Expanding the Chemistry of Actinide Metallocene Bromides. Synthesis, Properties and Molecular Structures of the Tetravalent and Trivalent Uranium Bromide Complexes: (C5Me4R)2UBr2, (C5Me4R)2U(O-2,6-iPr2C6H3)(Br), and [K(THF)][(C5Me4R)2UBr2] (R = Me, Et)
Abstract
:1. Introduction
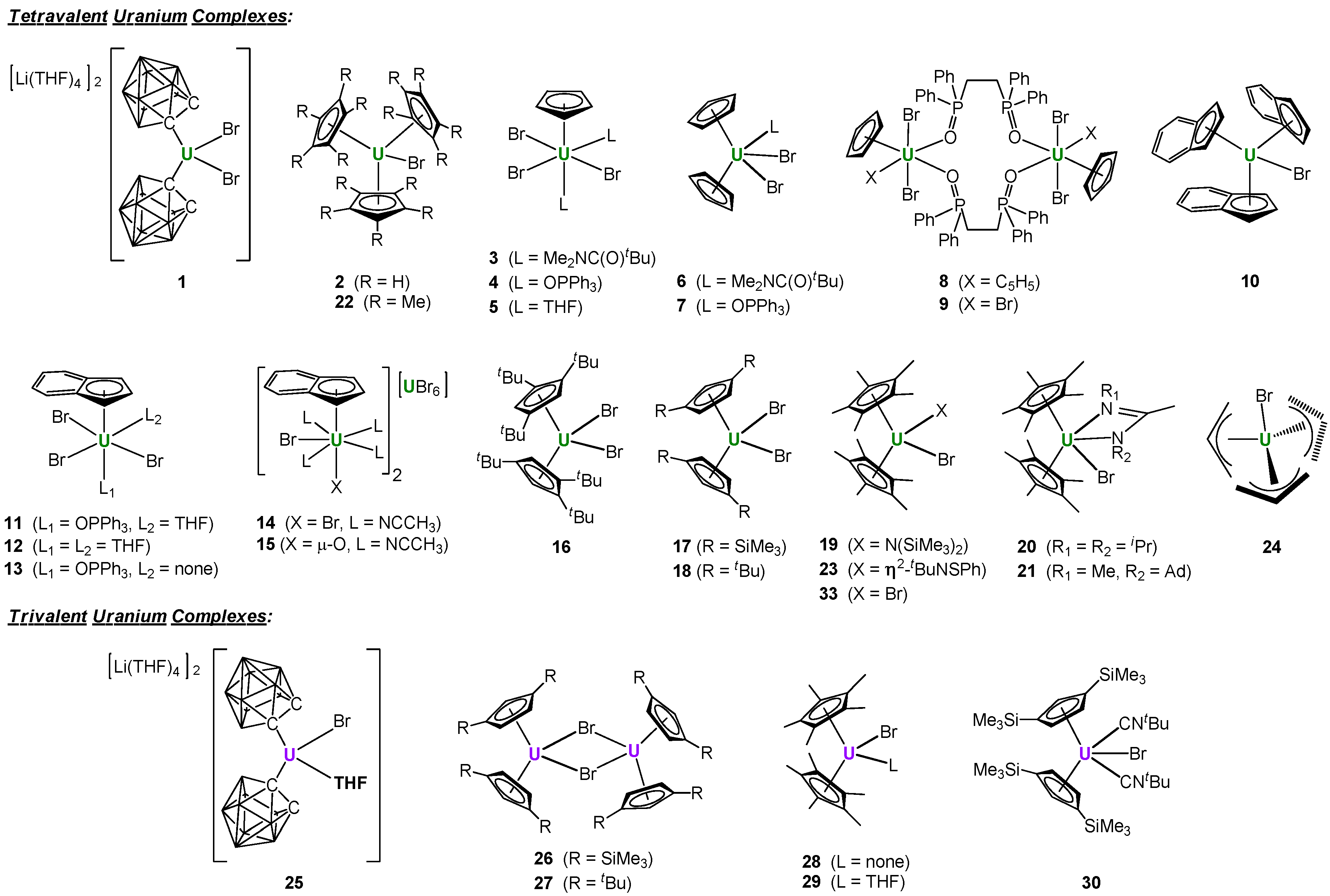
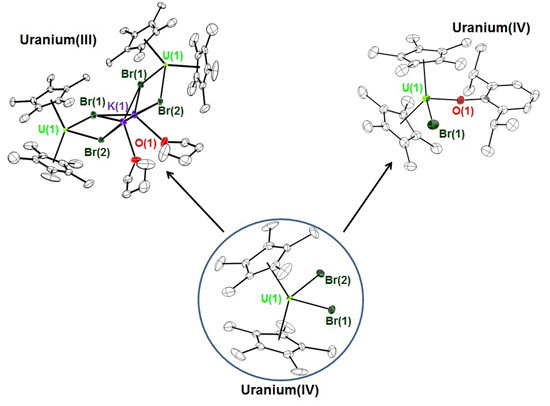
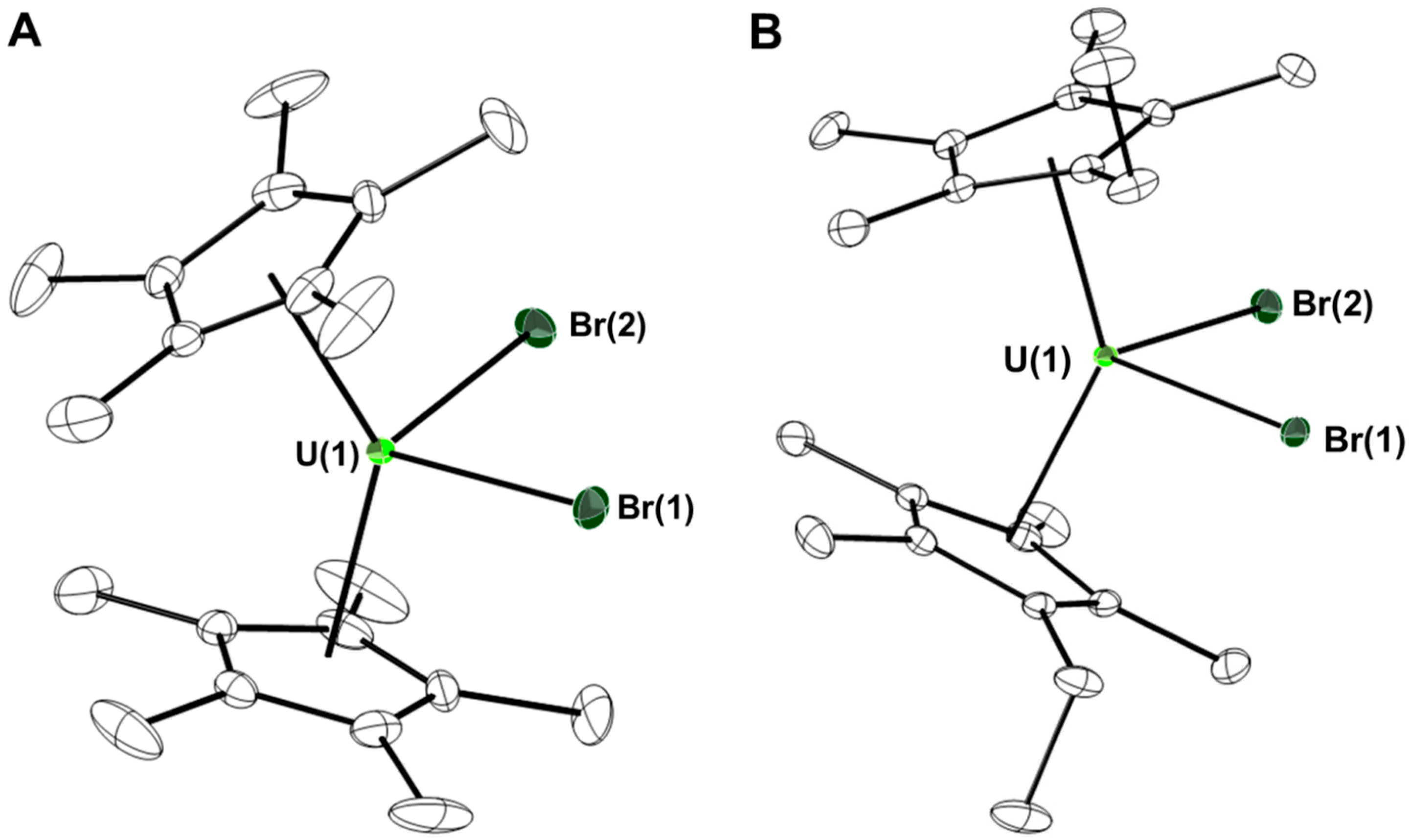
2. Results and Discussion
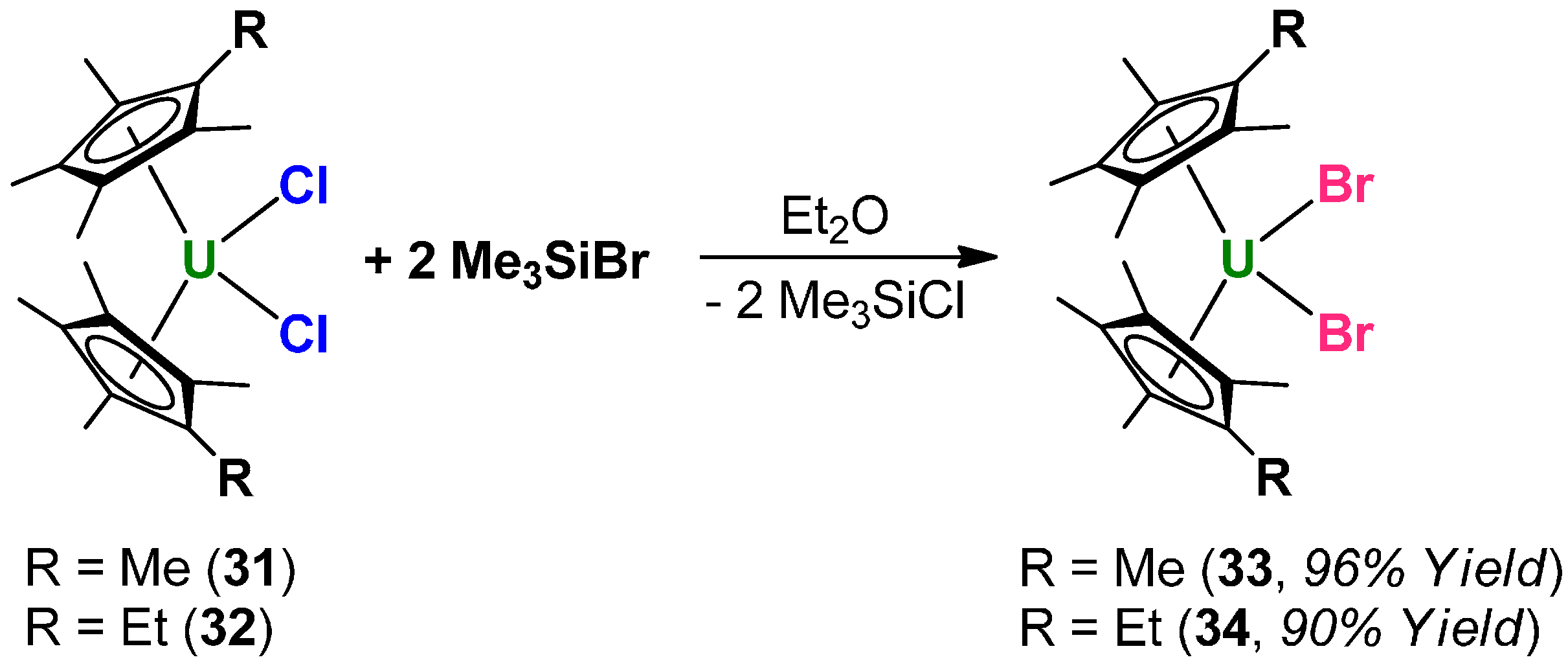
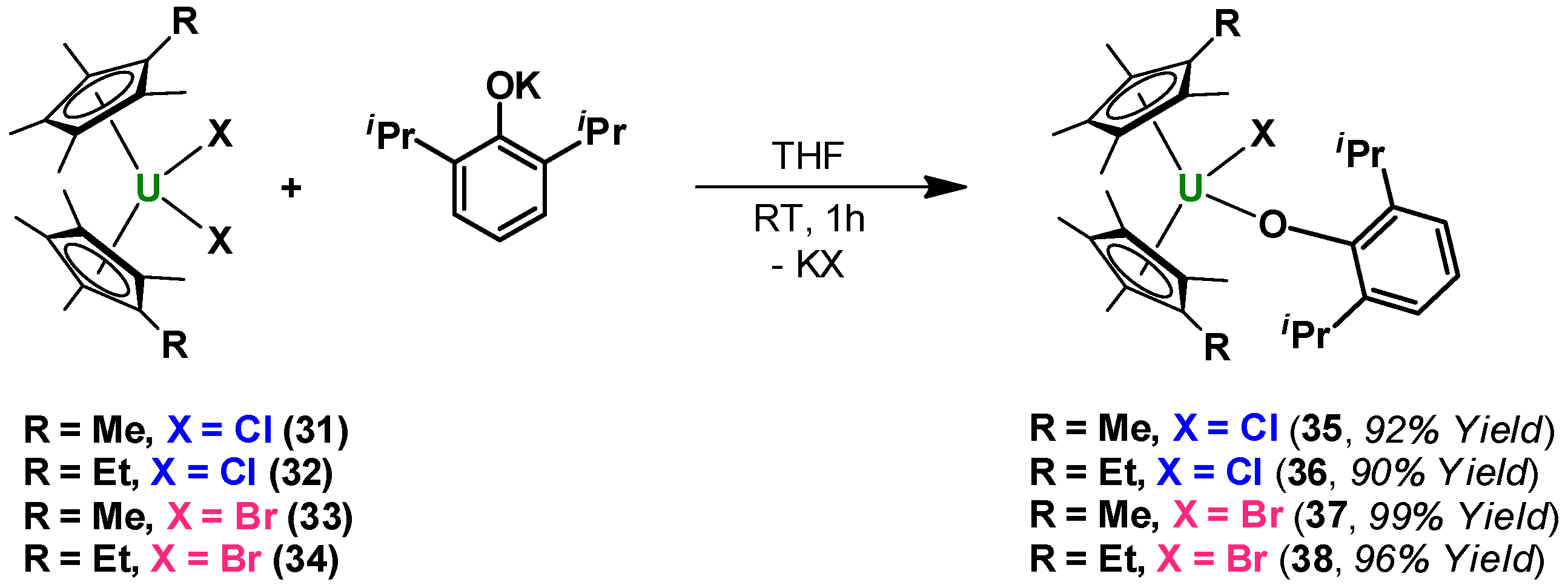

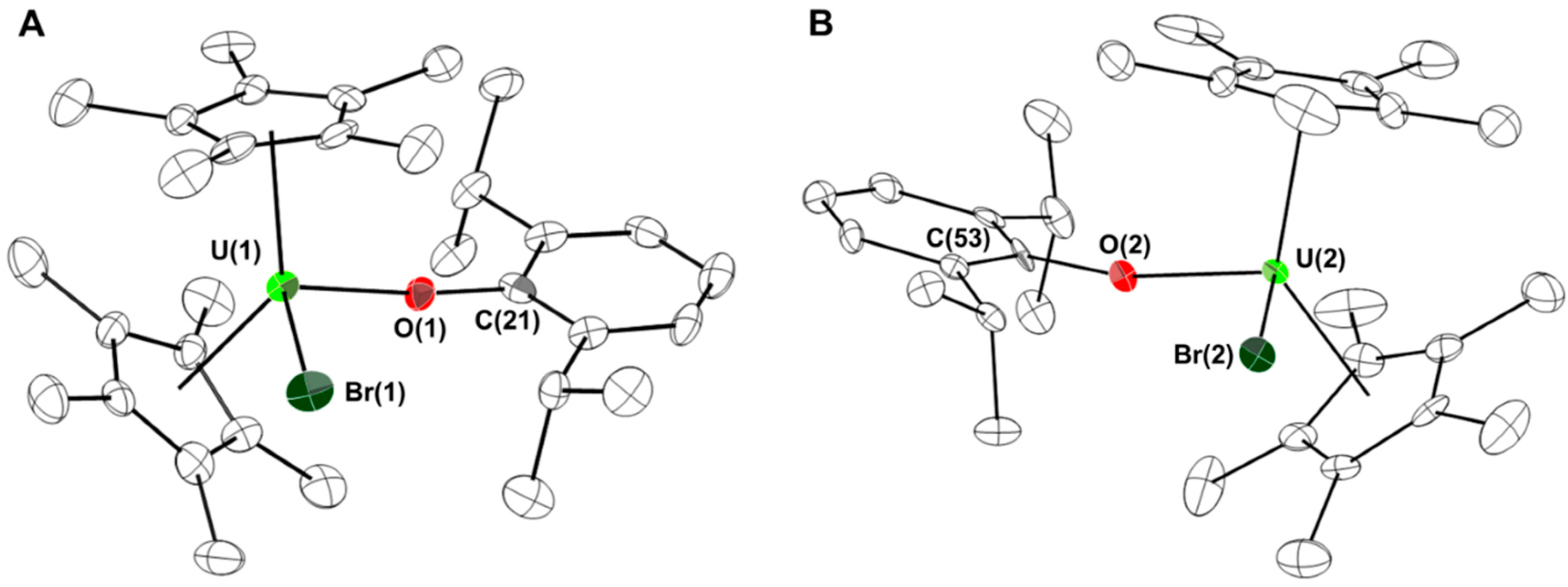

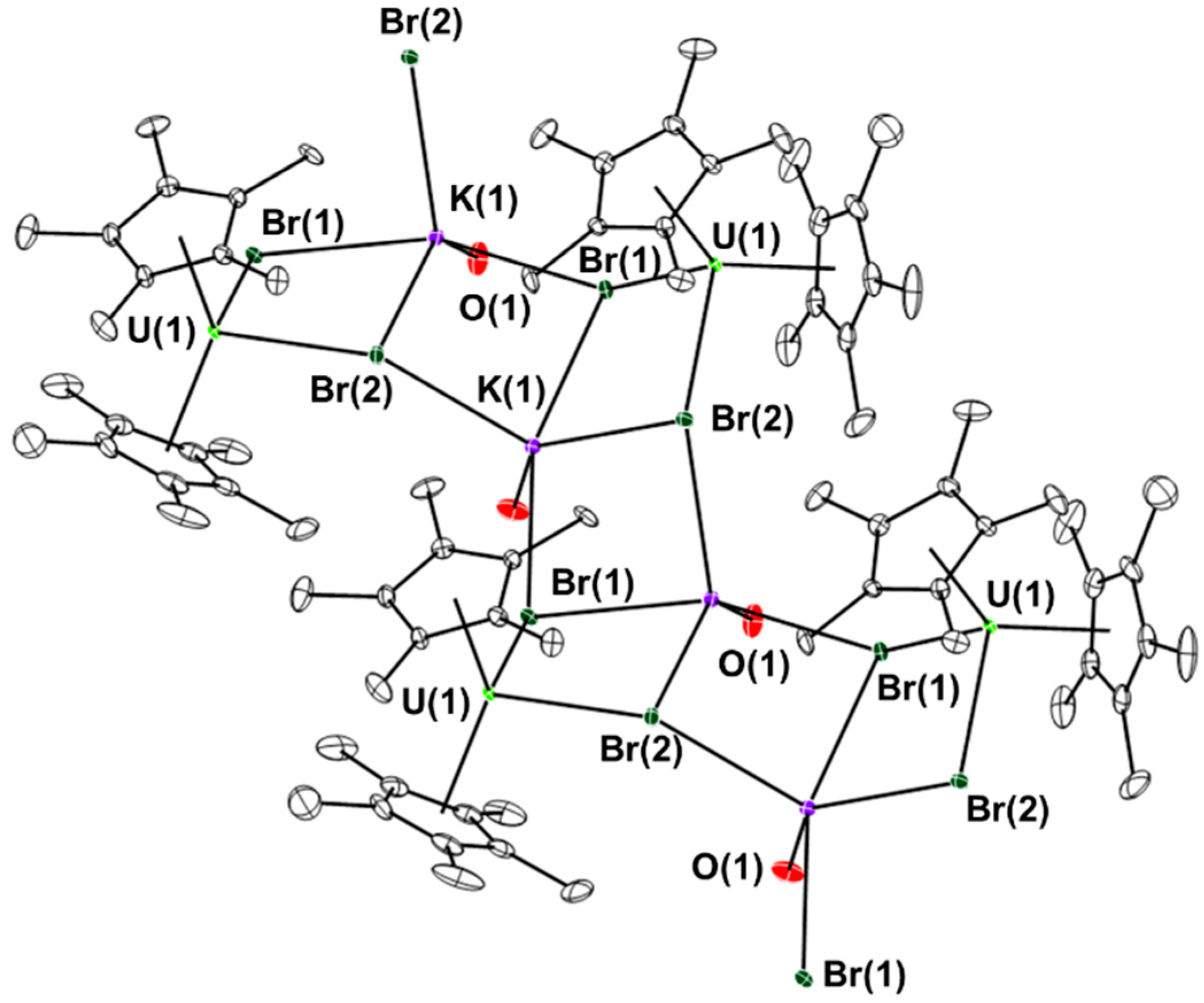

3. Experimental Section
3.1. General Synthetic Considerations
3.2. Materials
3.3. Caution
3.4. Synthetic Procedures
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spirlet, M.R.; Rebizant, J.; Apostolidis, C.; Kanellakopulos, B. Bis(cyclopentadienyl) actinide(IV) compounds. I. The structure of dichlorobis(pentamethyl-η5-cyclopentadienyl)uranium(IV) and dichlorobis(pentamethyl-η5-cyclopentadienyl)thorium(IV). Acta Crystallogr. Sect. C Cryst. Commun. 1992, 48, 2135–2137. [Google Scholar] [CrossRef]
- Graves, C.R.; Schelter, E.J.; Cantat, T.; Scott, B.L.; Kiplinger, J.L. A mild protocol to generate uranium(IV) mixed-ligand metallocene complexes using copper(I) iodide. Organometallics 2008, 27, 5371–5378. [Google Scholar] [CrossRef]
- Fagan, P.J.; Manriquez, J.M.; Maatta, E.A.; Seyam, A.M.; Marks, T.J. Synthesis and properties of bis(pentamethylcyclopentadienyl) actinide hydrocarbyls and hydrides. A new class of highly reactive f-element organometallic compounds. J. Am. Chem. Soc. 1981, 103, 6650–6667. [Google Scholar] [CrossRef]
- Lukens, W.W., Jr.; Beshouri, S.M.; Blosch, L.L.; Stuart, A.L.; Andersen, R.A. Preparation, solution behavior, and solid-state structures of (1,3-R2C5H3)2UX2, where R is CMe3 or SiMe3 and X is a one-electron ligand. Organometallics 1999, 18, 1235–1246. [Google Scholar] [CrossRef]
- Zi, G.; Jia, L.; Werkema, E.L.; Walter, M.D.; Gottfriedsen, J.P.; Andersen, R.A. Preparation and reactions of base-free bis(1,2,4-tri-tert-butylcyclopentadienyl)uranium oxide, Cp′2UO. Organometallics 2005, 24, 4251–4264. [Google Scholar] [CrossRef]
- Blake, P.C.; Lappert, M.F.; Taylor, R.G.; Atwood, J.L.; Hunter, W.E.; Zhang, H. Synthesis, spectroscopic properties and crystal structures of [ML2Cl2] [M = Th or U; L = η-C5H3(SiMe3)2-1,3] and [UL2X2] (X = Br, I or BH4). Dalton Trans. 1995, 3335–3341. [Google Scholar] [CrossRef]
- Blake, P.C.; Lappert, M.F.; Taylor, R.G.; Atwood, J.L.; Zhang, H. Some aspects of the coordination and organometallic chemistry of thorium and uranium (MIII, MIV, UV) in +3 and +4 oxidation states. Inorg. Chim. Acta 1987, 139, 13–20. [Google Scholar] [CrossRef]
- Thomson, R.K.; Graves, C.R.; Scott, B.L.; Kiplinger, J.L. Organometallic uranium(IV) fluoride complexes: Preparation using protonolysis chemistry and reactivity with trimethylsilyl reagents. Dalton Trans. 2010, 39, 6826–6831. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, D.; Haswell, C.M.; Scott, B.L.; Miller, R.L.; Nielsen, J.B.; Abney, K.D. New dicarbollide complexes of uranium. Inorg. Chem. 1996, 35, 1425–1426. [Google Scholar] [CrossRef] [PubMed]
- Spirlet, M.R.; Rebizant, J.; Apostolidis, C.; Andreetti, G.D.; Kanellakopulos, B. Structure of tris(cyclopentadienyl)uranium bromide. Acta Crystallogr. C 1989, 45, 739–741. [Google Scholar] [CrossRef]
- Goffart, J.; Fuger, J.; Gilbert, B.; Hocks, L.; Duyckaerts, G. On the indenyl compounds of actinide elements Part I: Triindenylthorium bromide and triindenyluranium bromide. Inorg. Nucl. Chem. Lett. 1975, 11, 569–583. [Google Scholar] [CrossRef]
- Goffart, J.; Piret-Meunier, J.; Duyckaerts, G. On the indenyl compounds of actinide elements: Part V: Some oxygen-donor complexes of indenyl actinide halides. Inorg. Nucl. Chem. Lett. 1980, 16, 233–244. [Google Scholar] [CrossRef]
- Spirlet, M.R.; Rebizant, J.; Goffart, J. Structure of tris(1-3-η-Indenyl)uranium bromide. Acta Crystallogr. C 1987, 43, 354–355. [Google Scholar] [CrossRef]
- Bagnall, K.W.; Edwards, J. Uranium(IV) substituted poly(pyrazol-1-yl) borate complexes. J. Less Common Met. 1976, 48, 159–165. [Google Scholar] [CrossRef]
- Bagnall, K.W.; Edwards, J.; Tempest, A.C. Some oxygen-donor complexes of cyclopentadienyluranium(IV) halides. Dalton Trans. 1978, 295–298. [Google Scholar] [CrossRef]
- Beeckman, W.; Goffart, J.; Rebizant, J.; Spirlet, M.R. The first cationic indenyl-f-metal complexes with pentagonal bipyramidal geometry. Crystal structures of [C9H7UBr2(CH3CN)4]2+[UBr6]2− and [{C9H7UBr(CH3CN)4}2O]2+[UBr6]2−. J. Organomet. Chem. 1986, 307, 23–37. [Google Scholar] [CrossRef]
- Thomson, R.K.; Scott, B.L.; Morris, D.E.; Kiplinger, J.L. Synthesis, structure, spectroscopy and redox energetics of a series of uranium(IV) mixed-ligand metallocene complexes. C. R. Chim. 2010, 13, 790–802. [Google Scholar] [CrossRef]
- Evans, W.J.; Walensky, J.R.; Ziller, J.W. Reaction chemistry of the U3+ metallocene amidinate (C5Me5)2[iPrNC(Me)NiPr]U including the isolation of a uranium complex of a monodentate acetate. Inorg. Chem. 2010, 49, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Walensky, J.R.; Ziller, J.W. Reactivity of methyl groups in actinide metallocene amidinate and triazenido complexes with silver and copper salts. Organometallics 2010, 29, 101–107. [Google Scholar] [CrossRef]
- Evans, W.J.; Nyce, G.W.; Johnston, M.A.; Ziller, J.W. How much steric crowding is possible in tris(η5-pentamethylcyclopentadienyl) complexes? Synthesis and structure of (C5Me5)3UCl and (C5Me5)3UF. J. Am. Chem. Soc. 2000, 122, 12019–12020. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Hankin, D.M.; Cafferkey, S.M.; Hursthouse, M.B. η2-Sulfenamido complexes of uranium. Dalton Trans. 2000, 1613–1615. [Google Scholar] [CrossRef]
- Lugli, G.; Mazzei, A.; Poggio, S. High 1,4-cis-polybutadiene by uranium catalysts, 1. Tris(π-allyl)uranium halide catalysts. Makromol. Chem. 1974, 175, 2021–2027. [Google Scholar] [CrossRef]
- Lukens, W.W., Jr.; Beshouri, S.M.; Stuart, A.L.; Andersen, R.A. Solution structure and behavior of dimeric uranium(III) metallocene halides. Organometallics 1999, 18, 1247–1252. [Google Scholar] [CrossRef]
- Blake, P.C.; Lappert, M.F.; Taylor, R.G.; Atwood, J.L.; Hunter, W.E.; Zhang, H. A complete series of uranocene(III) halides [(UCp″2X)n] [X = F, Cl, Br, or I; Cp″ = η5-C5H3(SiMe3)2]; Single-crystal X-ray structure determinations of the chloride and bromide (n = 2, X− = μ-Cl, μ-Br). Chem. Commun. 1986, 1394–1395. [Google Scholar] [CrossRef]
- De Rege, F.M.; Smith, W.H.; Scott, B.L.; Nielsen, J.B.; Abney, K.D. Electrochemical properties of bis(dicarbollide)uranium dibromide. Inorg. Chem. 1998, 37, 3664–3666. [Google Scholar] [CrossRef] [PubMed]
- Beshouri, S.M.; Zalkin, A. Bis[η5-bis(trimethylsilyl)cyclopentadienyl]bromouranium(III) bis(tert-butyl isocyanide). Acta Crystallogr. C 1989, C45, 1221–1222. [Google Scholar] [CrossRef]
- Finke, R.G.; Hirose, Y.; Gaughan, G. (C5Me5)2UCl·tetrahydrofuran. Oxidative-addition and related reactions. Chem. Commun. 1981, 232–234. [Google Scholar] [CrossRef]
- Maynadié, J.; Berthet, J.-C.; Thuéry, P.; Ephritikhine, M. An unprecedented type of linear metallocene with an f-element. J. Am. Chem. Soc. 2006, 128, 1082–1083. [Google Scholar] [CrossRef] [PubMed]
- Maynadie, J.; Berthet, J.-C.; Thuery, P.; Ephritikhine, M. From bent to linear uranium metallocenes: Influence of counterion, solvent, and metal ion oxidation state. Organometallics 2006, 25, 5603–5611. [Google Scholar] [CrossRef]
- Monreal, M.J.; Thomson, R.K.; Cantat, T.; Travia, N.E.; Scott, B.L.; Kiplinger, J.L. UI4(1,4-dioxane)2, [UCl4(1,4-dioxane)]2, and UI3(1,4-dioxane)1.5: Stable and versatile starting materials for low- and high-valent uranium chemistry. Organometallics 2011, 30, 2031–2038. [Google Scholar] [CrossRef]
- Schelter, E.J.; Veauthier, J.M.; Graves, C.R.; John, K.D.; Scott, B.L.; Thompson, J.D.; Pool-Davis-Tournear, J.A.; Morris, D.E.; Kiplinger, J.L. 1,4-Dicyanobenzene as a scaffold for the preparation of bimetallic actinide complexes exhibiting metal–metal communication. Chem. Eur. J. 2008, 14, 7782–7790. [Google Scholar] [CrossRef] [PubMed]
- LeMarechal, J.F.; Villiers, C.; Charpin, P.; Nierlich, M.; Lance, M.; Vigner, J.; Ephritikhine, M. Anionic tricyclopentadienyluranium(III) complexes. Crystal structure of [Cp3UClUCp3][Na(18-Crown-6)(THF)2] (Cp = η-C5H5). J. Organomet. Chem. 1989, 379, 259–269. [Google Scholar] [CrossRef]
- Du Preez, J.G.H.; Gellatly, B.J.; Jackson, G.; Nassimbeni, L.R.; Rodgers, A.L. The chemistry of uranium. Part 23. Isomorphous tetrachlorobis(hexamethylphosphoramide)uranium(IV) and tetrabromobis(hexamethylphosphoramide)uranium(IV). Inorg. Chim. Acta 1978, 27, 181–184. [Google Scholar] [CrossRef]
- Du Preez, J.G.H.; Zeelie, B.; Casellato, U.; Graziani, R. The chemistry of uranium. Part 34. Structural and thermal properties of UX4L2 complexes (X = Cl and Br; L = N,N,N′,N′-tetramethylurea). Inorg. Chim. Acta 1986, 122, 119–126. [Google Scholar] [CrossRef]
- Du Preez, J.G.H.; Rohwer, H.E.; van Brecht, B.J.A.M.; Zeelie, B.; Casellato, U.; Graziani, R. The chemistry of uranium. Part 41. Complexes of uranium tetrahalide, with emphasis on iodide, and triphenylphosphine oxide, tris(dimethylamino)phosphine oxide or tris(pyrrolidinyl)phosphine oxide. Crystal structure of tetrabromobis[tris(pyrrolidinyl)phosphine oxide]uranium(IV). Inorg. Chim. Acta 1991, 189, 67–75. [Google Scholar]
- Du Preez, J.G.H.; Gouws, L.; Rohwer, H.; van Brecht, B.J.A.M.; Zeelie, B.; Casellato, U.; Graziani, R. The chemistry of uranium. Part 42. Cationic uranium(IV) complexes UX2L4Y2 (X = Cl, Br or I; Y = ClO4 or BPh4; L = bulky strong neutral O-donor ligand) and [U(ClO4)4(OAsPh3)4]: Crystal structures of [UX2L4][BPh4]2 (L = tris(pyrrolidine-1-yl)phosphine oxide, X = Br or I). Dalton Trans. 1991, 2585–2593. [Google Scholar] [CrossRef]
- Zych, E.; Starynowicz, P.; Lis, T.; Drozdzynski, J. Crystal structure and spectroscopic properties of ammonium bis(acetonitrile)pentaaquadibromouranium dibromide. Polyhedron 1993, 12, 1661–1667. [Google Scholar] [CrossRef]
- Berthet, J.-C.; Siffredi, G.; Thuéry, P.; Ephritikhine, M. Controlled chemical reduction of uranyl salts into UX4(MeCN)4 (X = Cl, Br, I) with Me3SiX reagents. Eur. J. Inorg. Chem. 2007, 2007, 4017–4020. [Google Scholar] [CrossRef]
- Caira, M.R.; de Wet, J.F.; Du Preez, J.G.H.; Gellatly, B.J. The crystal structures of hexahalouranates. I. Bis(triphenylethylphosphonium) hexachlorouranate(IV) and bis(triphenylethylphosphonium) hexabromouranate(IV). Acta Crystallogr. B 1978, 34, 1116–1120. [Google Scholar] [CrossRef]
- Schnaars, D.D.; Wu, G.; Hayton, T.W. Reactivity of UH3 with mild oxidants. Dalton Trans. 2008, 6121–6126. [Google Scholar] [CrossRef] [PubMed]
- Bombieri, G.; Benetollo, F.; Bagnall, K.W.; Plews, M.J.; Brown, D. Triphenylphosphine oxide complexes of actinide tetrahalides. Crystal and molecular structure of trans-tetrabromobis(triphenylphosphine oxide)uranium(IV). Dalton Trans. 1983, 343–348. [Google Scholar] [CrossRef]
- De Wet, J.F.; Caira, M.R. Structure and bonding in octahedral uranium(IV) complexes of the type UX4·2L (X = halogen, L = unidentate, neutral oxygen donor). Part 1. The crystal structures of tetrabromobis(NN′-dimethyl-NN′-diphenylurea)uranium(IV) and α- and β-tetrachlorobis(NN′-dimethyl-NN′-diphenylurea)uranium(IV). Dalton Trans. 1986, 2035–2041. [Google Scholar] [CrossRef]
- De Wet, J.F.; Caira, M.R. Structure and bonding in octahedral uranium(IV) complexes of the type UX4·2L (X = halogen, L = unidentate, neutral oxygen donor). Part 2. The crystal structures of tetrachlorobis[tris(pyrrolidinyl)phosphine oxide]-, tetrachlorobis(di-isobutyl sulphoxide)-, and tetrabromobis(triphenylarsine oxide)-uranium(IV). Dalton Trans. 1986, 2043–2048. [Google Scholar] [CrossRef]
- Kepert, D.; Patrick, J.; White, A. Structure and stereochemistry in “f-block” complexes of high coordination number. VI. Crystallographic characterizations of bis[(1,1-dimethyl-3-oxobutyl)triphenyl-phosphonium] hexabromouranate(IV) and tetrabromobis[ethane-1,2-diylbis(diphenylarsine) oxide]uranium(IV). Aust. J. Chem. 1983, 36, 469–476. [Google Scholar]
- Jantunen, K.C.; Batchelor, R.J.; Leznoff, D.B. Synthesis, characterization, and organometallic derivatives of diamidosilyl ether thorium(IV) and uranium(IV) halide complexes. Organometallics 2004, 23, 2186–2193. [Google Scholar] [CrossRef]
- Evans, W.J.; Miller, K.A.; Ziller, J.W.; Greaves, J. Analysis of uranium azide and nitride complexes by atmospheric pressure chemical ionization mass spectrometry. Inorg. Chem. 2007, 46, 8008–8018. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, H.M.; Ziller, J.W.; Anwander, R.; Evans, W.J. Reactivity of (C5Me5)2UMe2 and (C5Me5)2UMeCl toward group 13 alkyls. Organometallics 2009, 28, 1173–1179. [Google Scholar] [CrossRef]
- Thomson, R.K.; Graves, C.R.; Scott, B.L.; Kiplinger, J.L. Noble reactions for the actinides: Safe gold-based access to organouranium and azido complexes. Eur. J. Inorg. Chem. 2009, 2009, 1451–1455. [Google Scholar] [CrossRef]
- Thomson, R.K.; Graves, C.R.; Scott, B.L.; Kiplinger, J.L. Straightforward and efficient oxidation of tris(aryloxide) and tris(amide) uranium(III) complexes using copper(I) halide reagents. Inorg. Chem. Commun. 2011, 14, 1742–1744. [Google Scholar] [CrossRef]
- Thomson, R.K.; Graves, C.R.; Scott, B.L.; Kiplinger, J.L. Uncovering alternate reaction pathways to access uranium(IV) mixed-ligand aryloxide-chloride and alkoxide-chloride metallocene complexes: Synthesis and molecular structures of (C5Me5)2U(O-2,6-iPr2C6H3)(Cl) and (C5Me5)2U(O-tBu)(Cl). Inorg. Chim. Acta 2011, 369, 270–273. [Google Scholar] [CrossRef]
- Graves, C.R.; Yang, P.; Kozimor, S.A.; Vaughn, A.E.; Clark, D.L.; Conradson, S.D.; Schelter, E.J.; Scott, B.L.; Thompson, J.D.; Hay, P.J.; et al. Organometallic uranium(V)-imido halide complexes: From synthesis to electronic structure and bonding. J. Am. Chem. Soc. 2008, 130, 5272–5285. [Google Scholar] [CrossRef] [PubMed]
- Gowda, B.T.; Foro, S.; Shakuntala, K. Potassium N-bromo-4-chlorobenzenesulfonamidate monohydrate. Acta Crystallogr. E 2011, 67, m962. [Google Scholar] [CrossRef] [PubMed]
- Tonshoff, C.; Merz, K.; Bucher, G. Azidocryptands—Synthesis, structure, and complexation properties. Org. Biomol. Chem. 2005, 3, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.M.; Nawaratna, G.U.; Beatty, A.M.; Duggan, P.J.; Smith, B.D. Transport of alkali halides through a liquid organic membrane containing a ditopic salt-binding receptor. Inorg. Chem. 2004, 43, 5902–5907. [Google Scholar] [CrossRef] [PubMed]
- Jaroschik, F.; Nief, F.; le Goff, X.-F.; Ricard, L. Isolation of stable organodysprosium(II) complexes by chemical reduction of dysprosium(III) precursors. Organometallics 2007, 26, 1123–1125. [Google Scholar] [CrossRef]
- Fender, N.S.; Fronczek, F.R.; John, V.; Kahwa, I.A.; McPherson, G.L. Unusual luminescence spectra and decay dynamics in crystalline supramolecular [(A18C6)4MBr4][TlBr4]2 (A = Rb, K; M = 3d element) complexes. Inorg. Chem. 1997, 36, 5539–5547. [Google Scholar] [CrossRef]
- Danis, J.A.; Lin, M.R.; Scott, B.L.; Eichhorn, B.W.; Runde, W.H. Coordination trends in alkali metal crown ether uranyl halide complexes: The series [A(Crown)]2[UO2X4] where A = Li, Na, K and X = Cl, Br. Inorg. Chem. 2001, 40, 3389–3394. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-Y.; Liu, B.; Liu, X.-M.; Yang, J.-H. Syntheses, structures and magnetic properties of two heterometallic carbonates: K2Li[Cu(H2O)2Ru2(CO3)4X2]·5H2O (X = Cl, Br). CrystEngComm 2013, 15, 7936–7942. [Google Scholar] [CrossRef]
- Linti, G.; Li, G.; Pritzkow, H. On The chemistry of gallium: Part 19. Synthesis and crystal structure analysis of novel complexes containing Ga–FeCp(CO)2-fragments. J. Organomet. Chem. 2001, 626, 82–91. [Google Scholar] [CrossRef]
- Wu, F.; Tong, H.; Li, Z.; Lei, W.; Liu, L.; Wong, W.-Y.; Wong, W.-K.; Zhu, X. A white phosphorescent coordination polymer with Cu2I2 alternating units linked by benzo-18-crown-6. Dalton Trans. 2014, 43, 12463–12466. [Google Scholar] [CrossRef] [PubMed]
- Gibney, B.R.; Wang, H.; Kampf, J.W.; Pecoraro, V.L. Structural evaluation and solution integrity of alkali metal salt complexes of the manganese 12-metallacrown-4 (12-MC-4) structural type. Inorg. Chem. 1996, 35, 6184–6193. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, Q.-G.; Li, S.-N.; Xia, R.; Xiang, H.-J.; Jiang, Y.-C.; Hu, M.-C. Two anionic [CuI6X7]nn− (X = Br and I) chain-based organic–inorganic hybrid solids with N-substituted benzotriazole ligands. J. Solid State Chem. 2010, 183, 1150–1158. [Google Scholar] [CrossRef]
- Amo-Ochoa, P.; Jiménez-Aparicio, R.; Perles, J.; Torres, M.R.; Gennari, M.; Zamora, F. Structural diversity in paddlewheel dirhodium(II) compounds through ionic interactions: Electronic and redox properties. Cryst. Growth Des. 2013, 13, 4977–4985. [Google Scholar] [CrossRef]
- Yan, H.; Jang, H.B.; Lee, J.-W.; Kim, H.K.; Lee, S.W.; Yang, J.W.; Song, C.E. A chiral-anion generator: Application to catalytic desilylative kinetic resolution of silyl-protected secondary alcohols. Angew. Chem. Int. 2010, 49, 8915–8917. [Google Scholar] [CrossRef] [PubMed]
- Molcanov, K.; Kojic-Prodic, B.; Babic, D.; Zilic, D.; Rakvin, B. Stabilisation of tetrabromo- and tetrachlorosemiquinone (bromanil and chloranil) anion radicals in crystals. CrystEngComm 2011, 13, 5170–5178. [Google Scholar] [CrossRef]
- Neumüller, B.; Gahlmann, F. Mesityltrifluorogallate. Die kristallstrukturen von Cs[MesGaF3] und K[MesInBr3]. Z. Anorg. Allg. Chem. 1993, 619, 1897–1904. [Google Scholar] [CrossRef]
- Schelter, E.J.; Wu, R.; Scott, B.L.; Thompson, J.D.; Morris, D.E.; Kiplinger, J.L. Mixed valency in a uranium multimetallic complex. Angew. Chem. Int. 2008, 47, 2993–2996. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Kozimor, S.A.; Hillman, W.R.; Ziller, J.W. Synthesis and structure of the bis(tetramethylcyclopentadienyl)uranium metallocenes (C5Me4H)2UMe2, (C5Me4H)2UMeCl, [(C5Me4H)2U][(μ-η6:η1-Ph)(μ-η1:η1-Ph)BPh2], and [(C5Me4)SiMe2(CH2CH=CH2)]2UI(THF). Organometallics 2005, 24, 4676–4683. [Google Scholar] [CrossRef]
- Mehdoui, T.; Berthet, J.-C.; Thuery, P.; Salmon, L.; Riviere, E.; Ephritikhine, M. Lanthanide(III)/actinide(III) differentiation in the cerium and uranium complexes [M(C5Me5)2(L)]0,+ (L = 2,2′-bipyridine, 2,2′:6′,2″-terpyridine): Structural, magnetic, and reactivity studies. Chem. Eur. J. 2005, 11, 6994–7006. [Google Scholar] [CrossRef] [PubMed]
- Mehdoui, T.; Berthet, J.-C.; Thuery, P.; Ephritikhine, M. The distinct affinity of cyclopentadienyl ligands towards trivalent uranium over lanthanide ions. evidence for cooperative ligation and back-bonding in the actinide complexes. Dalton Trans. 2005, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Mehdoui, T.; Berthet, J.-C.; Thuery, P.; Ephritikhine, M. The remarkable efficiency of N-heterocyclic carbenes in lanthanide(III)/actinide(III) differentiation. Chem. Commun. 2005, 2860–2862. [Google Scholar] [CrossRef] [PubMed]
- Fagan, P.J.; Manriquez, J.M.; Marks, T.J.; Day, C.S.; Vollmer, S.H.; Day, V.W. Synthesis and properties of a new class of highly reactive trivalent actinide organometallic compounds. derivatives of bis(pentamethylcyclopentadienyl)uranium(III). Organometallics 1982, 1, 170–180. [Google Scholar] [CrossRef]
- Cendrowski-Guillaume, S.M.; Lance, M.; Nierlich, M.; Vigner, J.; Ephritikhine, M. New actinide hydrogen transition metal compounds. Synthesis of [K(C12H24O6)][(η-C5Me5)2(Cl)UH6Re(PPh3)2] and the crystal structure of its benzene solvate. Chem. Commun. 1994, 1655–1656. [Google Scholar] [CrossRef]
- Evans, W.J.; Kozimor, S.A.; Ziller, J.W. Methyl displacements from cyclopentadienyl ring planes in sterically crowded (C5Me5)3M complexes. Inorg. Chem. 2005, 44, 7960–7969. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Kozimor, S.A.; Ziller, J.W. Bis(pentamethylcyclopentadienyl) U(III) oxide and U(IV) oxide carbene complexes. Polyhedron 2004, 23, 2689–2694. [Google Scholar] [CrossRef]
- Evans, W.J.; Kozimor, S.A.; Ziller, J.W.; Kaltsoyannis, N. Structure, reactivity, and density functional theory analysis of the six-electron reductant, [(C5Me5)2U]2(μ-η6:η6-C6H6), synthesized via a new mode of (C5Me5)3M reactivity. J. Am. Chem. Soc. 2004, 126, 14533–14547. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Nyce, G.W.; Forrestal, K.J.; Ziller, J.W. Multiple syntheses of (C5Me5)3U. Organometallics 2002, 21, 1050–1055. [Google Scholar] [CrossRef]
- Roger, M.; Belkhiri, L.; Thuéry, P.; Arliguie, T.; Fourmigué, M.; Boucekkine, A.; Ephritikhine, M. Lanthanide(III)/actinide(III) differentiation in mixed cyclopentadienyl/dithiolene compounds from X-ray diffraction and density functional theory analysis. Organometallics 2005, 24, 4940–4952. [Google Scholar] [CrossRef]
- Arliguie, T.; Lescop, C.; Ventelon, L.; Leverd, P.C.; Thuéry, P.; Nierlich, M.; Ephritikhine, M. C–H and C–S bond cleavage in uranium(III) thiolato complexes. Organometallics 2001, 20, 3698–3703. [Google Scholar] [CrossRef]
- Boisson, C.; Berthet, J.C.; Ephritikhine, M.; Lance, M.; Nierlich, M. Synthesis and crystal structure of [U(η-C5Me5)2(OC4H8)2][BPh4], the first cationic cyclopentadienyl compound of uranium(III). J. Organomet. Chem. 1997, 533, 7–11. [Google Scholar] [CrossRef]
- Manriquez, J.M.; Fagan, P.J.; Marks, T.J.; Vollmer, S.H.; Day, C.S.; Day, V.W. Pentamethylcyclopentadienyl organoactinides. trivalent uranium organometallic chemistry and the unusual structure of bis(pentamethylcyclopentadienyl)uranium monochloride. J. Am. Chem. Soc. 1979, 101, 5075–5078. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Lalancette, J.M.; Rollin, G.; Dumas, P. Metals intercalated in graphite. I. Reduction and oxidation. Can. J. Chem. 1972, 50, 3058–3062. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Killough, J.M. Reactions of potassium-graphite. J. Am. Chem. Soc. 1978, 100, 2126–2134. [Google Scholar] [CrossRef]
- Thomson, R.K.; Cantat, T.; Scott, B.L.; Morris, D.E.; Batista, E.R.; Kiplinger, J.L. Uranium azide photolysis results in C–H bond activation and provides evidence for a terminal uranium nitride. Nat. Chem. 2010, 2, 723–729. [Google Scholar] [CrossRef] [PubMed]
- APEX II 1.08, Bruker AXS, Inc.: Madison, WI, USA, 2004; 53719.
- SAINT+ 7.06, Bruker AXS, Inc.: Madison, WI, USA, 2003; 53719.
- SADABAS 2.03, University of Göttingen: Göttingen, Germany, 2001.
- SHELXTL 5.10, Bruker AXS, Inc.: Madison, WI, USA, 1997; 53719.
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichtscheidl, A.G.; Pagano, J.K.; Scott, B.L.; Nelson, A.T.; Kiplinger, J.L. Expanding the Chemistry of Actinide Metallocene Bromides. Synthesis, Properties and Molecular Structures of the Tetravalent and Trivalent Uranium Bromide Complexes: (C5Me4R)2UBr2, (C5Me4R)2U(O-2,6-iPr2C6H3)(Br), and [K(THF)][(C5Me4R)2UBr2] (R = Me, Et). Inorganics 2016, 4, 1. https://doi.org/10.3390/inorganics4010001
Lichtscheidl AG, Pagano JK, Scott BL, Nelson AT, Kiplinger JL. Expanding the Chemistry of Actinide Metallocene Bromides. Synthesis, Properties and Molecular Structures of the Tetravalent and Trivalent Uranium Bromide Complexes: (C5Me4R)2UBr2, (C5Me4R)2U(O-2,6-iPr2C6H3)(Br), and [K(THF)][(C5Me4R)2UBr2] (R = Me, Et). Inorganics. 2016; 4(1):1. https://doi.org/10.3390/inorganics4010001
Chicago/Turabian StyleLichtscheidl, Alejandro G., Justin K. Pagano, Brian L. Scott, Andrew T. Nelson, and Jaqueline L. Kiplinger. 2016. "Expanding the Chemistry of Actinide Metallocene Bromides. Synthesis, Properties and Molecular Structures of the Tetravalent and Trivalent Uranium Bromide Complexes: (C5Me4R)2UBr2, (C5Me4R)2U(O-2,6-iPr2C6H3)(Br), and [K(THF)][(C5Me4R)2UBr2] (R = Me, Et)" Inorganics 4, no. 1: 1. https://doi.org/10.3390/inorganics4010001
APA StyleLichtscheidl, A. G., Pagano, J. K., Scott, B. L., Nelson, A. T., & Kiplinger, J. L. (2016). Expanding the Chemistry of Actinide Metallocene Bromides. Synthesis, Properties and Molecular Structures of the Tetravalent and Trivalent Uranium Bromide Complexes: (C5Me4R)2UBr2, (C5Me4R)2U(O-2,6-iPr2C6H3)(Br), and [K(THF)][(C5Me4R)2UBr2] (R = Me, Et). Inorganics, 4(1), 1. https://doi.org/10.3390/inorganics4010001