Fibroblast Response to Cyclo- and Organic Phosphate Solutions: A Cytotoxicity Study
Abstract
1. Introduction
2. Results
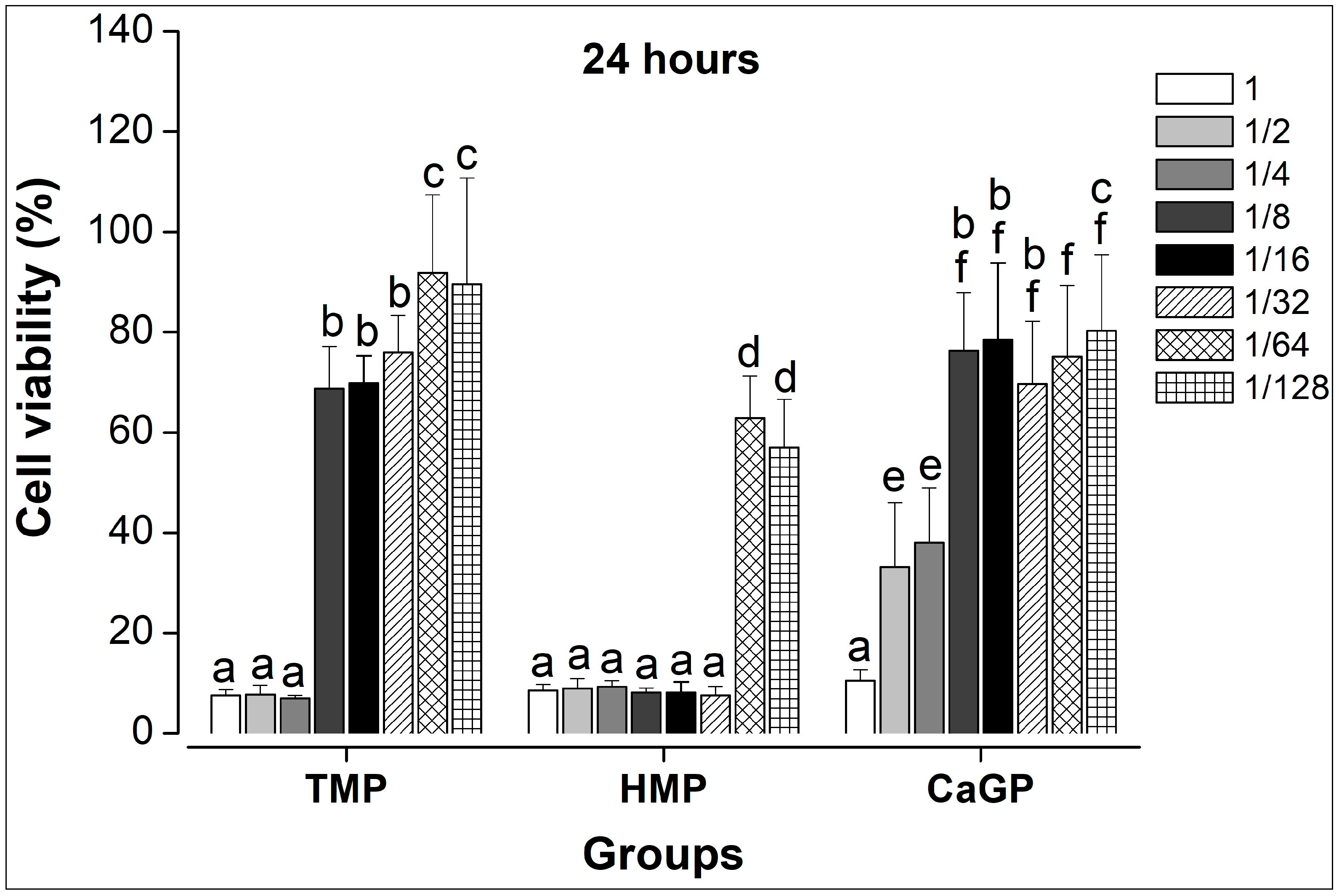
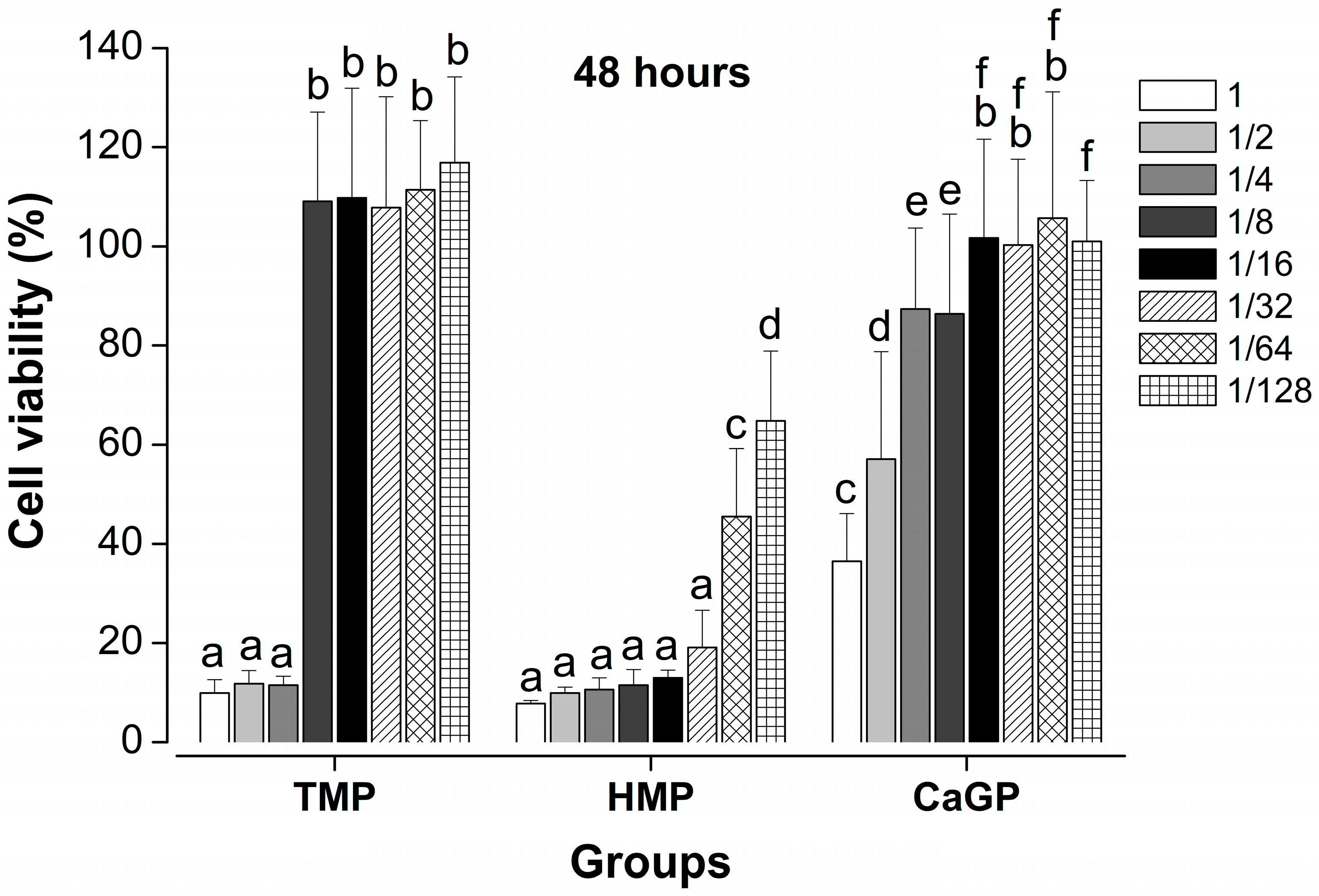
2.1. Evaluation of Cell Viability
2.2. Determination of the pH of the Dilutions
3. Discussion
4. Materials and Methods
4.1. Preparation of Solutions
4.2. Assessment of Cell Viability
4.3. Determination of pH of Dilutions
4.4. Statistical Analysis
5. Conclusions
- HMP dilutions resulted in significantly lower cell viability compared to the other compounds, regardless of the incubation period.
- TMP maintained higher cell viability from the 1/8 dilution onward, regardless of the incubation time.
- TMP and CaGP show lower cytotoxicity at higher dilutions than HMP, suggesting that they may be promising compounds for the development of new biomaterials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s global oral health status report 2022: Actions, discussion and implementation. Oral Dis. 2024, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.C.; Worthington, H.V.; Walsh, T.; Chong, L.Y. Fluoride gels for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2015, 15, CD002280. [Google Scholar] [CrossRef]
- Marinho, V.C.; Chong, L.Y.; Worthington, H.V.; Walsh, T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2016, 7, CD002284. [Google Scholar] [CrossRef]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Marinho, V.C.; Jeroncic, A. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019, 3, CD007868. [Google Scholar] [CrossRef]
- Tenuta, L.M.; Nóbrega, D.F.; Mei, M.L. Chapter 9.1: The Use of Fluorides in the Control of Coronal Caries. Monogr. Oral Sci. 2023, 31, 129–148. [Google Scholar]
- Mohammadipour, H.S.; Maghrebi, Z.F.; Ramezanian, N.; Ahrari, F.; Daluyi, R.A. The effects of sodium hexametaphosphate combined with other remineralizing agents on the staining and microhardness of early enamel caries: An in vitro modified pH-cycling model. Dent. Res. J. 2019, 16, 398–406. [Google Scholar]
- Greenfield, S.; Clift, M. General properties of the condensed phosphates. In Analytical Chemistry of the Condensed Phosphates; Belcher, R., Freiser, H., Eds.; Elsevier: San Diego, CA, USA, 1975; pp. 1–36. [Google Scholar]
- Kulaev, I.; Vagabov, V.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates, 2nd ed.; John Wiley & Sons: Newark, NJ, USA, 2005. [Google Scholar]
- Schröder, H.C.; Müller, W.E.G. Inorganic Polyphosphates: Biochemistry, Biology, Biotechnology; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Manna, C.M.; Nassar, M.Y.; Tofan, D.; Chakarawet, K.; Cummins, C.C. Facile synthesis of mononuclear early transition-metal complexes of κ3cyclo-tetrametaphosphate ([P4O12]4−) and cyclo-trimetaphosphate ([P3O9]3−). Dalton Trans. 2014, 28, 1509–1518. [Google Scholar] [CrossRef]
- Durmuş, E.; Kölüş, T.; Çoban, E.; Yalçınkaya, H.; Ülker, H.E.; Çelik, I. In vitro determination of the remineralizing potential and cytotoxicity of non-fluoride dental varnish containing bioactive glass, eggshell, and eggshell membrane. Eur. Arch. Paediatr. Dent. 2023, 24, 229–239. [Google Scholar] [CrossRef]
- Nagata, M.E.; Delbem, A.C.B.; Báez-Quintero, L.C.; Danelon, M.; Sampaio, C.; Monteiro, D.R.; Wiegand, A.; Pessan, J.P. Effect of fluoride gels with nano-sized sodium trimetaphosphate on the in vitro remineralization of caries lesions. J. Appl. Oral Sci. 2023, 31, e20230155. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.; Qi, Y.; Niu, L.N.; Elshafiy, S.; Mao, J.; Breschi, L.; Pashley, D.H.; Tay, F.R. The use of sodium trimetaphosphate as a biomimetic analog of matrix phosphoproteins for remineralization of artificial caries-like dentin. Dent. Mater. 2011, 27, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.G.; Danelon, M.; Pessan, J.P.; Figueiredo, L.R.; Camargo, E.R.; Delbem, A.C.B. Surface free energy of enamel treated with sodium hexametaphosphate, calcium and phosphate. Arch. Oral Biol. 2018, 90, 108–112. [Google Scholar] [CrossRef]
- Harnett, E.M.; Alderman, J.; Wood, T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids Surf. B Biointerfaces 2007, 55, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Hosida, T.Y.; Pessan, J.P.; Cavazana, T.P.; Sampaio, C.; de Morais, L.A.; Monteiro, D.R.; Delbem, A.C.B. Effects of Sodium Hexametaphosphate and Fluoride on the pH and Inorganic Components of Streptococcus mutans and Candida albicans Biofilm after Sucrose Exposure. Antibiotics 2022, 3, 1044. [Google Scholar] [CrossRef]
- Sampaio, C.; Delbem, A.C.B.; Hosida, T.Y.; de Morais, L.A.; Fernandes, A.V.P.; Souza Neto, F.N.; de Camargo, E.R.; Monteiro, D.R.; Pessan, J.P. Effects of nano-sized sodium hexametaphosphate on the viability, metabolism, matrix composition, and structure of dual-species biofilms of Streptococcus mutans and Candida albicans. Biofouling 2022, 38, 321–330. [Google Scholar] [CrossRef]
- Torsakul, P.; Rirattanapong, P.; Prapansilp, W. The Remineralization Effect of Calcium Glycerophosphate in Fluoride Mouth Rinse on Demineralized Primary Enamel: An in vitro Study. J. Int. Soc. Prev. Community Dent. 2023, 13, 410–415. [Google Scholar] [CrossRef]
- Cavazana, T.P.; Hosida, T.Y.; Sampaio, C.; de Morais, L.A.; Monteiro, D.R.; Pessan, J.P.; Delbem, A.C.B. Calcium glycerophosphate and fluoride affect the pH and inorganic composition of dual-species biofilms of Streptococcus mutans and Candida albicans. J. Dent. 2021, 115, 103844. [Google Scholar] [CrossRef]
- Lynch, R.M. Calcium glycerophosphate and caries: A review of the literature. Int. Dent. J. 2004, 54, 310–314. [Google Scholar] [CrossRef]
- Lanigan, R.S. Final report on the safety assessment of sodium metaphosphate, sodium trimetaphosphate, and sodium hexametaphosphate. Int. J. Toxicol. 2001, 20, 75–89. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & Scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Haggerty, A.E.; Oudega, M. Biomaterials for spinal cord repair. Neurosci. Bull. 2013, 29, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. The evolution of biomaterials research. Mater. Today 2013, 16, 408–409. [Google Scholar] [CrossRef]
- Gu, L.S.; Kim, J.; Kim, Y.K.; Liu, Y.; Dickens, S.H.; Pashley, D.H.; Ling, J.Q.; Tay, F.R. A chemical phosphorylation-inspired design for type I collagen biomimetic remineralization. Dent. Mater. 2010, 26, 1077–1089. [Google Scholar] [CrossRef]
- Luka, B.; Duerrschnabel, A.; Neumaier, S.; Schlueter, N.; Vach, K. Interaction between Hexametaphosphate, Other Active Ingredients of Toothpastes, and Erosion-Abrasion in Enamel in vitro. Caries Res. 2023, 57, 265–275. [Google Scholar] [CrossRef]
- Cavazana, T.P.; Hosida, T.Y.; Sampaio, C.; de Morais, L.A.; Monteiro, D.R.; Pessan, J.P.; Delbem, A.C.B. The Activity of Calcium Glycerophosphate and Fluoride against Cariogenic Biofilms of Streptococcus mutans and Candida albicans Formed In Vitro. Antibiotics 2023, 20, 422. [Google Scholar] [CrossRef]
- Rose, R. The role of calcium in oral streptococcal aggregation and the implications for biofilm formation and retention. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2000, 1475, 76–82. [Google Scholar] [CrossRef]
- Körstgens, V.; Flemming, H.-C.; Wingender, J.; Borchard, W. Influence of calcium ions on the mechanical properties of a model biofilm of mucoid Pseudomonas aeruginosa. Water Sci. Technol. 2001, 43, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Changgen, L.; Yongxin, L. Selective flotation of scheelite from calcium minerals with sodium oleate as a collector and phosphates as modifiers. II. The mechanism of the interaction between phosphate modifiers and minerals. Int. J. Miner. Process. 1983, 10, 219–235. [Google Scholar] [CrossRef]
- Choi, I.K.; Wen, W.W.; Smith, R.W. The effect of a long chain phosphate on the adsorption of collectors on kaolinite. Miner. Eng. 1993, 6, 1191–1197. [Google Scholar] [CrossRef]
- Kura, G.; Ohashi, S.; Kura, S. Complex formation of cyclic phosphate anions with bivalent cátions. J. Inorg. Nucl. Chem. 1974, 36, 1605–1609. [Google Scholar] [CrossRef]
- Larsen, M.J.; Fejerskov, O. Chemical and structural challenges in remineralization of dental enamel lesions. Scand. J. Dent. Res. 1989, 97, 285–296. [Google Scholar] [CrossRef]
- Enax, J.; Fandrich, P.; Schulze Zur Wiesche, E.; Epple, M. The Remineralization of Enamel from Saliva: A Chemical Perspective. Dent. J. 2024, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Hannig, M. Interaction between Saliva, Pellicle, and Dental Erosion. Monogr. Oral Sci. 2025, 33, 128–148. [Google Scholar] [PubMed]
- Omelon, S.; Georgiou, J.; Henneman, Z.J.; Wise, L.M.; Sukhu, B.; Hunt, T.; Wynnyckyj, C.; Holmyard, D.; Bielecki, R.; Grynpas, M.D. Control of vertebrate skeletal mineralization by polyphosphates. PLoS ONE 2009, 4, e5634. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.N.S.; Morais, L.A.; Gorup, L.F.; Ribeiro, L.S.; Martins, T.J.; Hosida, T.Y.; Francatto, P.; Barbosa, D.B.; Camargo, E.R.; Delbem, A.C.B. Facile Synthesis of PVP-Coated Silver Nanoparticles and Evaluation of Their Physicochemical, Antimicrobial and Toxic Activity. Colloids Interfaces 2023, 7, 66. [Google Scholar] [CrossRef]

| Groups | Dilutions | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 1/2 | 1/4 | 1/8 | 1/16 | 1/32 | 1/64 | 1/132 | |
| TMP | 7.68 (0.43) | 7.86 (0.38) | 7.95 (0,37) | 7.98 (0.37) | 8.01 (0.34) | 8.02 (0.35) | 8.04 (0.35) | 8.02 (0.36) |
| HMP | 6.38 (0.10) | 6.77 (0.15) | 7.10 (0.22) | 7.47 (0.27) | 7.76 (0.32) | 7.94 (0.35) | 7.98 (0.37) | 7.78 (0.49) |
| CaGP | 7.60 (0.20) | 7.57 (0.15) | 7.59 (0.16) | 7.68 (0.19) | 7.75 (0.24) | 7.81 (0.26) | 7.89 (0.27) | 7.79 (0.41) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Morais, L.A.; Delbem, A.C.B.; Sampaio, C.; de Aguiar, V.B.; Guisso, L.P.; Ferraresso, L.F.O.T.; Pessan, J.P.; Hosida, T.Y. Fibroblast Response to Cyclo- and Organic Phosphate Solutions: A Cytotoxicity Study. Inorganics 2025, 13, 309. https://doi.org/10.3390/inorganics13090309
de Morais LA, Delbem ACB, Sampaio C, de Aguiar VB, Guisso LP, Ferraresso LFOT, Pessan JP, Hosida TY. Fibroblast Response to Cyclo- and Organic Phosphate Solutions: A Cytotoxicity Study. Inorganics. 2025; 13(9):309. https://doi.org/10.3390/inorganics13090309
Chicago/Turabian Stylede Morais, Leonardo Antônio, Alberto Carlos Botazzo Delbem, Caio Sampaio, Vitória Bittencourt de Aguiar, Luigi Pedrini Guisso, Lucas Fernando Oliveira Tomáz Ferraresso, Juliano Pelim Pessan, and Thayse Yumi Hosida. 2025. "Fibroblast Response to Cyclo- and Organic Phosphate Solutions: A Cytotoxicity Study" Inorganics 13, no. 9: 309. https://doi.org/10.3390/inorganics13090309
APA Stylede Morais, L. A., Delbem, A. C. B., Sampaio, C., de Aguiar, V. B., Guisso, L. P., Ferraresso, L. F. O. T., Pessan, J. P., & Hosida, T. Y. (2025). Fibroblast Response to Cyclo- and Organic Phosphate Solutions: A Cytotoxicity Study. Inorganics, 13(9), 309. https://doi.org/10.3390/inorganics13090309







