Abstract
The coordination of the bifunctional ligand 4-mercaptoaniline with aqueous tin(II) metal ion was studied. A coordination polymer was synthesized when an aqueous solution of SnCl2 was treated with 4-MA. The crystalline material is stable under atmospheric conditions retaining its oxidation state. However, when submerged in a solution saturated with oxygen, the compound oxidizes to a mononuclear tin(IV) complex. Both the compounds were characterized by single crystal X-ray diffraction studies. Although the structure of the tin(IV) complex was previously reported, crystal structure of this compound was redetermined.
Keywords:
tin(II); tin(IV); coordination complex; polymer; metal sulfide; 4-Aminothiophenol; mercaptoaniline; aqueous; synthesize; redox 1. Introduction
Recently, we have investigated various separation techniques in order to isolate metal ions from aqueous waste streams. Methods that can yield recyclable treated material are of interest. One such technique involves the use of 4-mercaptoaniline (4-MA) as a precipitant. The precipitated material from this method can be recycled as metal sulfides.
4-MA is commonly used to conduct surface enhanced Raman spectroscopy (SERS) [1], as the metal-binding terminal end of conductive nanowires [2], and to synthesize gold nanoparticles [3]. Sulfur serves as a “soft” ligand (base) with affinity for “soft” metals (acids), according to hard soft acid base (HSAB) theory. Sulfur can coordinate both in terminal and bridging fashion to a metal center [4]. We are exploring the feasibility of using 4-MA as a precipitating agent to treat aqueous metal ion waste streams. 4-MA is known to exist as a zwitterion in both solid and pure liquid forms [5].
Tin is a group 14 metal with common oxidation states of +2 and +4. It finds many applications in fuel cells [6], batteries [7] and photovoltaics [8] to chemical sensors [9], semiconducting nanowires [10], and window coatings [11]. Many types of catalysis utilizing tin species have been reported [12], including hydrogenations [13], cross-coupling reactions [14], oxidation of carbon monoxide [15] and ethanol [16] and the reduction of carbon dioxide [17] and NOx [18]. Tin oxides [19] and tin sulfides [20] are well known and characterized for their structures and activities.
Previously Eichhöfer and co-workers reported the synthesis of a one dimensional polymer compound (2) that was formed by one tin(II) and two thiophenol units per monomer [21]. One thiolate bridges the tin backbone while the other “sticks out” from the chain. This synthesis was carried out in dimethyl ether (DME) and PhSSiMe3 was used as a source of thiophenol moiety. Our studies on separation techniques demand execution in water, hence we have studied the coordination of tin(II) in aqueous medium with ethanolic 4-MA under ambient conditions. We also examined the coordination of tin(II) in nonaqueous solution under ambient conditions. Studies with tin(IV) and thiophenols have also been carried out in nonaqueous medium at elevated temperature, using the disulfide form of MA [22,23]. Complexes of inorganic or organic mercury and 4-MA have been synthesized, yielding polymeric substances [24].
We studied the application of 4-MA as a mercury(II) precipitant using cold vapor atomic absorption spectroscopy (CVAAS). We suspected that there could be some chemical interference due to coordination of excess 4-MA (from mercury treatment) to tin(II) used in CVAAS. The method for mercury analysis using this technique requires the use of tin(II) ion to reduce mercury(II) to mercury(0) followed by atomic absorption spectroscopy. Since tin(II) is similarly “soft” as mercury(II) we anticipated that it was a good candidate for complexation with 4-MA. We were able to remove over 98% of tin(IV) from a 6000 ppm synthetic waste stream. In this article we report a study on the coordination nature of tin(II) chloride with 4-MA in ambient conditions.
2. Results and Discussion
The tin solution used in CVAAS is acidic and hence, we treated SnCl2 in 10% v/v HCl with ethanolic 4-MA solution. This yielded no precipitation. However, when an acid-free solution of SnCl2 was treated with ethanolic 4-MA, precipitation did occur. We were also interested in learning if tin(II) can be oxidized to tin(IV) when coordinating with 4-MA and thereby making tin(II) unavailable for the reduction of mercury(II).
When colorless aqueous solution of SnCl2 is treated with ethanolic 4-MA, solution color changes to yellow. If this solution is shaken, microcrystalline material begins to form. Diffraction quality crystals can be formed by taking the un-disturbed solution and storing it overnight in the fridge. It must be noted that in every synthesis of tin(II) complex attempted, both tin(II) and tin(IV) complexes formed. Single crystal X-ray diffraction analysis of such crystals revealed the polymeric structure of compound 1 and tetrahedral mononuclear compound 2. Table 1 contains the crystallographic data of compounds 1 and 2.

Table 1.
Crystallographic data for compound 1 and 2.
| Compound | 1 | 2 |
|---|---|---|
| Empirical formula | C12H15ClN2OS2Sn | C24H26Cl2N4S4Sn |
| Formula weight | 421.52 g mol−1 | 688.32 g mol−1 |
| Temperature | 273(2) K | 273(2) K |
| Wavelength | 0.71075 Å | 0.71075 Å |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P 2(1)/c | C 2/c |
| Unit cell dimensions | a = 17.328(14) Å; α = 90° | a = 31.03(2) Å; α = 90° |
| Volume | 1521(2) Å3 | 2871(4) Å3 |
| Z | 4 | 4 |
| Density (calculated) | 1.840 Mg/m3 | 1.593 Mg/m3 |
| Absorption coefficient | 2.122 mm−1 | 1.388 mm−1 |
| F(000) | 832 | 1384 |
| Crystal size | 0.29 × 0.16 × 0.03 mm3 | 0.23 × 0.15 × 0.14 mm3 |
| Theta range for data collection | 2.049 to 27.418° | 2.643 to 27.482° |
| Index ranges | −20 ≤ h ≤ 22, −15 ≤ k ≤ 15, −9 ≤ l ≤ 9 | −40 ≤ h ≤ 38, −10 ≤ k ≤ 10, −15 ≤ l ≤ 15 |
| Reflections collected | 8800 | 11610 |
| Independent reflections | 3443 [R(int) = 0.0726] | 3281 [R(int) = 0.0427] |
| Completeness to theta = 27.418° | 98.9% | 99.9% |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 1.00 and 0.870 | 1.000 and 0.829 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3443/12/200 | 3281/0/211 |
| Goodness-of-fit on F2 | 1.064 | 1.074 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0536, wR2 = 0.1091 | R1 = 0.0321, wR2 = 0.0798 |
| R indices (all data) | R1 = 0.0974, wR2 = 0.1091 | R1 = 0.0373, wR2 = 0.0832 |
| Extinction coefficient | n/a | n/a |
| Largest diff. peak and hole | 0.806 and −1.030 e.Å−3 | 0.465 and −0.838 e.Å−3 |
The 1-D coordination polymer of tin and 4-MA can also be synthesized by layering ethanolic 4-MA on top of anhydrous SnCl2. This yields better crystals than the aqueous route. Compound 1 can be converted to complex 2 by dissolving complex 1 in acetone and bubbling air through the solution overnight (Scheme 1). Whether simple stoichiometric variation could lead to the formation of 2 was also investigated. When anhydrous SnCl2 is treated by layering ethanolic 4-MA in a 4:1 ligand to metal ratio and the material was allowed to crystallize overnight in the fridge, both compounds 1 and 2 form. Compound 2 can also be synthesized by treating SnCl4·5H2O in the same manner with 4-MA (Scheme 1).
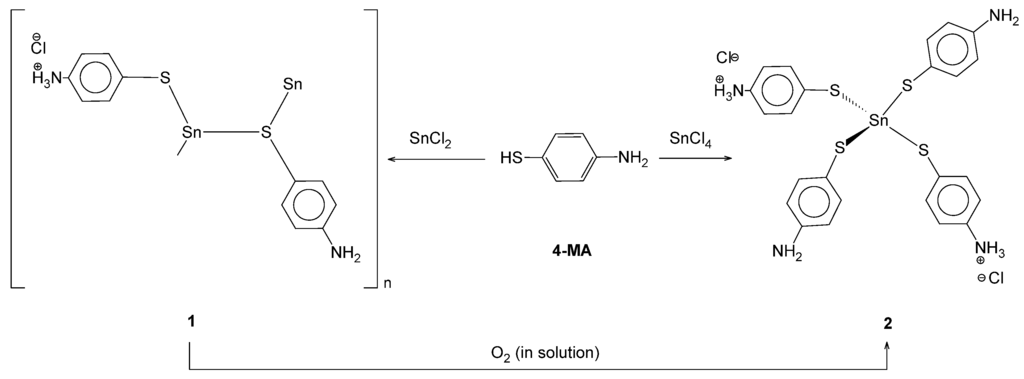
Scheme 1.
Synthesis of one dimensional coordination polymer (followed by its subsequent decomposition to the tin(IV) complex).
For the 1-D polymeric tin(II) complex we report trigonal pyramidal geometry around the tin(II) center. The polymer backbone of the tin(II) complex is composed of alternating tin-sulfur bonds in a “zipper” shape (when viewed along the b axis). The bonds between the tin center and bridging sulfur are two distinct lengths; the “primary” μ sulfur-tin bond length is 2.594(3) Å and the “secondary” μ sulfur-tin bond length is 2.702(2) Å (Table 2). These bridging ligands each have a terminal amine group. Each tin(II) center has an additional terminal 4-MA ligand. Unlike the bridging ligands, the terminal amine groups of the terminal ligands are protonated and paired with chloride counter anions. The distance between tin and sulfur of two neighboring strands is 3.778 Å. As mentioned, Eichhöfer’s group previously synthesized a tin-containing 1-D coordination polymer which utilized thiophenol as the bridge [21] between two neighboring tin. They also report distorted trigonal pyramidal geometry around tin. The S1–Sn1 bond length in this compound was 2.518(2) Å, the S2-Sn1 bond length was 2.577(2) Å and the S2'–Sn1 bond length was 2.731(2) Å. The reported S1–Sn1–S2 angle is 77.16(6)°, the S1–Sn1–S2' angle is 92.68(6)°, and the S2–Sn1–S2' angle is 87.58(5)°.

Table 2.
Selected bond distance and bond angles for compounds 1 and 2.
| 1 | 2 | ||
|---|---|---|---|
| Bond lengths, Å | |||
| Sn(1)–S(1) | 2.563(3) | Sn(1)–S(1) | 2.399(1) |
| Sn(1)–S(2) | 2.702(2) | Sn(1)–S(2) | 2.395(1) |
| Bond angles, ° | |||
| S(1)–Sn(1)–S(2) | 92.28(6) | S(2)–Sn(1)–S(1) | 101.73(5) |
Wang and co-workers have previously synthesized and characterized compound 2 [Sn(4-SC6H4NH2)2(4-SC6H4NH3)2Cl2] [25]. Complex 2 is a mono-nuclear compound with tetrahedral coordination around tin. Two 4-MA moieties contain terminal amine groups and two of them contain terminal ammonium groups with associated chloride counter ions. The Sn–S2 bond length (for each amine-terminal ligand) is 2.3955(12) Å; the Sn-S1 length (for each ammonium-terminal ligand) is 2.3989(13) Å. The sulfur-tin-sulfur angle in the asymmetric unit is 101.73(5)°. Figure 1a,b are the ORTEP diagrams of compound 1 and compound 2, respectively.
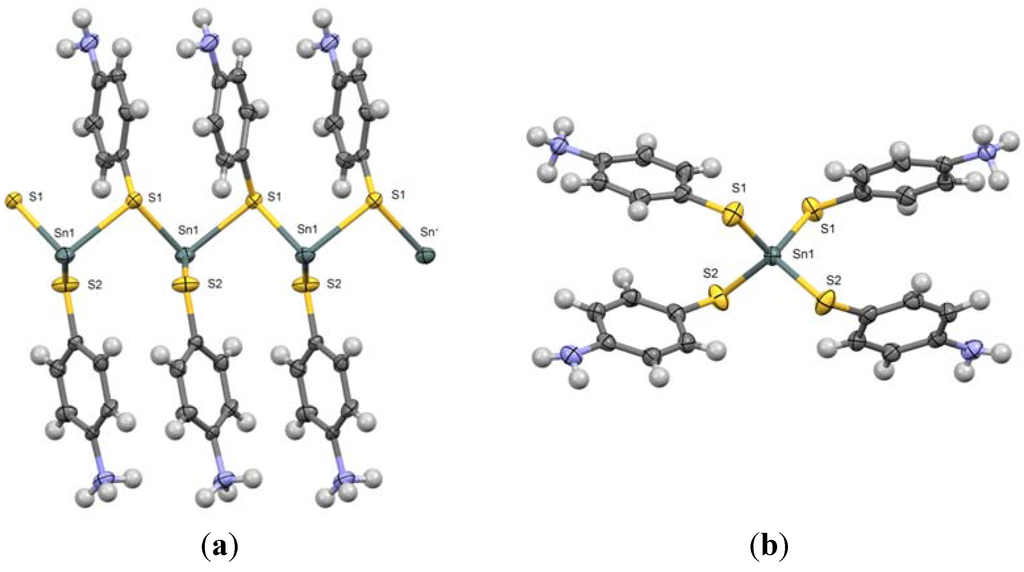
Figure 1.
(a) ORTEP diagram of Sn (II) containing one dimensional polymer (1) and (b) ORTEP diagram of Sn(IV) containing mononuclear complex (2).
3. Experimental Section
3.1. General
Both complex 1 and complex 2 were synthesized under ambient conditions. Reagents were obtained commercially and used without further purification. XRD data were collected on a Rigaku (The Woodlands, TX, USA) XtaLAB mini. Infrared spectra were recorded on a Thermo Scientific Nicolet (Madison, WI, USA) iS10 with smart iTR accessory.
3.2. Synthesis of [{Sn(µ-4-C6H4NH3S)(4-C6H4NH2S)}Cl∙H2O]n
The tin(II) complex (1) was prepared by two routes.
Route 1: 0.0759 g (0.4 mmol) SnCl2 was dissolved in 1 mL of deionized water to which 0.1056 g (0.8 mmol) 4-MA dissolved in 1.5 mL of ethanol was added. The two components were mixed thoroughly and small yellow crystals quickly formed. The contents were dried in vacuo then washed with diethyl ether and washed with acetone until colorless wash was obtained (7 × 15 mL). Some of compound 1 will be converted to compound 2 during workup. However, in a separate experiment we have determined that compound 2 has very limited solubility in acetone, compared to compound 1. In order to selectively isolate compound 3 from potential mixture of compound 1 and compound 2, the mixture was washed with acetone. All of the acetone washes were combined and solvent was removed under reduced pressure. Dry solid yielded 33.9% complex 1.
Route 2: 0.0844 g (0.4 mmol) of SnCl2 was added to a round bottom flask along with 0.1203 g (1 mmol) 4-MA dissolved in 15 mL ethanol and mixed vigorously. The mixture was dried under vacuum then washed with diethyl ether to remove excess 4-MA. The remaining mixture was washed with acetone to isolate tin(II) complex. This acetone fraction was then dried under vacuum. Dry solid yielded 40.3% complex 1; IR (cm−1) 3333, 3247, 3158, 3047, 2921, 2851, 2745, 2585.
3.3. Synthesis of [Sn(4-SC6H4NH2)2(4-SC6H4NH3)2Cl2]
The tin(IV) complex (2) was prepared by two routes as well.
Route 1: A solution was prepared by dissolving 0.1003 g (0.2 mmol) of compound 1 in 10 mL of acetone. A gentle stream of air was bubbled through the solution for 12 h. This method resulted in an impure product containing compounds 1 and 2.
Route 2: 0.0509 g SnCl4·5H2O (0.1 mmol) was combined with 0.0736 g (0.6 mmol) ethanolic 4-MA in a vial. A yellow solution forms and, upon further disturbance, small yellow crystals precipitate from solution. This mixture was dried under vacuum then washed with water (3 × 1 mL), followed by further washing with acetone and diethyl ether. The dry solid yielded 64.2% of compound 2. IR (cm−1) 3332, 3245, 3158, 3047, 2825, 2744, 2585.
3.4. X-ray Crystallography
A greenish yellow platelet crystal of compound 1 having dimensions of 0.29 × 0.16 × 0.03 mm and a yellow prism crystal of compound 2 having dimensions of 0.23 × 0.15 × 0.14 mm were attached to a MiTeGen (Ithaca, NY, USA) loop by Apiezon (Manchester, UK) grease and mounted on a Rigaku Mercury (The Woodlands, TX, USA) 375R/M CCD (XtaLAB mini) diffractometer using graphite monochromated Mo-Kα radiation (0.71075 Å) at 273 K. The crystals were positioned at 49.50 mm from the CCD system. Data were collected and processed using Crystal Clear (Rigaku, The Woodlands, TX, USA). The data were corrected for Lorentz and polarization effects. The structures of both the complexes were solved using direct methods with SHELXS97 program [26]. The non-hydrogen atoms were refined with anisotropic thermal parameters. The hydrogen atoms were included in calculated positions (riding model) with Uiso set at 1.2 times the Ueq of the parent atom. The structures were refined on F2 by full-matrix least-squares using SHELXL97 [26]. Crystallographic data for compounds 1 and 2 have been deposited with the Cambridge Crystallographic Data Centre (CCDC). The CCDC deposition number for compound 1 is 1009828. The CCDC deposition number for compound 2 is 1027160. This data can be obtained free of charge from www.ccdc.cam.ac.uk/data_request/cif.
4. Conclusions
A new one dimensional coordination polymer of tin(II) and 4-mercaptoaniline was synthesized by a simple procedure under ambient conditions. The compound was studied for structural insight. The tin center has a distorted trigonal pyramidal geometry, where one of the ligands coordinates in a terminal fashion, whereas, the other two ligands are bridging the tin centers in μ2 fashion, giving rise to one dimensional polymeric structure. The terminal ligand had the amine group protonated with a chloride counter anion. Upon treatment with oxygen this polymer oxidized to a mononuclear tin(IV) complex. The tin center for this complex has a tetrahedral geometry with two of the four ligands having protonated amine groups with chloride counter anions.
Acknowledgments
TAS acknowledges the partial support of this work by a grant from the US DOE, fund number 222174-13407.
Author Contributions
Both authors contributed to all the sections of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Osawa, M.; Matsuda, N.; Yoshii, K.; Uchida, I. Charge transfer resonance Raman process in surface-enhanced Raman scattering from p-aminothiophenol adsorbed on silver: Herzberg-Teller contribution. J. Phys. Chem. 1994, 98, 12702–12707. [Google Scholar] [CrossRef]
- Ho Choi, S.; Kim, B.; Frisbie, C.D. Electrical resistance of long conjugated molecular wires. Science 1994, 320, 1482–1486. [Google Scholar] [CrossRef]
- Johnson, S.R.; Evans, S.D.; Brydson, R. Influence of a terminal functionality on the physical properties of surfactant-stabilized gold nanoparticles. Langmuir 1998, 14, 6639–6647. [Google Scholar] [CrossRef]
- Zemlyanskii, N.N.; Borisova, I.V.; Kuznetsova, M.G.; Khrustalev, E.N.; Antipin, M.Y.; Ustynyuk, Y.A.; Lunin, E.E.; Eaborn, C.; Hill, M.S.; Smith, J.D. New Stable Germylenes, Stannylenes, and Related Compounds II. Bis(butylthio)tin(II) and ate-Complexes [(Me3Si)3CE(μ-SBu)2Li(THF)2] (E = Ge, Sn). Synthesis and Structure. Russ. J. Org. Chem. 2003, 39, 491–500. [Google Scholar] [CrossRef]
- Jetti, R.K.R.; Boese, R.; Thakur, T.S.; Vangala, V.R.; Desiraju, G.R. Proton transfer and N(+)–H···S(−) hydrogen bonds in the crystal structure of 4-aminothiophenol. Chem. Commun. 2004, 2526–2527. [Google Scholar] [CrossRef]
- Vidu, R.; Plapcianu, C.; Bartha, C. Multivalence Ce and Sn Oxide Doped Materials with Controlled Porosity for Renewable Energy Applications. Ind. Eng. Chem. Res. 2014, 53, 7829–7839. [Google Scholar] [CrossRef]
- Wang, H.; Rogach, A. Hierarchical SnO2 nanostructures: Recent advances in design, synthesis, and applications. Chem. Mater. 2013, 26, 123–133. [Google Scholar] [CrossRef]
- Tahar, R.; Ban, T. Tin doped indium oxide thin films: Electrical properties. J. Appl. Phys. 1998, 83, 2631–2645. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Gopel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius’ J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Barth, S.; Hernandez-Ramirez, F.; Holmes, J.D.; Romano-Rodriguez, A. Synthesis and applications of one-dimensional semiconductors. Prog. Mater. Sci. 2010, 55, 563–627. [Google Scholar] [CrossRef]
- Hamberg, C. Evaporated tin-doped indium oxide films: Basic optical properties and applications to energy efficient windows. J. Appl. Phys. 1986, 60, 123–159. [Google Scholar] [CrossRef]
- Singh, O.M.; Devi, L.R. Stannous chloride as a versatile catalyst in organic synthesis. Mini. Rev. Org. Chem. 2013, 10, 84–96. [Google Scholar] [CrossRef]
- Thomas, J.M.; Johnson, B.F.G.; Raja, R.; Sankar, G.; Midgley, P.A. High-performance nanocatalysts for single-step hydrogenations. Acc. Chem. Res. 2003, 36, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Espinet, P.; Echavarren, A.M. The mechanisms of the Stille reaction. Angew. Chem. Int. Ed. Engl. 2004, 43, 4704–4734. [Google Scholar] [PubMed]
- Sun, Y.F.; Lei, F.C.; Gao, S.; Pan, B.; Zhou, J.F.; Xie, Y. Atomically thin tin dioxide sheets for efficient catalytic oxidation of carbon monoxide. Angew. Chem. Int. Ed. Engl. 2013, 52, 10569–10572. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Sun, G.; Xin, Q. Effect of alloying degree in PtSn catalyst on the catalytic behavior for ethanol electro-oxidation. Electrochim. Acta 2009, 54, 1511–1518. [Google Scholar] [CrossRef]
- Wu, J.; Risalvato, F.G.; Ma, S.; Zhou, X.-D. Electrochemical reduction of carbon dioxide III. The role of oxide layer thickness on the performance of Sn electrode in a full electrochemical cell. J. Mater. Chem. A 2014, 2, 1647. [Google Scholar] [CrossRef]
- Park, P.W.; Kung, H.H.; Kim, D.; Kung, M.C. Characterization of SnO2/Al2O3 Lean NOx Catalysts. J. Catal. 1999, 184, 440–454. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Ozin, G. New directions in tin sulfide materials chemistry. J. Mater. Chem. 1998, 8, 1099–1108. [Google Scholar] [CrossRef]
- Eichhöfer, A.; Jiang, J. 1-d-Tin(II) Phenylchalcogenolato Complexes ∞ 1[Sn(EPh)2] (E = S, Se, Te)—Synthesis, Structures, Quantum Chemical Studies and Thermal Behaviour. Eur. J. Inorg. Chem. 2010, 2010, 410–418. [Google Scholar] [CrossRef]
- Buttrus, N.H.; Suliman, M.M.; Al-Allaf, T.A.K. Synthesis of new Tin(IV) compounds of substituted diphenylsulfide derivatives and their complexes with some neutral ligands. Synth. React. Inorg. Met. Chem. 2001, 31, 837–848. [Google Scholar] [CrossRef]
- Wuyts, Y.; Vangindertaelen, A. The quadrivalence of tin and its mercaptides. Bull. Soc. Chim. Belg. 1921, 30, 323–328. [Google Scholar]
- Almagro, X.; Clegg, W. Schiff bases derived from mercury (II)-aminothiolate complexes as metalloligands for transition metals. J. Organomet. Chem. 2001, 623, 137–148. [Google Scholar] [CrossRef]
- Wang, L.S.; Zhang, J.J.; Du, W.X.; Hu, S.M.; Fu, R.-B.; Xia, S.-Q.; Wu, X.-T.; Xiang, S.-C.; Li, Y.-M.; Fu, Z.-Y. Syntheses and characterizations of (4-NH2C6H4S)4Sn and [(4-NH2C6H4S)2(4-NH3C6H4S)2Sn]Cl2. Chin. J. Struct. Chem. 2005, 24, 79–83. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).