Experimental and Theoretical Studies of the Factors Affecting the Cycloplatination of the Chiral Ferrocenylaldimine (SC)-[(η5-C5H5)Fe{(η5-C5H4)–C(H)=N–CH(Me)(C6H5)}]
Abstract
:1. Introduction
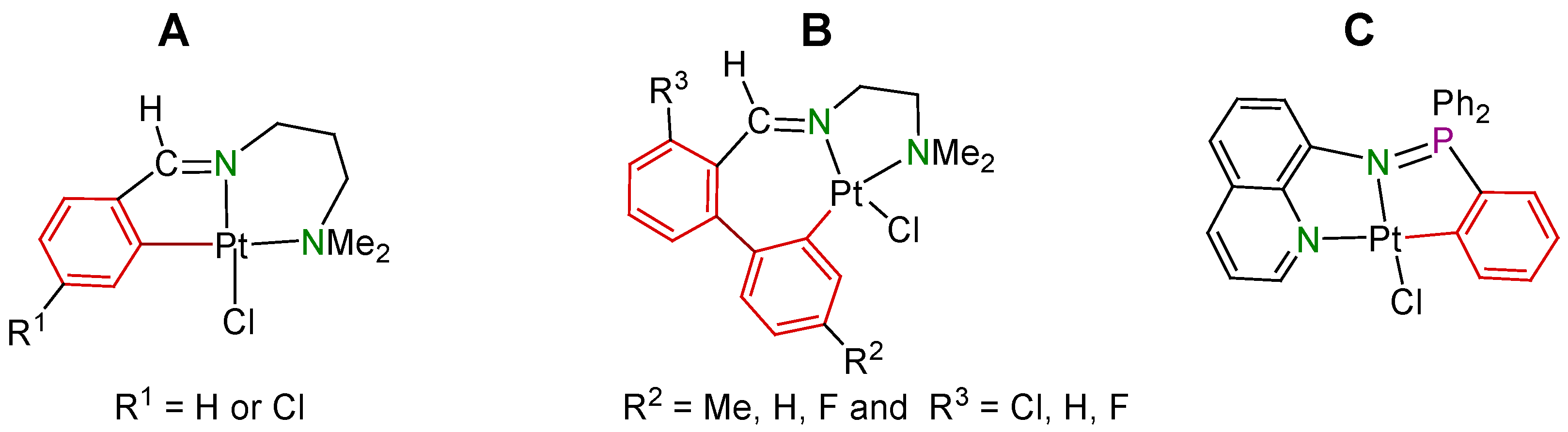
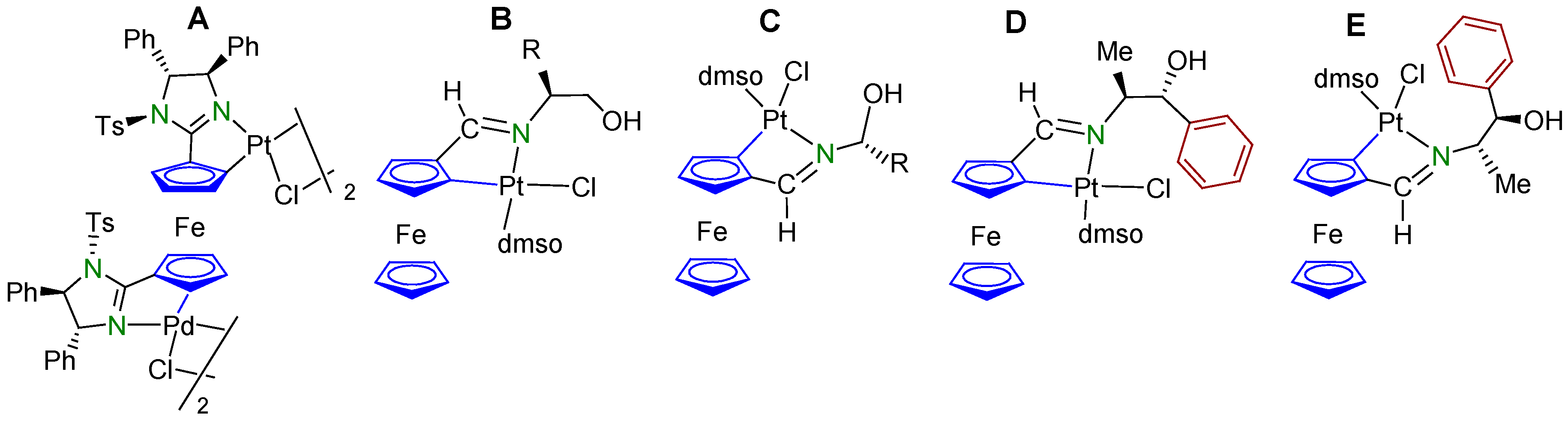
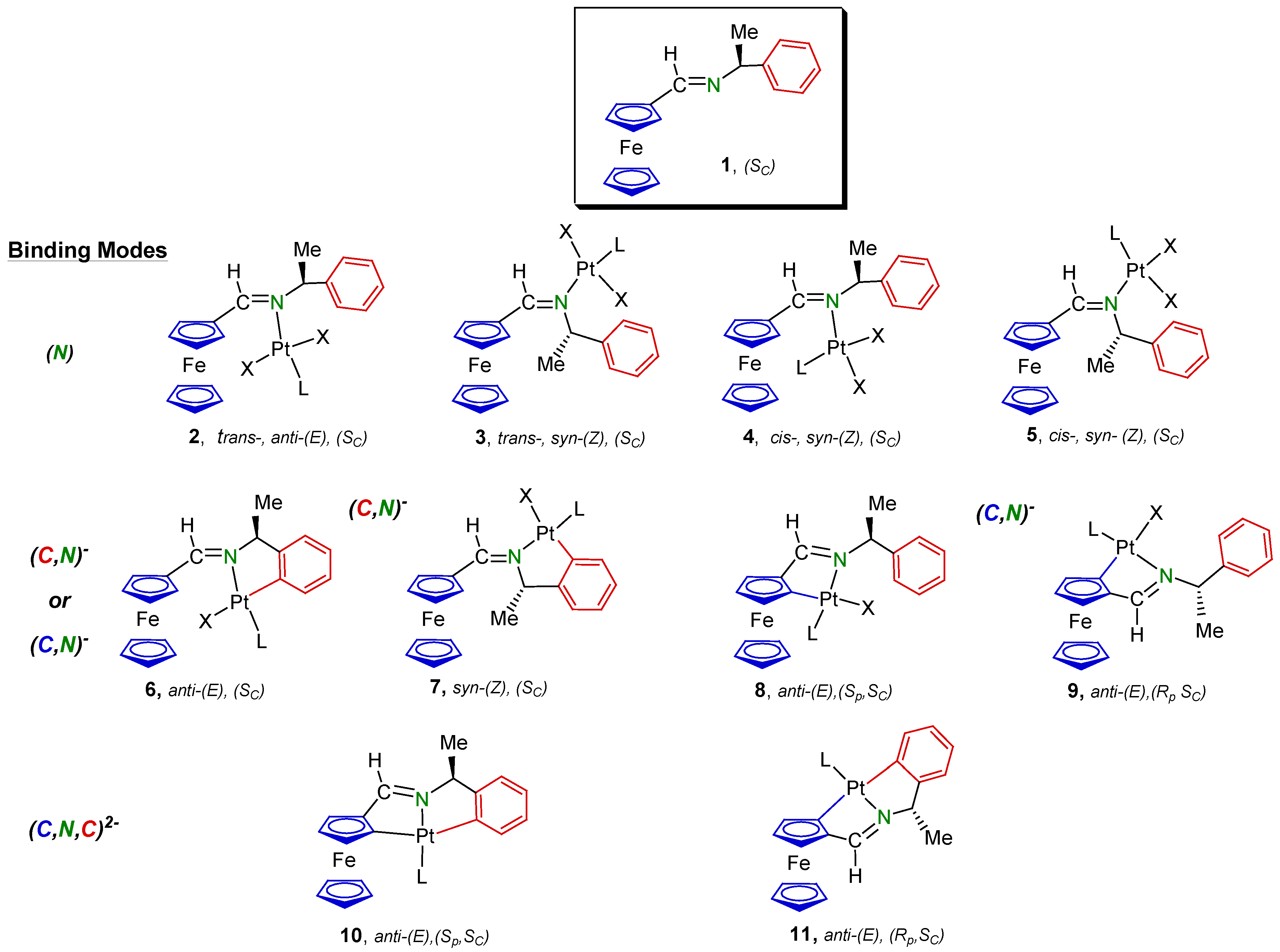
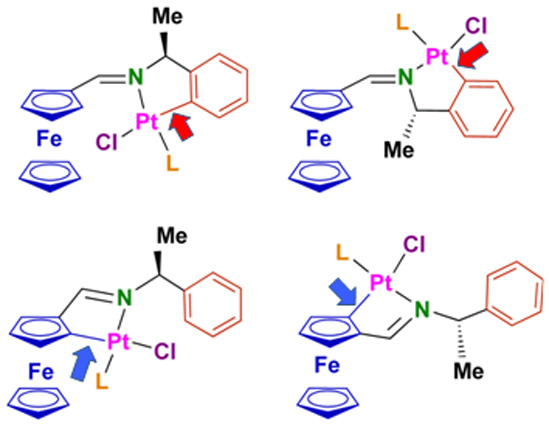
2. Results and Discussion
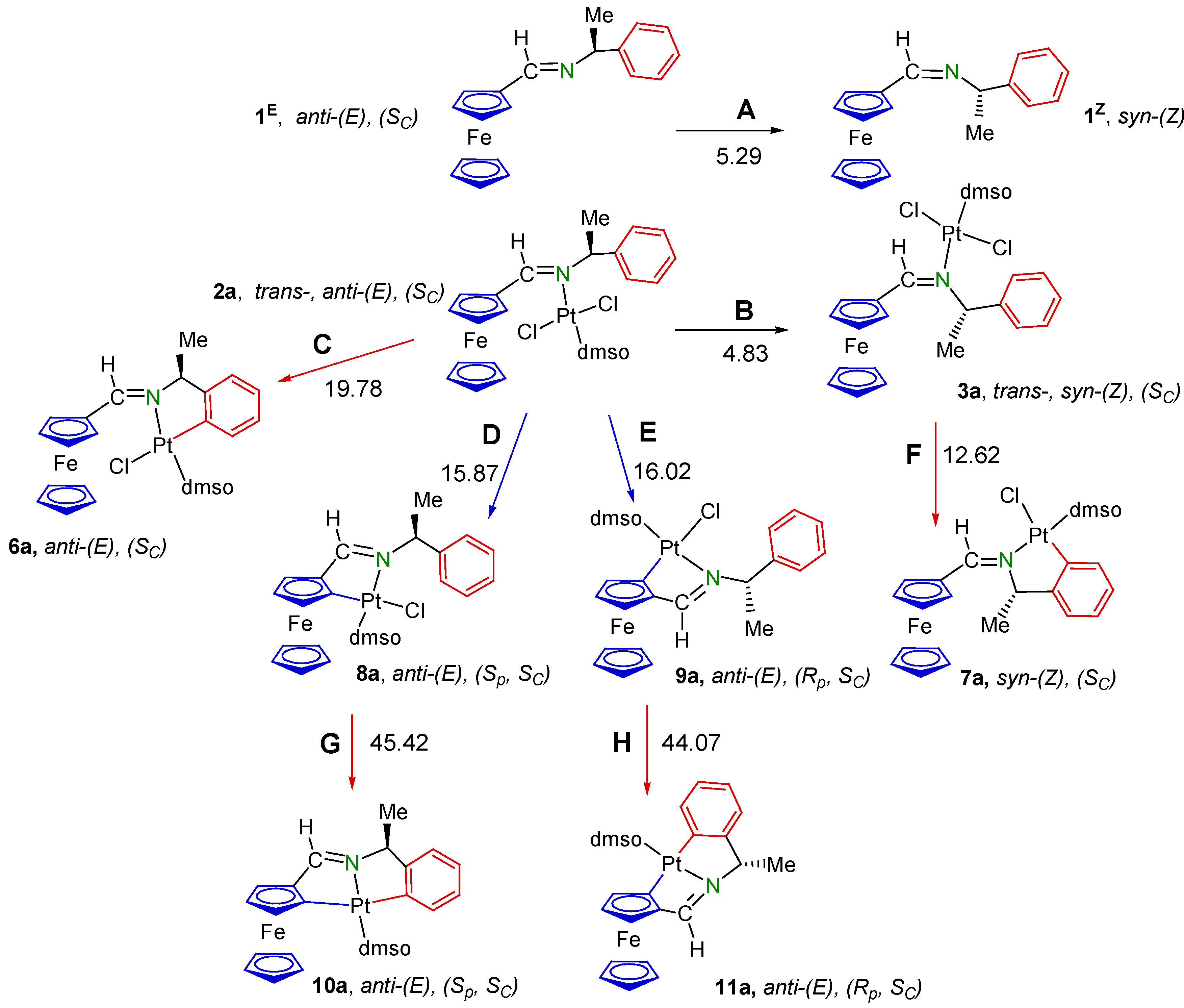
2.1. Synthesis and Characterization of the Compounds
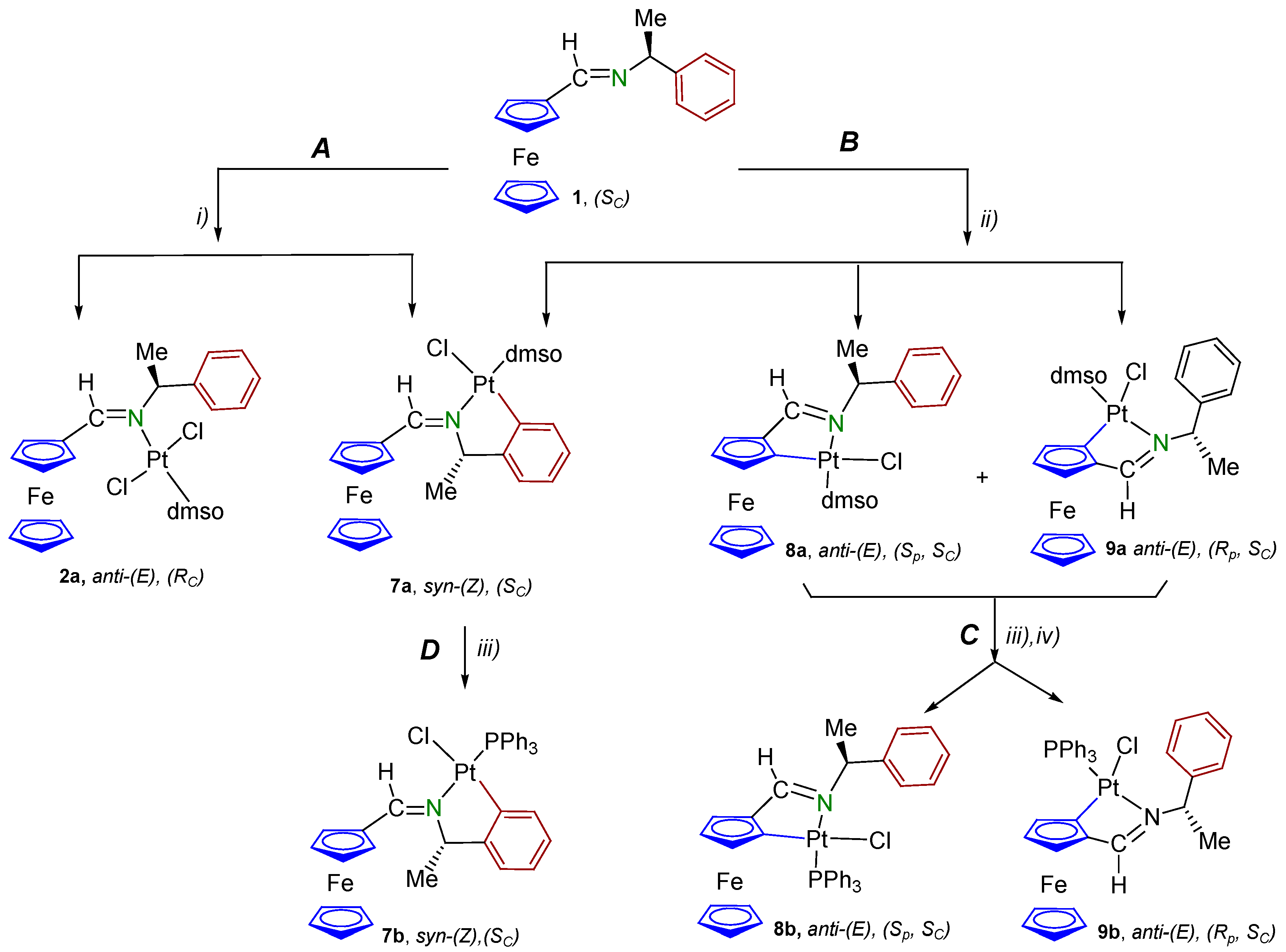
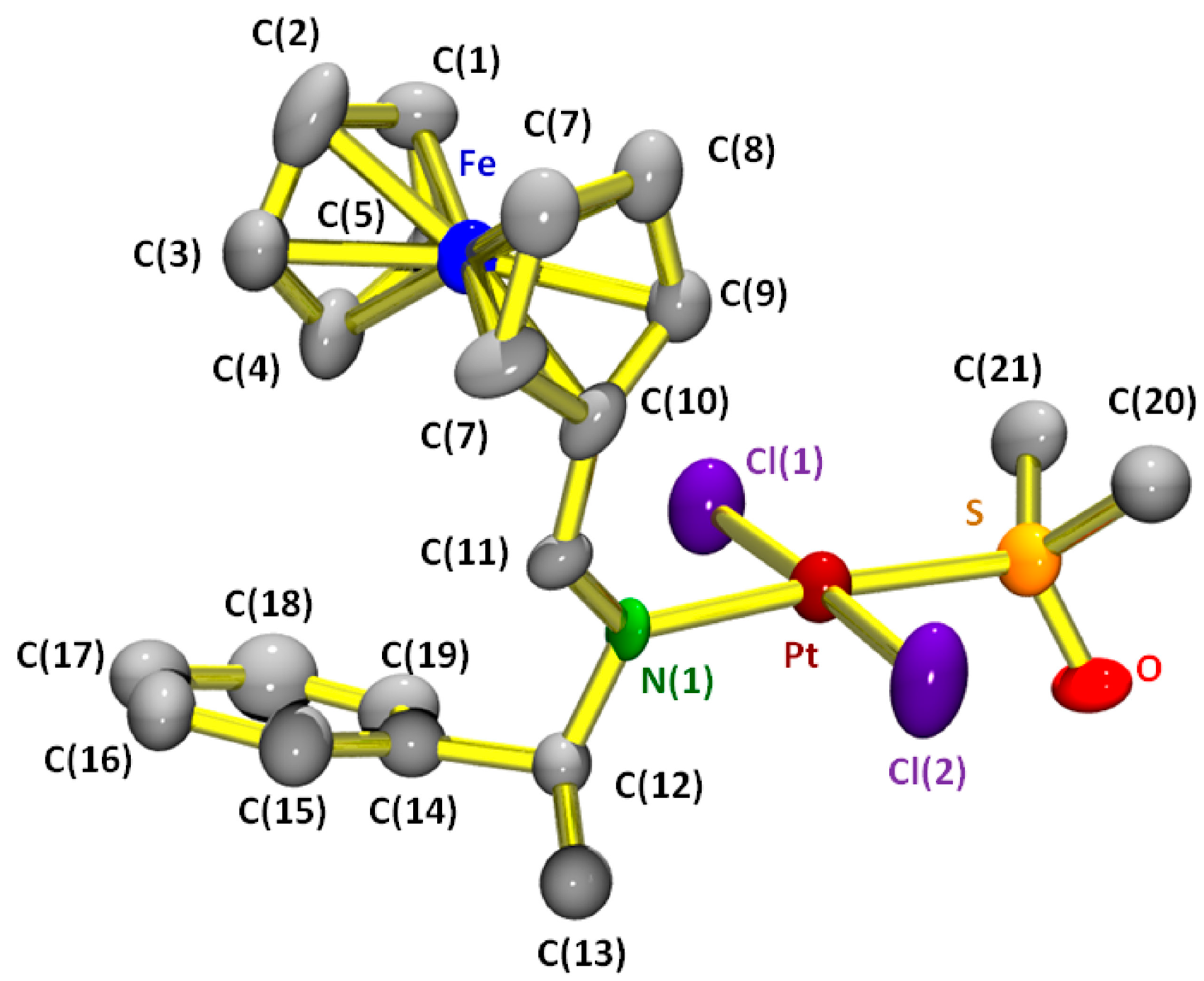
2.2. Study of the Cytotoxic Activity
| Compound | Binding mode of 1 | Cytotoxic activity, IC50 | Electrochemical data | ||||
|---|---|---|---|---|---|---|---|
| MCF7 | MDA-MB231 | Epa | Epc | ΔE | Ipa/Ipc | ||
| 2a | N | >100 | 76 ± nd | 0.076 | 0.006 | 0.070 | 1.08 |
| 7b | (CPh, N)− | 37 ± 4 | 29 ± 3 | 0.101 | 0.038 | 0.063 | 1.10 |
| 8b | (CFc, N)− | 11.4 ± 1.1 | 5.8 ± 0.8 | −0.127 | −0.197 | 0.070 | 0.98 |
| 9b | (CFc, N)− | 9.2 ± 0.9 | 4.3 ± 0.6 | −0.157 | −0.228 | 0.071 | 0.97 |
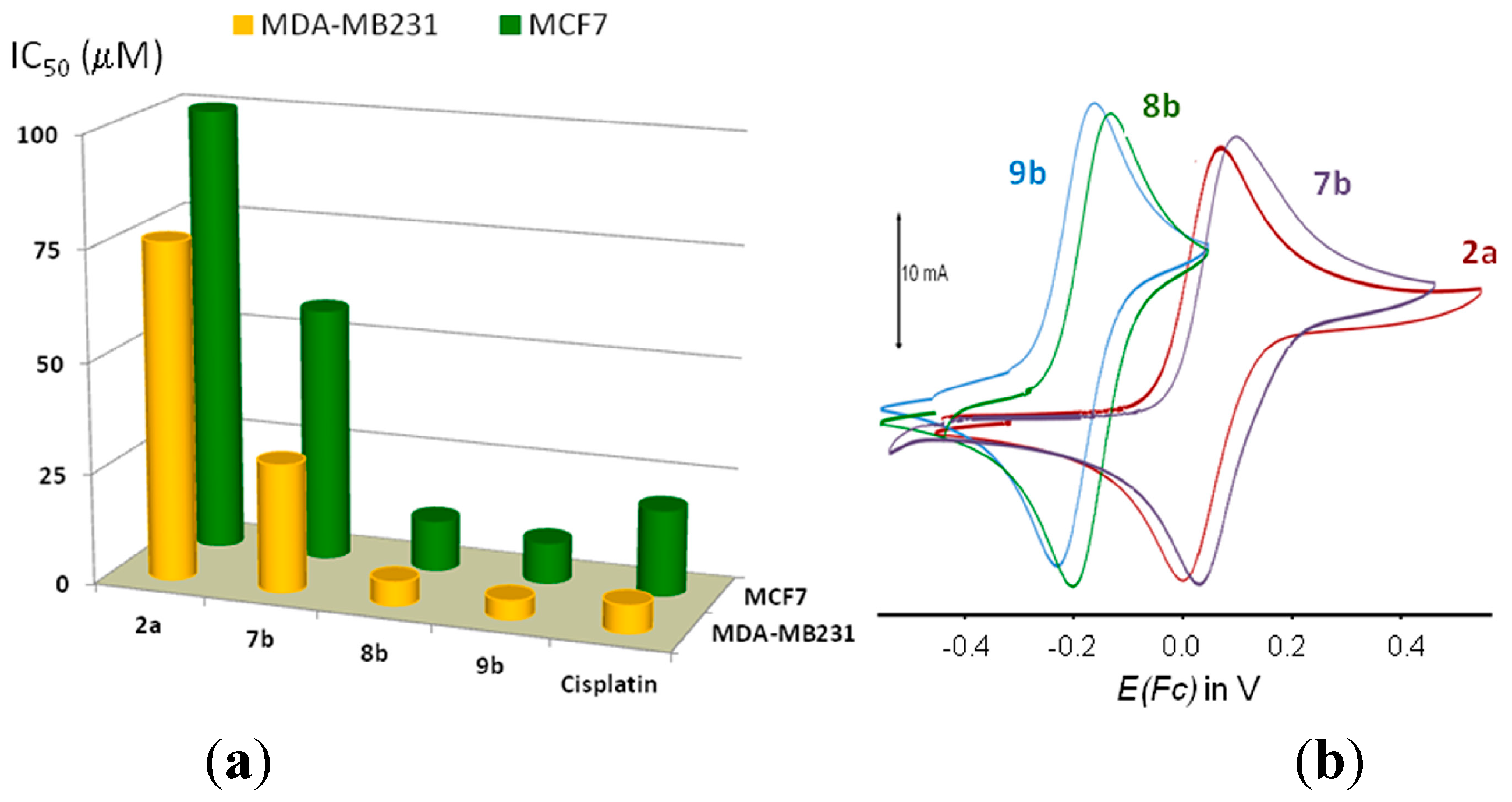
2.3. Electrochemical Properties
2.4. Theoretical Studies
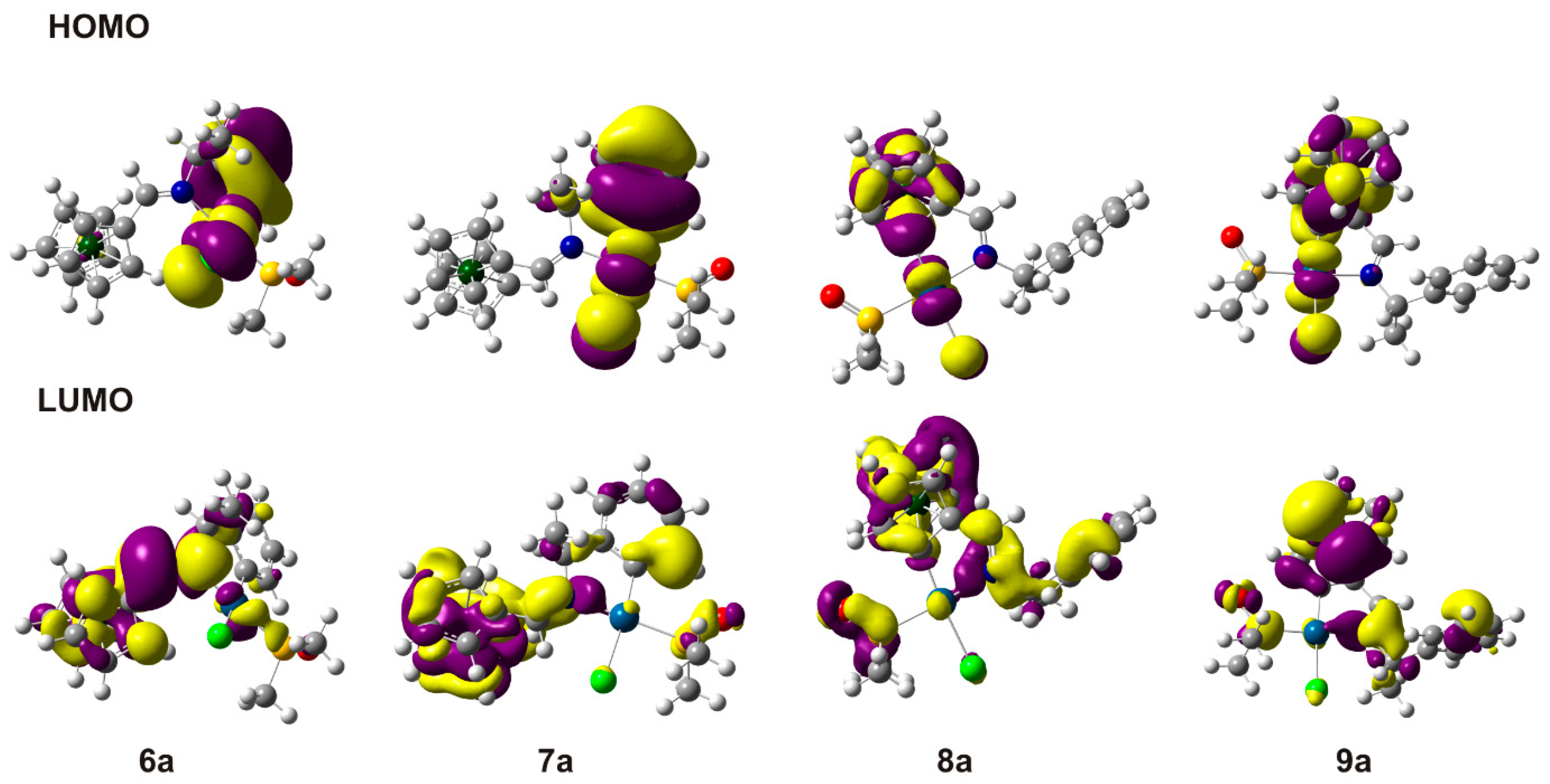
| Compound | Binding Mode of 1 | EHOMO | ELUMO | ELUMO–EHOMO |
|---|---|---|---|---|
| 6a | (CPh, N)− | −0.204 | −0.058 | 0.146 |
| 7a | (CPh, N)− | −0.204 | −0.062 | 0.142 |
| 8a | (CFc, N)− | −0.198 | −0.054 | 0.144 |
| 9a | (CFc, N)− | −0.193 | −0.057 | 0.136 |
3. Experimental Section
3.1. Preparation of the Compounds
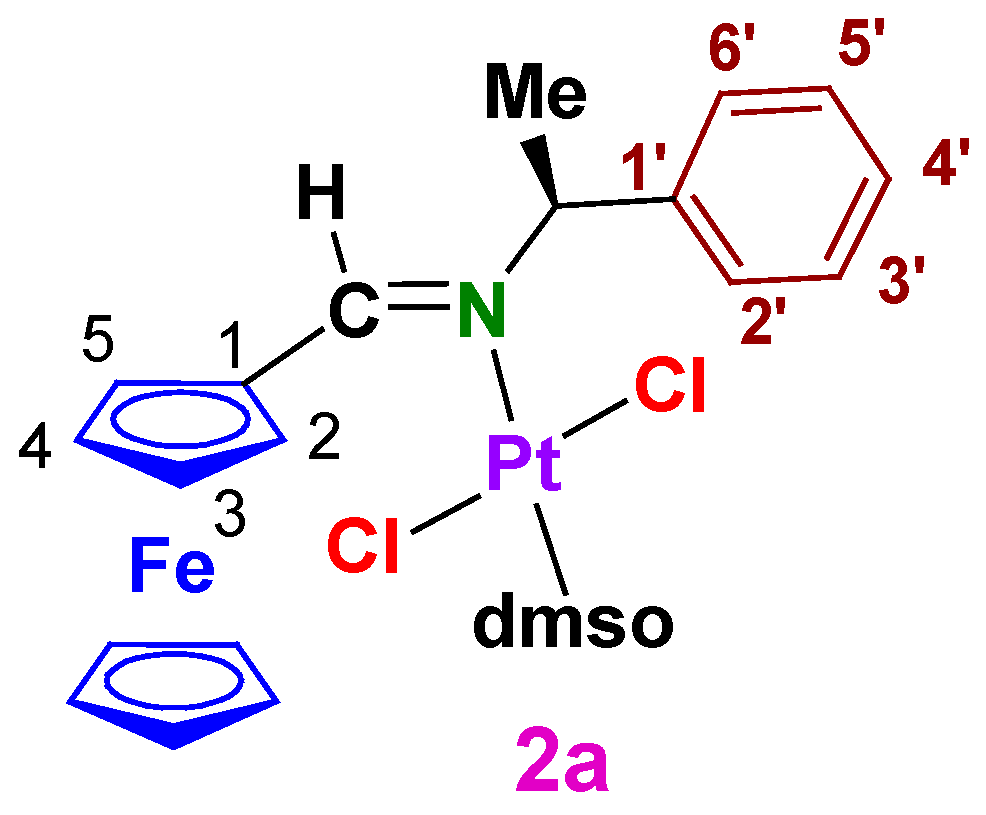
3.1.1. trans-(SC)-[Pt{κ1-N[FcC(H)=N−CH(Me)(C6H5)]}Cl2(dmso)] (2a)
3.1.2. (SC)-[Pt{κ2-C,N[(C6H4)(Me)CHN=C(H)Fc]}Cl(dmso)] (7a)
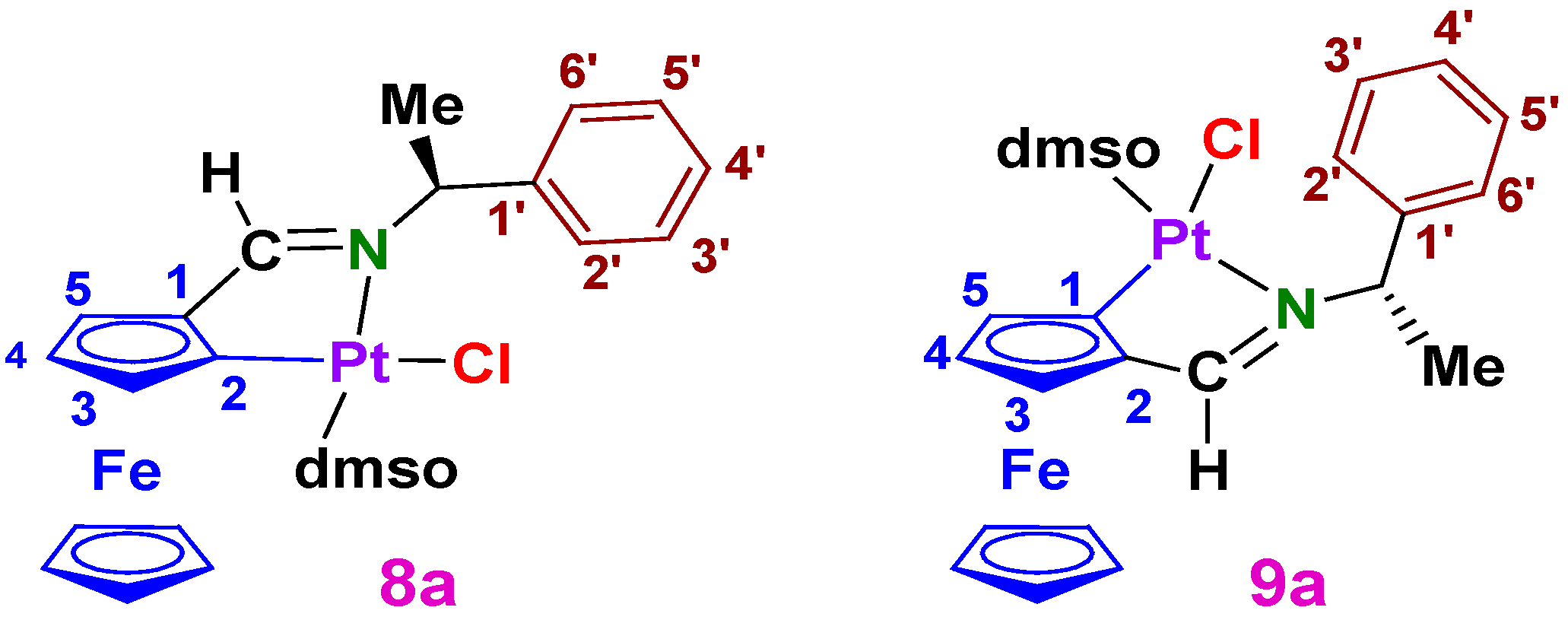
3.1.3. Synthesis of II {a Mixture of the (Sp, SC) and (Rp, SC) Diastereomers [Pt{κ2-C,N[(η5-C5H3)−CH=N−{CH(Me)(C6H5)}]Fe(η5-C5H5)}Cl(dmso)] (8a and 9a, respectively)
3.1.4. (SC)-[Pt{κ2-C,N[(C6H4)CH(Me)−N=C(H)Fc]}Cl(PPh3)] (7b)
3.1.5. Preparation of the Mixture of the Diastereomers of [Pt{κ2-C,N[(η5C5H3)−CH=N−CH(Me)(C6H5)]Fe(η5-C5H5)}Cl(PPh3)] (8b and 9b)
3.1.6. Separation of the Diastereomers of [Pt{κ2-C,N[(η5-C5H3)−CH=N−CH(Me)(C6H4)]Fe(η5-C5H5)}Cl(PPh3)] (8b and 9b)
4. Conclusions
Acknowledgments
Author Contributions
Appendix
A1. Materials and Methods
A1.1. General
| Structural Parameter | Compound 2a |
|---|---|
| Crystal dimmensions/mm × mm × mm | 0.2 × 0.1 × 0.1 |
| Empirical formula | C21H25Cl2FeNO2Pt |
| Formula weight | 661.32 |
| T/K | 293(2) |
| λ/Å | 0.71073 |
| Crystal system | Orthorhombic |
| Space group | P212121 |
| a/Å | 11.670(10) |
| b/Å | 13.2570(10) |
| c/Å | 14.8780(10) |
| α = β = γ/° | 90.0 |
| Volume/Å3 | 2202.6(3) |
| Z | 4 |
| Calculated density/Mg × m−3 | 1.994 |
| µ/mm−1 | 7.353 |
| F(000) | 1280 |
| Θ range for data collection/° | 2.06 to 31.72 |
| Limiting indices | 0 ≤ h ≤ 16, −19 ≤ k ≤ 19, −21 ≤ l ≤ 21 |
| Reflections collected/unique | 9069/6010 [R(int) = 0.0705] |
| Completeness to Θ = 28.34° | 95.2% |
| Absorption correction | Empirical |
| Max. and min. Transmission | 0.48 and 0.42 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 6010/0/254 |
| Goodness-of-fit on F2 | 1.196 |
| Final R indices [I > 2σ(I)] | R1 = 0.0418, wR2 = 0.1301 |
| R indices (all data) | R1 = 0.514, wR2 = 0.1414 |
| Absolute struture parameter | 0.00(2) |
| Largest diff. peak and hole/e Å−3 | 1.522 and −1.764 |
A1.2. Crystallography
A1.3. Biological Studies
A1.3.1. Cell Culture
A1.3.2. Cell Viability Assays
A1.4. Electrochemical Studies
A1.5. Theoretical Studies
A2. Summary of Characterization Data for Compounds under Study (Atom Numbering Schemes for Compounds 2a, 7a, Diastereomers 8a and 9a, 7b, 8b and 9b Are Presented in Figure A1, Figure A2, Figure A3, Figure A4, Figure A5 and Figure A6, Respectively)
A2.1. trans-(SC)-[Pt{κ1-N[FcC(H)=N–CH(Me)(C6H5)]}Cl2(dmso)] (2a)
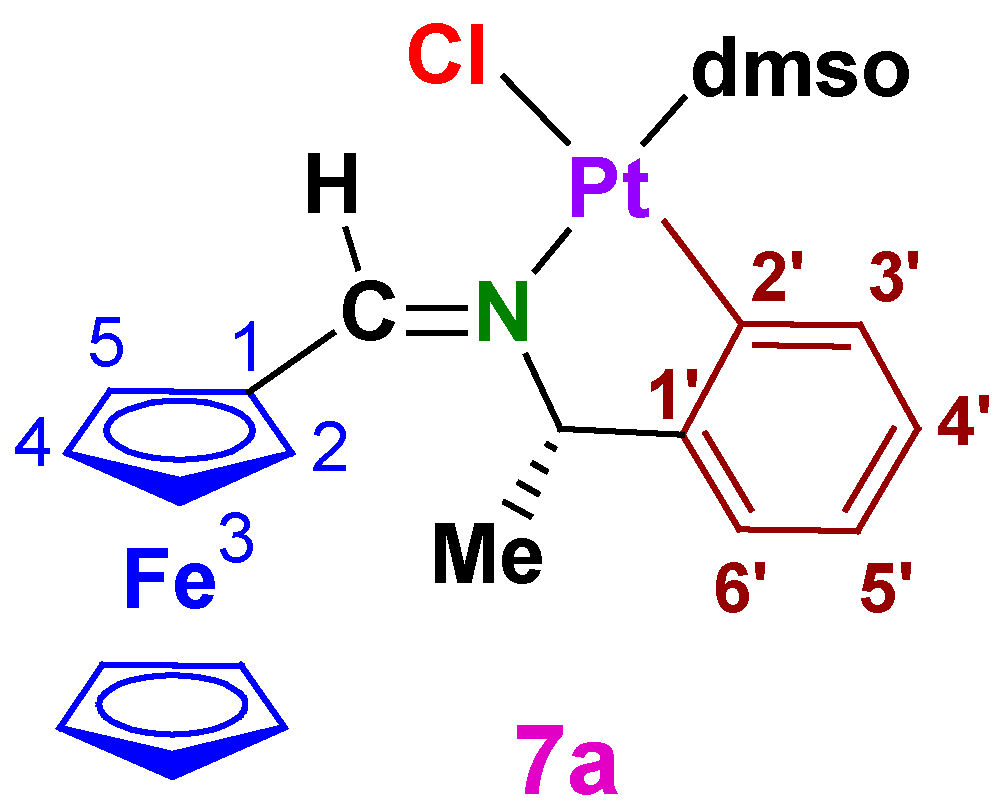
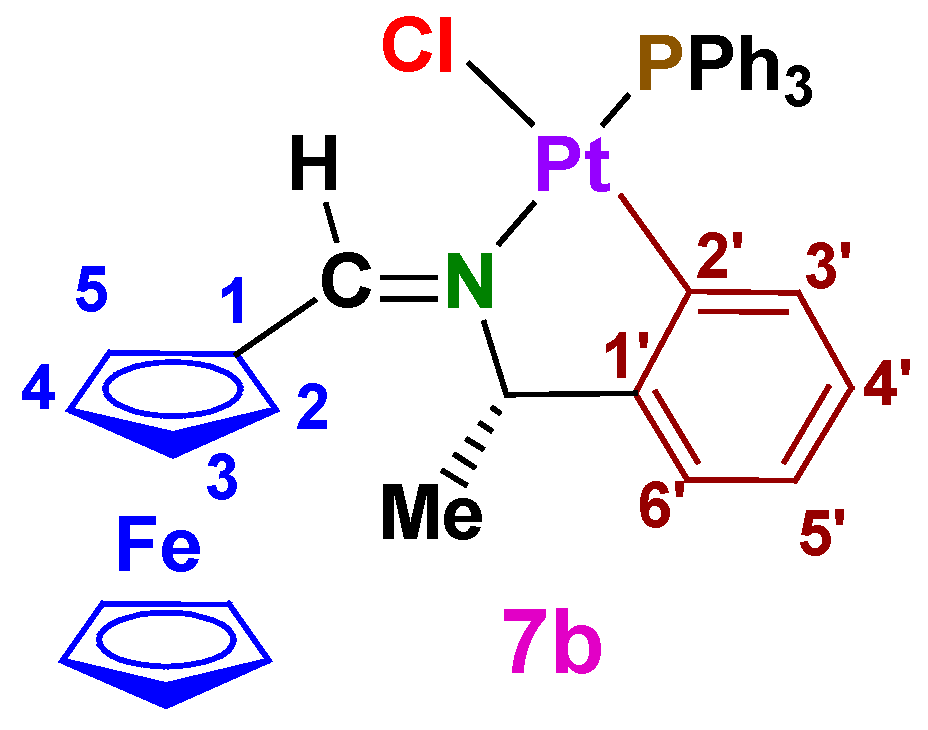
A2.2. (SC)-[Pt{κ2-C,N[(C6H4)CH(Me)–N=C(H)Fc]}Cl(dmso)] (7a)
A2.3. The mixture (II) of the Two Diastereomers of [Pt{{κ2-C,N[(η5-C5H3)–CH=N–{CH(Me)(C6H5)}]Fe(η5-C5H5)}Cl(dmso)] (8a and 9a)
A2.4. (SC)-[Pt{κ2-C,N[(C6H4)CH(Me)–N=C(H)Fc]}Cl(PPh3)] (7b)
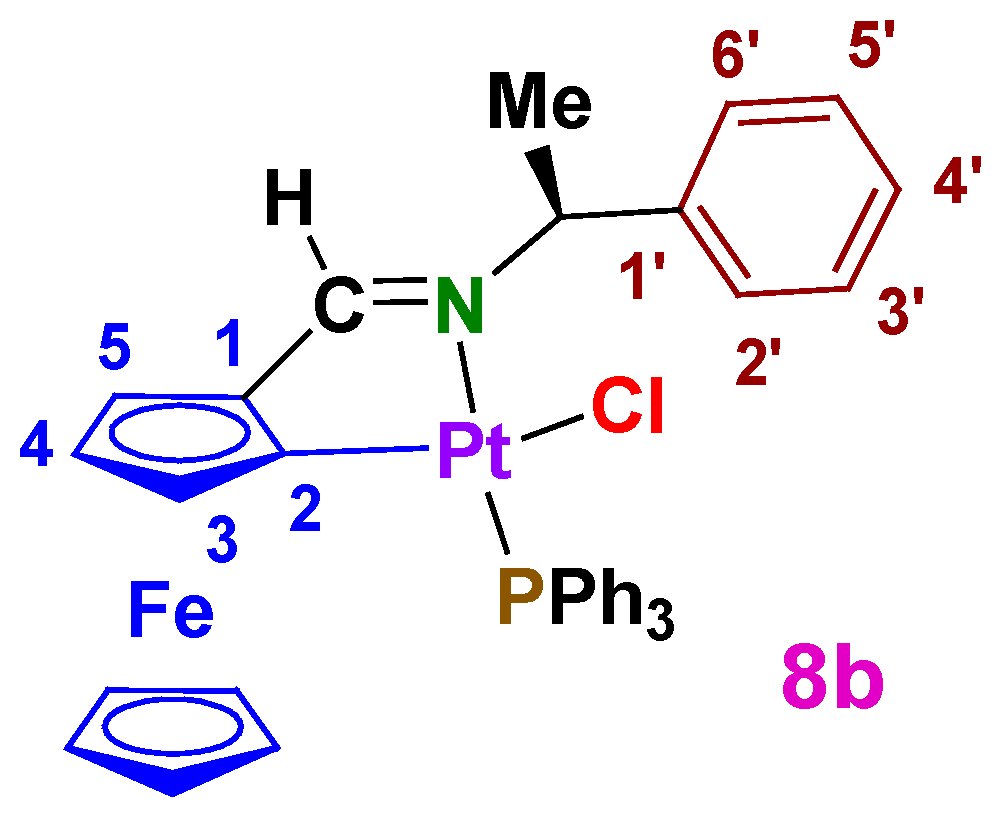
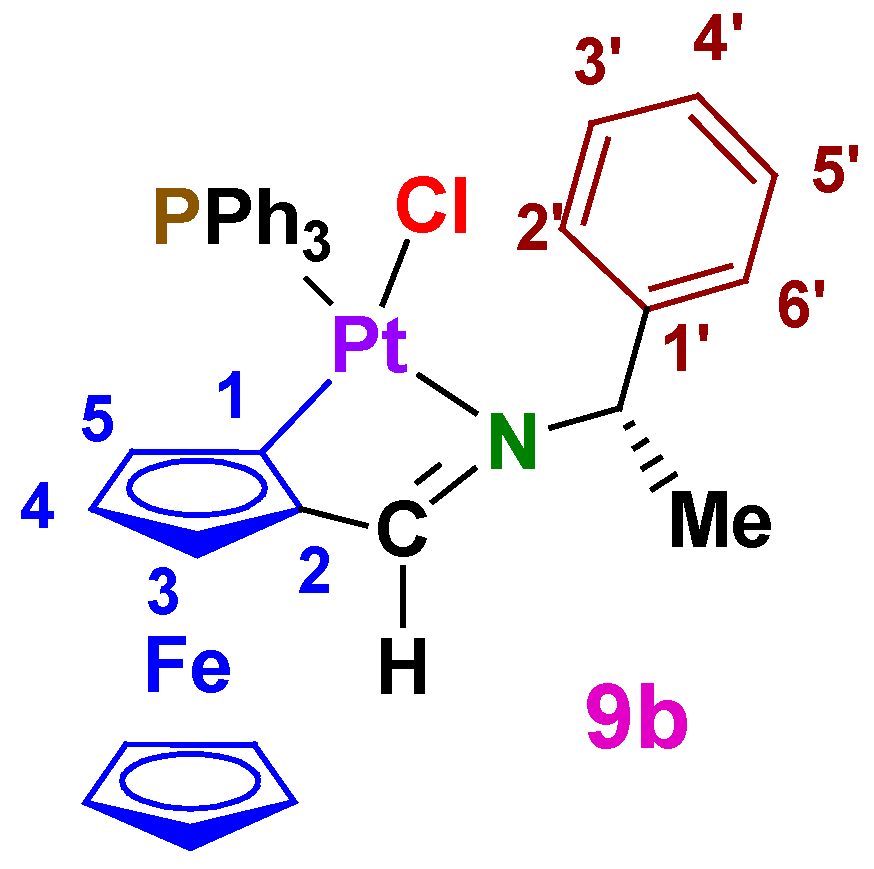
A2.5. Diastereomers of [Pt{κ2-C,N[(η5-C5H3)-CH=N-CH(Me)(C6H5)]Fe(η5-C5H5)}Cl(PPh3)] (8b and 9b)
Conflicts of Interest
References and Notes
- Crespo, M. Effect of fluorine substituents in intramolecular activation of carbon-fluorine and carbon-hydrogen bonds by platinum(II). Organometallics 2012, 31, 1216–1234. [Google Scholar] [CrossRef]
- Guerchais, V.; Ordronneau, L.; le Bozec, H. Recent developments in the field of metal complexes containing photochromic ligands: Modulation of linear and nonlinear optical properties. Coord. Chem. Rev. 2010, 254, 2533–2545. [Google Scholar] [CrossRef]
- Diez, A.; Lalinde, E.; Moreno, M.T. Heteropolynuclear cycloplatinated complexes: Structural and photophysical properties. Coord. Chem. Rev. 2011, 255, 2426–2477. [Google Scholar] [CrossRef]
- Kalinowski, J.; Fattori, V.; Cocchi, M.; Williams, J.A.G. Light-emitting devices based on organometallic platinum complexes as emitters. Coord. Chem. Rev. 2011, 255, 2401–2425. [Google Scholar] [CrossRef]
- Williams, J.A.G.; Develay, S.; Rochester, D.L.; Murphy, L. Optimising the luminescence of platinum(II) complexes and their application in organic light emitting devices (OLEDs). Coord. Chem. Rev. 2008, 252, 2596–2611. [Google Scholar] [CrossRef]
- Siu, P.K.-M.; Ma, D.-L.; Che, C.-M. Luminescent cyclometalated platinum(II) complexes with amino acid ligands for protein binding. Chem. Commun. 2005, 1025–1027. [Google Scholar]
- Sicilia, V.; Fuertes, S.; Martin, A.; Palacios, A. N-Assisted CPh−H Activation in 3,8-dinitro-6-phenylphenanthridine. New C,N-cyclometalated compounds of platinum(II): Synthesis, structure, and luminescence studies. Organometallics 2013, 32, 4092–4102. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Sun, C.; Helander, M.G.; Amarne, H.; Lu, Z.-H.; Wang, S. Enhancing Phosphorescence and Electrophosphorescence Efficiency of Cyclometalated Pt(II) Compounds with Triarylboron. Adv. Funct. Mat. 2010, 20, 3426–3439. [Google Scholar] [CrossRef]
- Rausch, A.F.; Murphy, L.; Williams, J.A.G.; Yersin, H. Improving the Performance of Pt(II) Complexes for Blue Light Emission by Enhancing the Molecular Rigidity. Inorg. Chem. 2012, 51, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Gandioso, A.; Valle-Sistac, J.; Rodriguez, L.; Crespo, M.; Font-Bardia, M. Platinum(II) Compounds Containing Cyclometalated Tridentate Ligands: Synthesis, Luminescence Studies, and a Selective Fluoro for Methoxy Substitution. Organometallics 2014, 33, 561–570. [Google Scholar] [CrossRef]
- Arias, A.; Forniés, J.; Fortuño, C.; Martin, A.; Latronico, M.; Mastrorilli, P.; Todisco, S.; Gallo, V. Formation of P–C Bond through Reductive Coupling between Bridging Phosphido and Benzoquinolinate Groups. Isolation of Complexes of the Pt(II)/Pt(IV)/Pt(II) Sequence. Inorg. Chem. 2012, 51, 12682–12696. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Pullarkat, S.A.; Tan, K.-W.; Li, Y.; Leung, P.-H. Enantioselective Diels-Alder Reaction of 3-Diphenylphosphinofuran with 1-Phenyl-3,4-dimethylphosphole and Subsequent Synthetic Manipulations of the Cycloadduct. Organometallics 2009, 28, 6254–6259. [Google Scholar] [CrossRef]
- Newman, C.P.; Casey-Green, K.; Clarkson, G.J.; Cave, G.W.V.; Errington, W.; Rourke, J.P. Cyclometallated platinum(II) complexes: oxidation to, and C–H activation by platinum(IV). Dalton Trans. 2007, 29, 3170–3182. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.R.; Fernández, J.; Giménez, N.; Lalinde, E.; Moreno, M.T.; Sánchez, S. Unexpected Formation of Ferrocenyl(vinyl)benzoquinoline Ligands by Oxidation of an Alkyne Benzoquinolate Platinum(II) Complex. Organometallics 2013, 32, 3943–3953. [Google Scholar]
- Xiao, X.-S.; Kwong, W.-L.; Guan, X.; Yang, C.; Lu, W.; Che, C.-M. Platinum(II) and Gold(III) Allenylidene Complexes: Phosphorescence, Self-Assembled Nanostructures and Cytotoxicity. Chem.-Eur. J. 2013, 19, 9457–9462. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, Y.; Lu, W.; Zhu, N.; Che, C.M. A Cyclometalated Platinum(II) Complex with a Pendent Pyridyl Motif as Solid-State Luminescent Sensor for Acidic Vapors. Chem.-Eur. J. 2011, 17, 4109–4112. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, K.; Łęczkowska, A.; Furrer, M.A.; Wu, Y.; Kuimova, K.K.; Therrien, B.; White, A.J.P.; Vilar, R. A Cyclometallated Platinum Complex as a Selective Optical Switch for Quadruplex DNA. Chem.-Eur. J. 2012, 18, 16277–16282. [Google Scholar] [CrossRef] [PubMed]
- Boixel, J.; Guerchais, V.; le Bozec, H.; Jacquemin, D.; Amar, A.; Boucekkine, A.; Colombo, A.; Dragonetti, C.; Marinotto, D.; Roberto, D.; et al. Second-Order NLO Switches from Molecules to Polymer Films Based on Photochromic Cyclometalated Platinum(II) Complexes. J. Am. Chem. Soc. 2014, 136, 5367–5375. [Google Scholar] [CrossRef] [PubMed]
- Koo, C.-K.; Lam, B.; Leung, S.-K.; Lam, M.H.-W.; Wong, W.-Y. A “Molecular Pivot-Hinge” Based on the pH-Regulated Intramolecular Switching of Pt–Pt and π–π Interactions. J. Am. Chem. Soc. 2006, 128, 16434–16435. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; López, C.; Caubet, A.; Solans, X.; Font-Bardía, M. New Heterodimetallic Platinum(II) Complexes Potentially Useful as Molecular Switches. Eur. J. Inorg. Chem. 2008, 1599–1612. [Google Scholar]
- Ho, Y.-M.; Au, N.-P.B.; Wong, K.-L.; Chan, C.T.-L.; Kwok, W.-M.; Law, G.-L.; Tang, K.-K.; Wong, W.-Y.; Ma, C.-H.E.; Lam, M.H.-W. A lysosome-specific two-photon phosphorescent binuclear cyclometalated platinum(II) probe for in vivo imaging of live neurons. Chem. Commun. 2014, 50, 4161–4163. [Google Scholar] [CrossRef]
- Cocchi, M.; Kalinowski, J.; Fattori, V.; Williams, J.G.; Murphy, L. Control of magnetic-field effect on electro-luminescence in Alq3-based organic light emitting diodes. Appl. Phys. Lett. 2009, 94, 166104. [Google Scholar] [CrossRef]
- Zou, T.; Liu, J.; Lum, C.T.; Ma, C.; Chan, R.C.T.; Lok, C.-N.; Kwok, W.-M.; Che, C.-M. Luminescent Cyclometalated Platinum(II) Complex Forms Emissive Intercalating Adducts with Double-Stranded DNA and RNA: Differential Emissions and Anticancer Activities. Angew. Chem. Int. Ed. 2014, 53, 10119–10123. [Google Scholar] [CrossRef]
- Kalinowski, J.; Cocchi, M.; Virgili, D.; Fattori, V.; Williams, J.G. Mixing of Excimer and Exciplex Emission: A New Way to Improve White Light Emitting Organic Electrophosphorescent Diodes. Adv. Mater. 2007, 19, 4000–4005. [Google Scholar] [CrossRef]
- Yu, J.; Luo, J.; Chen, Q.; He, K.; Meng, F.; Deng, X.; Wang, Y.; Tan, H.; Jiang, H.; Zhu, W. Synthesis and optoelectronic properties of a novel dinuclear cyclometalated platinum(II) complex containing triphenylamine-substituted indolo[3,2-b]carbazole derivative in the single-emissive-layer WPLEDs. Tetrahedron 2014, 70, 1246–1251. [Google Scholar] [CrossRef]
- Escolà, A.; Crespo, M.; Quirante, J.; Cortés, R.; Jayaraman, A.; Badia, J.; Baldomà, L.; Calvet, T.; Font-Bardia, M.; Cascante, M. Exploring the Scope of [Pt2(4-FC6H4)4(μ-SEt2)2] as a Precursor for New Organometallic Platinum(II) and Platinum(IV) Antitumor Agents. Organometallics 2014, 33, 1740–1750. [Google Scholar] [CrossRef]
- Cutillas, N.; Martínez, A.; Yellol, G.S.; Rodríguez, V.; Zamora, A.; Pedreño, M.; Donaire, A.; Janiak, C.; Ruiz, J. Anticancer C,N-Cycloplatinated(II) Complexes Containing Fluorinated Phosphine Ligands: Synthesis, Structural Characterization, and Biological Activity. Inorg. Chem. 2013, 52, 13529–13535. [Google Scholar] [CrossRef] [PubMed]
- Ruíz, J.; Vicente, C.; de Haro, C.; Espinosa, A. Synthesis and Antiproliferative Activity of a C,N-Cycloplatinated(II) Complex with a Potentially Intercalative Anthraquinone Pendant. Inorg. Chem. 2011, 50, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Cortés, R.; Crespo, M.; Davin, L.; Martin, R.; Quirante, J.; Ruiz, D.; Messeguer, R.; Calvis, C.; Baldomà, L.; Badia, J.; et al. Cyclopalladated primary amines: A preliminary study of antiproliferative activity through apoptosis induction. Eur. J. Med. Chem. 2012, 54, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Quirante, J.; Ruíz, D.; González, A.; López, C.; Cascante, M.; Cortés, R.; Messeguer, R.; Calvis, C.; Baldomà, L.; Pascual, A.; et al. Platinum(II) and palladium(II) complexes with (N,N′) and (C,N,N′)− ligands derived from pyrazole as anticancer and antimalarial agents: Synthesis, characterization and in vitro activities. Inorg. Biochem. 2011, 105, 1720–1728. [Google Scholar] [CrossRef]
- Ruíz, J.; Rodriguez, V.; Cutillas, N.; Espinosa, A.; Hannon, M. Novel C,N-chelate platinum(II) antitumor complexes bearing a lipophilic ethisterone pendant. J. Inorg. Biochem. 2011, 105, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.; Bosque, R.; Crespo, M.; Granell, J.; López, C.; Cortés, R.; Gonzalez, A.; Quirante, J.; Calvis, C.; Messeguer, R.; et al. Pt(II) complexes with (N,N') or (C,N,E)− (E = N,S) ligands: Cytotoxic studies, effect on DNA tertiary structure and structure–activity relationships. Bioorg. Med. Chem. 2013, 21, 4210–4217. [Google Scholar] [CrossRef] [PubMed]
- Frik, M.; Jiménez, J.; Vasilevski, V.; Carreira, M.; de Almeida, A.; Benoit, F.; Gascón, E.; Sanaú, M.; Casini, A.; Contel, M. Luminescent iminophosphorane gold, palladium and platinum complexes as potential anticancer agents. Inorg. Chem. Front. 2014, 1, 231–241. [Google Scholar] [CrossRef]
- Togni, A.; Hayashi, T. Ferrocenes. Homogeneous Catalysis, Organic Synthesis. Materials Science; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Stepnicka, P. Ferrocene. Ligands, Materials and Biomolecules; Wiley: Weinheim, Germany, 2008. [Google Scholar]
- López, C.; González, A.; Bosque, R.; Basu, P.K.; Font-Bardia, M.; Calvet, M.T. Platinum(II) and palladium(II) complexes derived from 1-ferrocenylmethyl-3,5-diphenylpyrazole. Coordination, cyclometallation or transannulation? RSC Adv. 2012, 2, 1986–2002. [Google Scholar]
- Wu, Y.; Huo, S.; Gong, J.; Cui, X.; Ding, L.; Ding, K.; Du, C.; Liu, Y.; Song, M. Studies on the cyclometallation of ferrocenylimines. J. Organomet. Chem. 2001, 637–639, 27–48. [Google Scholar]
- Dai, L.-X.; Hou, X. Chiral Ferrocenes in Asymmetric Catalysis; Wiley: Weinheim, Germany, 2010. [Google Scholar]
- Weiss, M.; Frey, W.; Peters, R. Asymmetric Synthesis of Heterobimetallic Planar Chiral Ferrocene Pallada-/Platinacycles and Their Application to Enantioselective Aza-Claisen Rearrangements. Organometallics 2012, 31, 6365–6372. [Google Scholar] [CrossRef]
- López, C.; Caubet, A.; Pérez, S.; Solans, X.; Font-Bardia, M. Easy access to diastereomerically pure platinacycles. Chem. Commun. 2004, 540–541. [Google Scholar]
- López, C.; Caubet, A.; Pérez, S.; Solans, X.; Font-Bardía, M.; Molins, M. Chiral Platinum(II) Compounds Containing Ferrocenyl Schiff Bases Acting as (N), (N,O)−, [C(sp2,ferrocene),N]− or [C(sp2,ferrocene),N,O]2− Ligands. Eur. J. Inorg. Chem. 2006, 3974–3984. [Google Scholar]
- Talancón, D.; López, C.; Font-Bardía, M.; Calvet, T.; Quirante, J.; Calvis, C.; Messeguer, R.; Cortés, R.; Cascante, M.; Baldomà, L.; et al. Diastereomerically pure platinum(II) complexes as antitumoral agents. The influence of the mode of binding {(N), (N,O)− or (C,N)−} of (1S,2R)-[(η5-C5H5)Fe{(η5-C5H4)CH=N−CH(Me)−CH(OH)C6H5}] and the arrangement of the auxiliary ligands. J. Inorg. Biochem. 2013, 118, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cortés, R.; Tarrado-Castellarnau, M.; Talancón, D.; López, C.; Link, W.; Ruiz, D.; Centelles, J.; Quirante, J.; Cascante, M. A novel cyclometallated Pt(II)–ferrocene complex induces nuclear FOXO3a localization and apoptosis and synergizes with cisplatin to inhibit lung cancer cell proliferation. Metallomics 2014, 6, 622–633. [Google Scholar] [CrossRef]
- Anderson, C.; Crespo, M.; Morris, J.; Tanski, J.M. Reactivity of cyclometallated platinum complexes with chiral ligands. J. Organomet. Chem. 2006, 691, 5635–5641. [Google Scholar] [CrossRef]
- Crespo, M.; Solans, X.; Font-Bardia, M. Cyclometallated platinum compounds with chiral imines. Crystal structure of [PtMe(2-FC6H3CH=N-(S)-CHMePh)(PPh3)]. Polyhedron 1998, 17, 3927–3934. [Google Scholar] [CrossRef]
- Krylova, L.F.; Pavlushko, T.A. Diastereomers of trans isomers of Pt(II) complexes with alanime and phenylalanine. Zhurnal Neorg. Khimii 2003, 48, 1790–1800. [Google Scholar]
- Ryabov, A.D.; Panyashkina, I.M.; Polyakov, V.A.; Fischer, A. Access to Central Carbon Chirality through Cycloplatination of 1-(2-Pyridinylthio)propanone by cis-[PtCl2(S-SOMe(p-tolyl))]. The Crystal Structure of (SsSc)-[Pt{py{SCHC(O)Me}-2}Cl(SOMe(p-tolyl))]. Organometallics 2002, 21, 1633–1636. [Google Scholar] [CrossRef]
- Platero-Prats, A.E.; Pérez, S.; López, C.; Solans, X.; van Leeuwen, P.W.N.M.; van Strijdonck, G.P.F.; Freixa, Z. Palladium(II)-allyl complexes containing chiral N-donor ferrocenyl ligands. J. Organomet. Chem. 2007, 692, 4215–4226. [Google Scholar] [CrossRef]
- Zucca, A.; Cinellu, M.A.; Minghetti, G.; Stoccoro, S.; Manassero, M. Dinuclear, Tricyclometallated Platinum(II) Derivatives—Substitution Reactions and Reactivity of the Platinum–Carbon Bonds. Eur. J. Inorg. Chem. 2004, 4484–4490. [Google Scholar]
- Kui, S.C.F.; Hung, F.F.; Lai, S.-L.; Yuan, M.-Y.; Kwok, C.-C.; Low, K.-H.; Chui, S.S.-Y.; Che, C.-M. Luminescent Organoplatinum(II) Complexes with Functionalized Cyclometalated C^N^C Ligands: Structures, Photophysical Properties, and Material Applications. Chem.-Eur. J. 2012, 18, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Crosby, S.H.; Clarkson, G.J.; Rourke, J.P. Reactions of a Platinum(II) Agostic Complex: Decyclometalation, Dicyclometalation, and Solvent-Switchable Formation of a Rollover Complex. Organometallics 2011, 30, 3603–3609. [Google Scholar] [CrossRef]
- Crosby, S.H.; Thomas, H.R.; Clarkson, G.J.; Rourke, J.P. Concerted reductive coupling of an alkyl chloride at Pt(IV). Chem. Commun. 2012, 48, 5775–5777. [Google Scholar] [CrossRef]
- Bernhardt, P.V.; Calvet, T.; Crespo, M.; Font-Bardía, M.; Jansat, S.; Martinez, M. New Insights in the Formation of Five- versus Seven-Membered Platinacycles: A Kinetico-Mechanistic Study. Inorg. Chem. 2013, 52, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Calvet, T.; Crespo, M.; Font-Bardía, M.; Jansat, S.; Martinez, M. Kinetico-Mechanistic Studies on Intramolecular C−X Bond Activation (X = Br, Cl) of Amino-Imino Ligands on Pt(II) Compounds. Prevalence of a Concerted Mechanism in Nonpolar, Polar, and Ionic Liquid Media. Organometallics 2012, 31, 4367–4373. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Cryst. Sect. B: Struct. Sci. 2002, B58, 380–388. [Google Scholar] [CrossRef]
- Crespo, M.; Martin, R.; Calvet, T.; Font-Bardia, M.; Solans, X. Novel platinum(II) compounds with N-benzylidenebenzylamines: Synthesis, crystal structures and the effect of cis or trans geometry on cycloplatination. Polyhedron 2008, 27, 2603–2612. [Google Scholar] [CrossRef]
- López, R.; Bosque, R.; Solans, X.; Font-Bardía, M. Steric effects in ferrocenylketimines and Aldimines. X-ray Crystal Structure of [Fe(η5-C5H5){(η5-C5H4)C(C6H5)=N–R'}] (with R' = C6H4-4-CH3 or C6H4-2-CH3). New J. Chem. 1996, 20, 1285–1292. [Google Scholar]
- López, C.; Bosque, R.; Solans, X.; Font-Bardía, M. Synthesis and Characterization of Optically Active Cyclopalladated Compounds containing Ferrocenylimines. Tetrahedron: Asymm. 1996, 7, 2527–2530. [Google Scholar] [CrossRef]
- Talancón, D.; López, C.; Font-Bardía, M.; Calvet, T.; Roubeau, O. Diastereomerically Pure Heterodi- and Heterotetrametallic (Pd and Pt) Compounds: A Study of the Effect Induced by the Binding Mode of a Ferrocene-Containing Ligand on Their Electrochemical Properties. Eur. J. Inorg. Chem. 2014, 213–220. [Google Scholar]
- López, C.; Bosque, R.; Sainz, D.; Solans, X.; Font-Bardia, M. A New Reagent for Chiral Recognition Containing a Five-Membered Palladacycle with a σ(Pd-Csp2, ferrocene) Bond. Organometallics 1997, 16, 3261–3266. [Google Scholar] [CrossRef]
- Gmelin Handbuch der Anorganischen Chemie. Ferrocen 1. In Eisen Organische Verbindunge; Slawisch, A. (Ed.) Springer-Verlag: Heildelberg, Germany, 1974; Teil A.
- Pregosin, P.S. Platinum-195 nuclear magnetic resonance. Coord. Chem. Rev. 1982, 44, 247–291. [Google Scholar] [CrossRef]
- Riera, X.; López, C.; Caubet, A.; Moreno, V.; Solans, X.; Font-Bardia, M. Platinum(II) and Palladium(II) Compounds Containing Chiral Thioimines. Eur. J. Inorg. Chem. 2001, 2135–2141. [Google Scholar]
- Shen, S.-L.; Shao, J.-H.; Luo, J.-Z.; Liu, J.-T.; Miao, J.-Y.; Zhao, B.-X. Novel chiral ferrocenylpyrazolo[1,5-a][1,4]diazepin-4-onederivatives—Synthesis, characterization and inhibition against lung cancer cells. Eur. J. Med. Chem. 2013, 63, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-L.; Zhu, J.; Li, M.; Zhao, B.-X.; Miao, J.-Y. Synthesis of ferrocenyl pyrazole-containing chiral aminoethanol derivatives and their inhibition against A549 and H322 lung cancer cells. Eur. J. Med. Chem. 2012, 54, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Joksović, M.D.; Marković, V.; Juranić, Z.D.; Stanjković, T.; Jovanović, L.S.; Damijanović, I.S.; Szécsényi, K.M.; Todorović, N.; Trifunovićc, S.; Vukiević, R.D. Synthesis, characterization and antitumor activity of novel N-substituted α-amino acids containing ferrocenyl pyrazole-moiety. J. Organomet. Chem. 2009, 694, 3935–3942. [Google Scholar] [CrossRef]
- Brown, E.R.; Sandifer, J.R. Physical Methods in Chemistry. Electrochemical Methods; Rossiter, B.W., Hamilton, J.H., Eds.; Wiley: New York, NY, USA, 1986; Volume 4, Chapter 4. [Google Scholar]
- Bosque, R.; López, C.; Sales, J. Substituent effects on the electrochemical behavior of iron(II) in Schiff bases derived from ferrocene and their cyclopalladated compounds. Inorg. Chim. Acta 1996, 244, 141–145. [Google Scholar] [CrossRef]
- Duivenvoorden, W.C.M.; Liu, Y.; Schatte, G.; Kraatz, H.-B. Synthesis of redox-active ferrocene pyrazole conjugates and their cytotoxicity in human mammary adenocarcinoma MCF-7 cells. Inorg. Chim. Acta 2005, 358, 3183–3189. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle_Salvetti correlation energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C. Gaussian 03; Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 20 June 2014).
- Price, J.H.; Williamson, A.N.; Schramm, R.F.; Wayland, B.B. Palladium(II) and platinum(II) alkyl sulfoxide complexes. Examples of sulfur-bonded, mixed sulfur- and oxygen-bonded, and totally oxygen-bonded complexes. Inorg. Chem. 1972, 11, 1280–1284. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 4th ed.; Butterworth–Heinemann: Oxford, UK, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXS A Computer Program for Determination of Crystal Structure; Univerversity of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELX97 A Computer Program for Determination of Crystal Structure; Univerversity of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Ibers, J.A.; Hamilton, W.C. International Tables of X-ray Crystallography; Kynoch Press: Birmingham, UK, 1974; Volume IV, pp. 99–100, 149. [Google Scholar]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Cryst. 1983, A39, 876–881. [Google Scholar] [CrossRef]
- Givens, K.T.; Kitada, S.; Chen, A.K.; Rothschiller, J.; Lee, D.A. Proliferation of human ocular fibroblasts: An assessment of in vitro colorimetric assays. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1856–1862. [Google Scholar]
- This signal was partially masked by the resonance due to the residual solvent.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, C.; Bosque, R.; Pujol, M.; Simó, J.; Sevilla, E.; Font-Bardía, M.; Messeguer, R.; Calvis, C. Experimental and Theoretical Studies of the Factors Affecting the Cycloplatination of the Chiral Ferrocenylaldimine (SC)-[(η5-C5H5)Fe{(η5-C5H4)–C(H)=N–CH(Me)(C6H5)}]. Inorganics 2014, 2, 620-648. https://doi.org/10.3390/inorganics2040620
López C, Bosque R, Pujol M, Simó J, Sevilla E, Font-Bardía M, Messeguer R, Calvis C. Experimental and Theoretical Studies of the Factors Affecting the Cycloplatination of the Chiral Ferrocenylaldimine (SC)-[(η5-C5H5)Fe{(η5-C5H4)–C(H)=N–CH(Me)(C6H5)}]. Inorganics. 2014; 2(4):620-648. https://doi.org/10.3390/inorganics2040620
Chicago/Turabian StyleLópez, Concepción, Ramón Bosque, Marta Pujol, Jonathan Simó, Eila Sevilla, Mercè Font-Bardía, Ramon Messeguer, and Carme Calvis. 2014. "Experimental and Theoretical Studies of the Factors Affecting the Cycloplatination of the Chiral Ferrocenylaldimine (SC)-[(η5-C5H5)Fe{(η5-C5H4)–C(H)=N–CH(Me)(C6H5)}]" Inorganics 2, no. 4: 620-648. https://doi.org/10.3390/inorganics2040620
APA StyleLópez, C., Bosque, R., Pujol, M., Simó, J., Sevilla, E., Font-Bardía, M., Messeguer, R., & Calvis, C. (2014). Experimental and Theoretical Studies of the Factors Affecting the Cycloplatination of the Chiral Ferrocenylaldimine (SC)-[(η5-C5H5)Fe{(η5-C5H4)–C(H)=N–CH(Me)(C6H5)}]. Inorganics, 2(4), 620-648. https://doi.org/10.3390/inorganics2040620