yao, Y.X.; Tan, D.L.; Fu, Q.; Bao, X.H. Silicatene thin films on palladium. Thin Solid Films 2008, 516, 3741–3750. [Google Scholar] [CrossRef]
- Kundu, M.; Murata, Y. Silicatene growth on nickel. Appl. Phys. Lett. 2002, 80, 1921–1929. [Google Scholar] [CrossRef]
- Stacchiola, D.; Kaya, S.; Weissenrieder, J.; Kuhlenbeck, H.; Shaikhutdinov, S.; Freund, H.J.; Sierka, M.; Todorova, T.K.; Sauer, J. Synthesis and structure of ultrathin aluminosilicate films. Angew. Chem. Int. Ed. 2006, 45, 7636–7641. [Google Scholar] [CrossRef] [PubMed]
- Gruendling, C.; Percher, J.A.; Goodman, D.W. Preparation of mixed Al/SiO2 thin films supported on Mo(100). Surf. Sci. 1994, 318, 97–112. [Google Scholar] [CrossRef]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burghaus, U. Adsorption of Water on Two-Dimensional Crystals: Water/Graphene and Water/Silicatene. Inorganics 2016, 4, 10. https://doi.org/10.3390/inorganics4020010
Burghaus U. Adsorption of Water on Two-Dimensional Crystals: Water/Graphene and Water/Silicatene. Inorganics. 2016; 4(2):10. https://doi.org/10.3390/inorganics4020010
Chicago/Turabian StyleBurghaus, Uwe. 2016. "Adsorption of Water on Two-Dimensional Crystals: Water/Graphene and Water/Silicatene" Inorganics 4, no. 2: 10. https://doi.org/10.3390/inorganics4020010
APA StyleBurghaus, U. (2016). Adsorption of Water on Two-Dimensional Crystals: Water/Graphene and Water/Silicatene. Inorganics, 4(2), 10. https://doi.org/10.3390/inorganics4020010




