Upconversion and Downconversion Luminescence of CaLaLiTeO6:Mn4+/Er3+ Phosphors for Dual-Mode Optical Thermometry and Anti-Counterfeiting Application
Abstract
1. Introduction
2. Results and Discussion
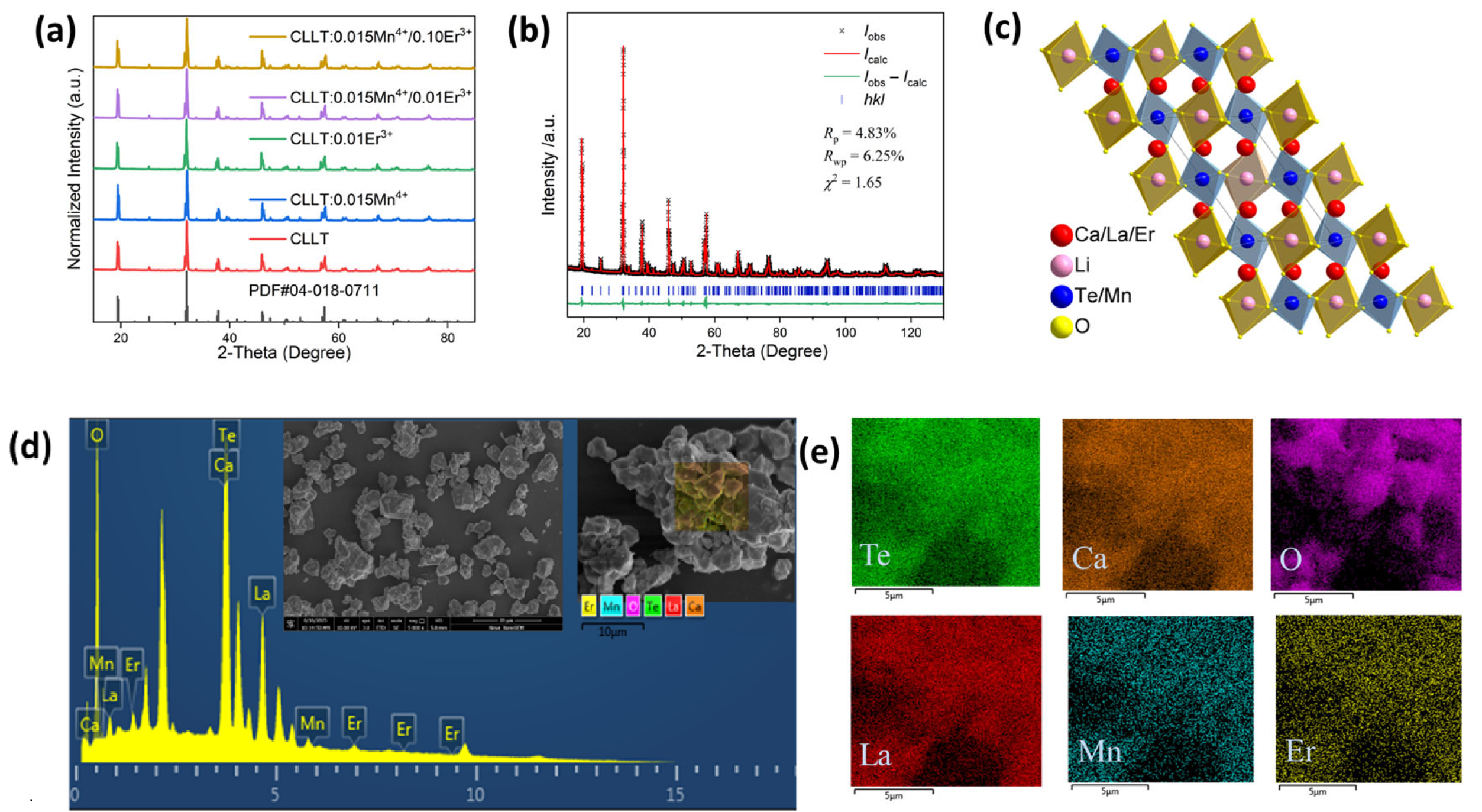
2.1. Crystalline Structure and Morphology
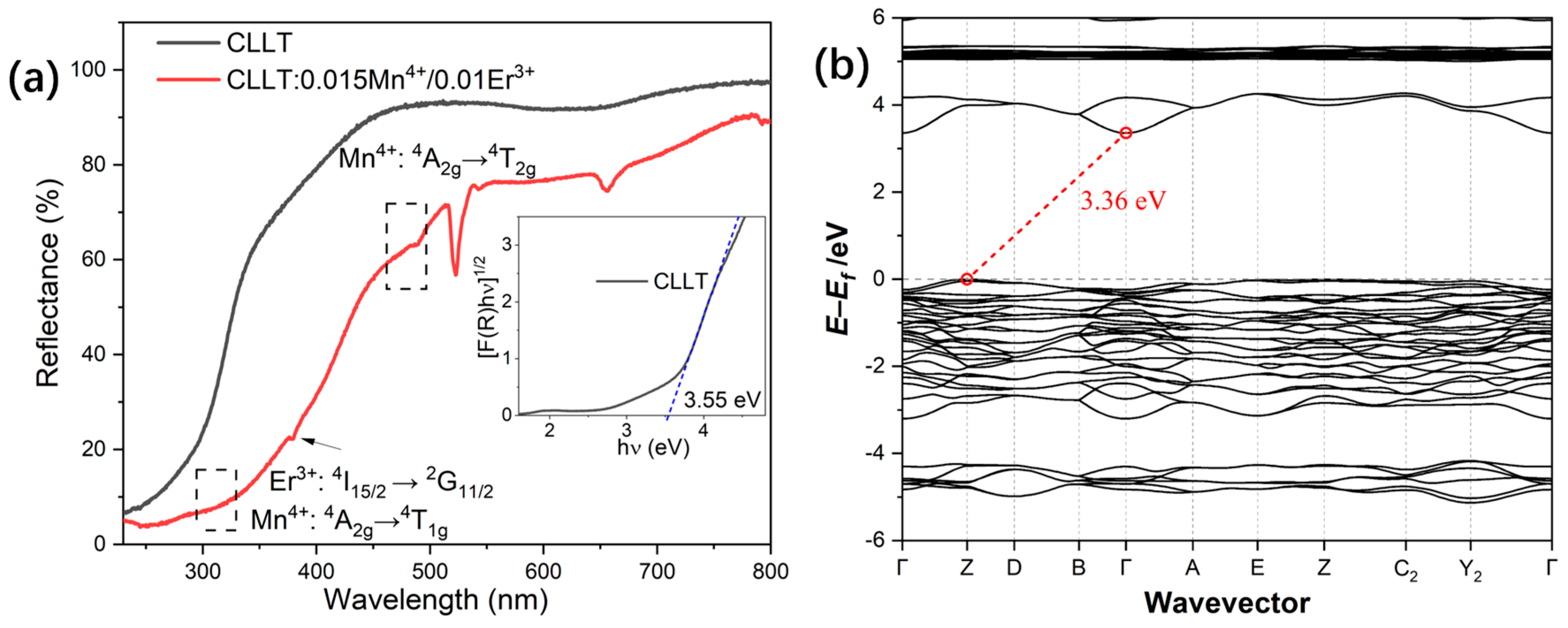
2.2. Diffuse Reflection Spectra and Electronic Band Structure
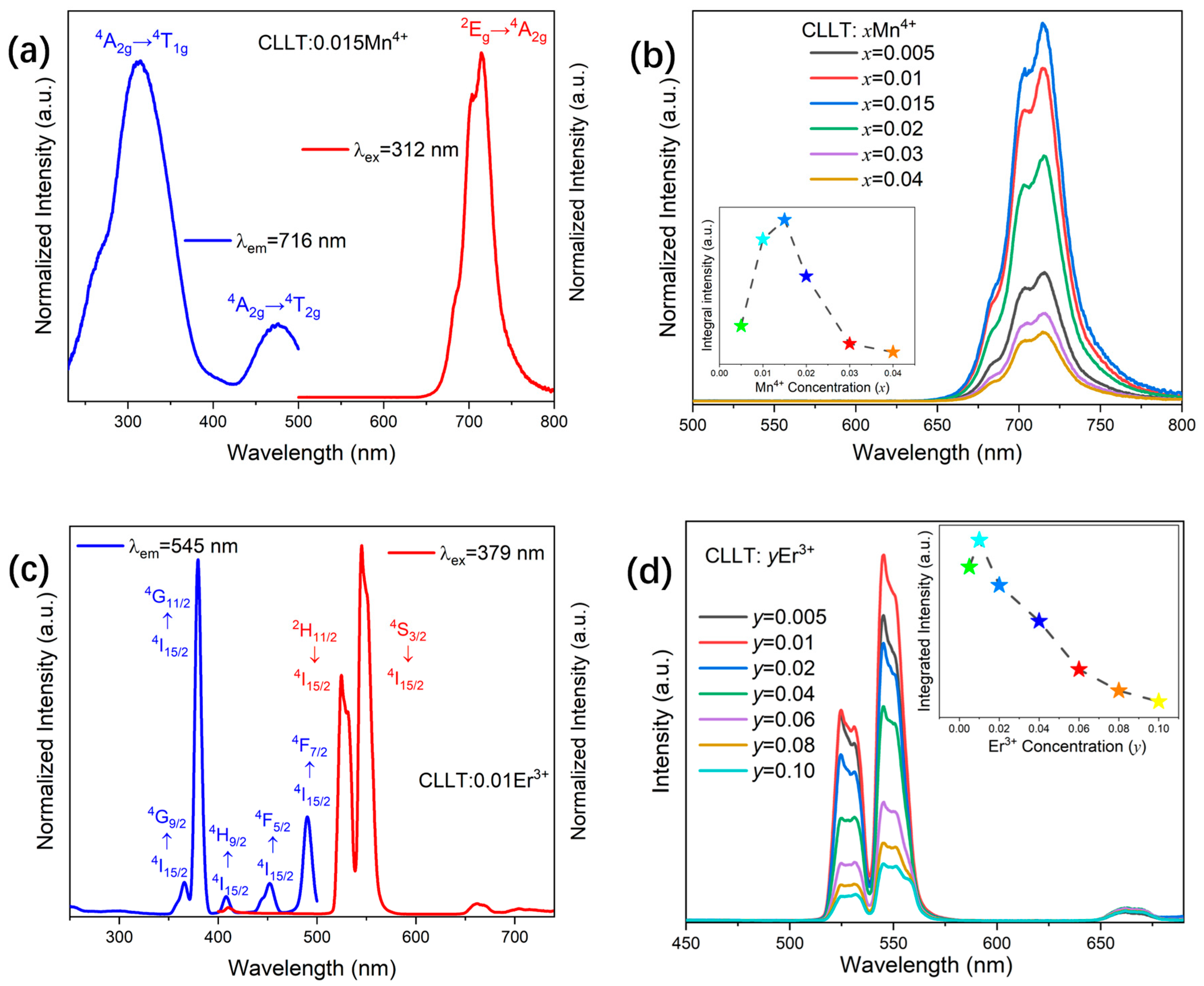
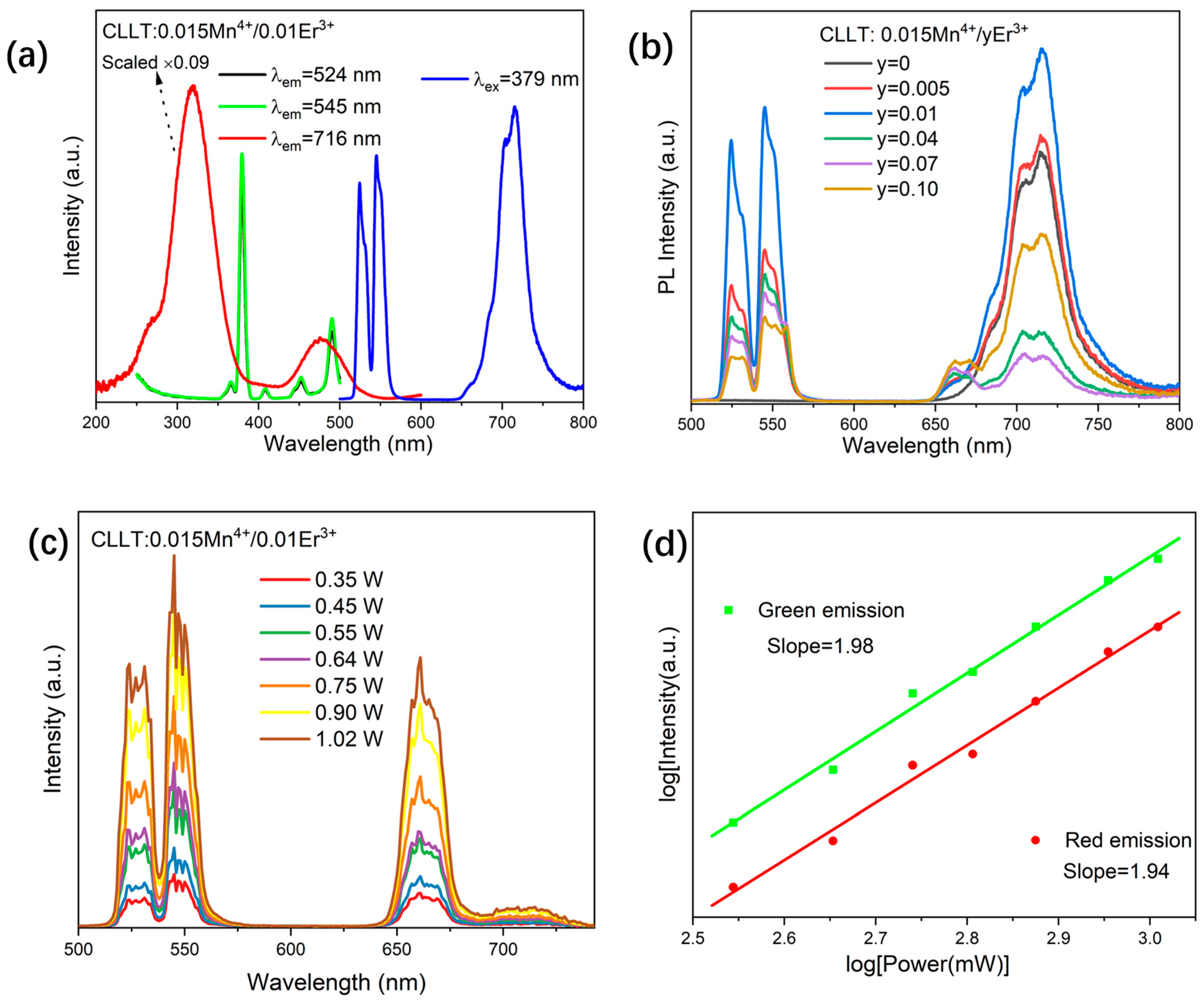
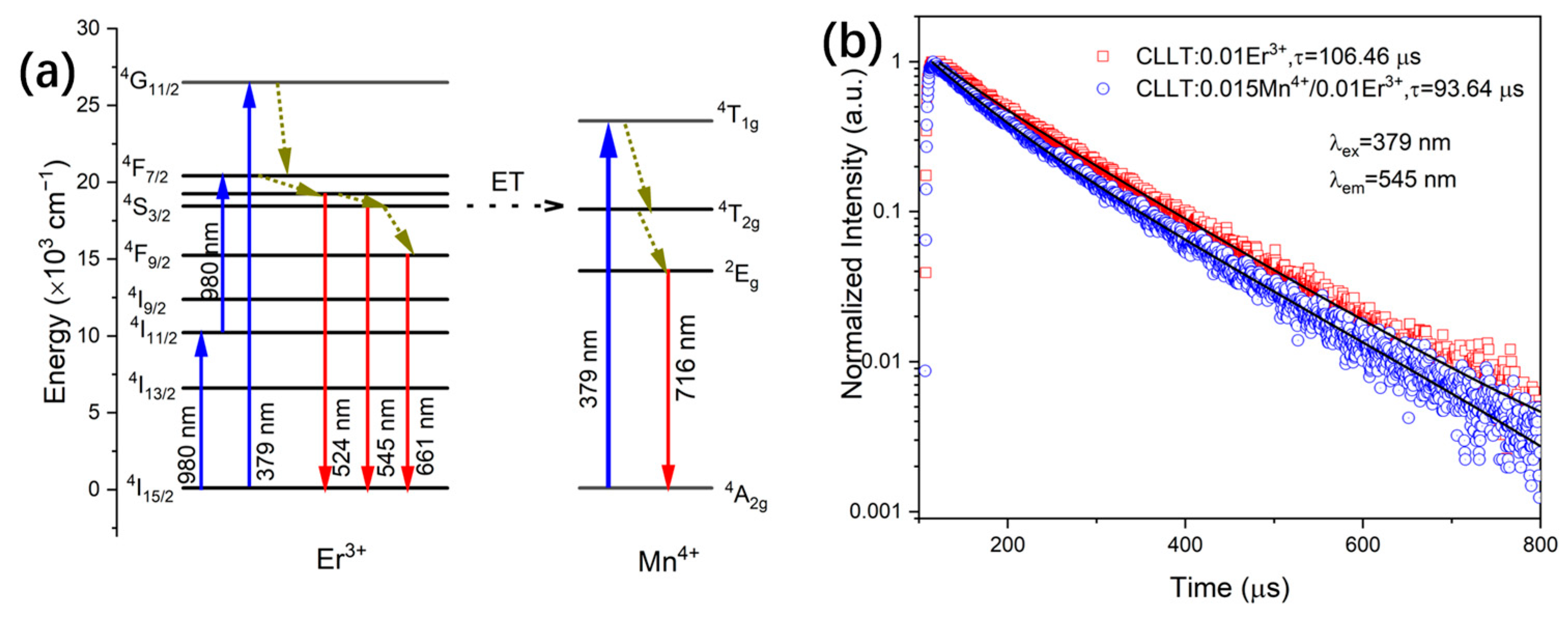
2.3. Photoluminescence Properties
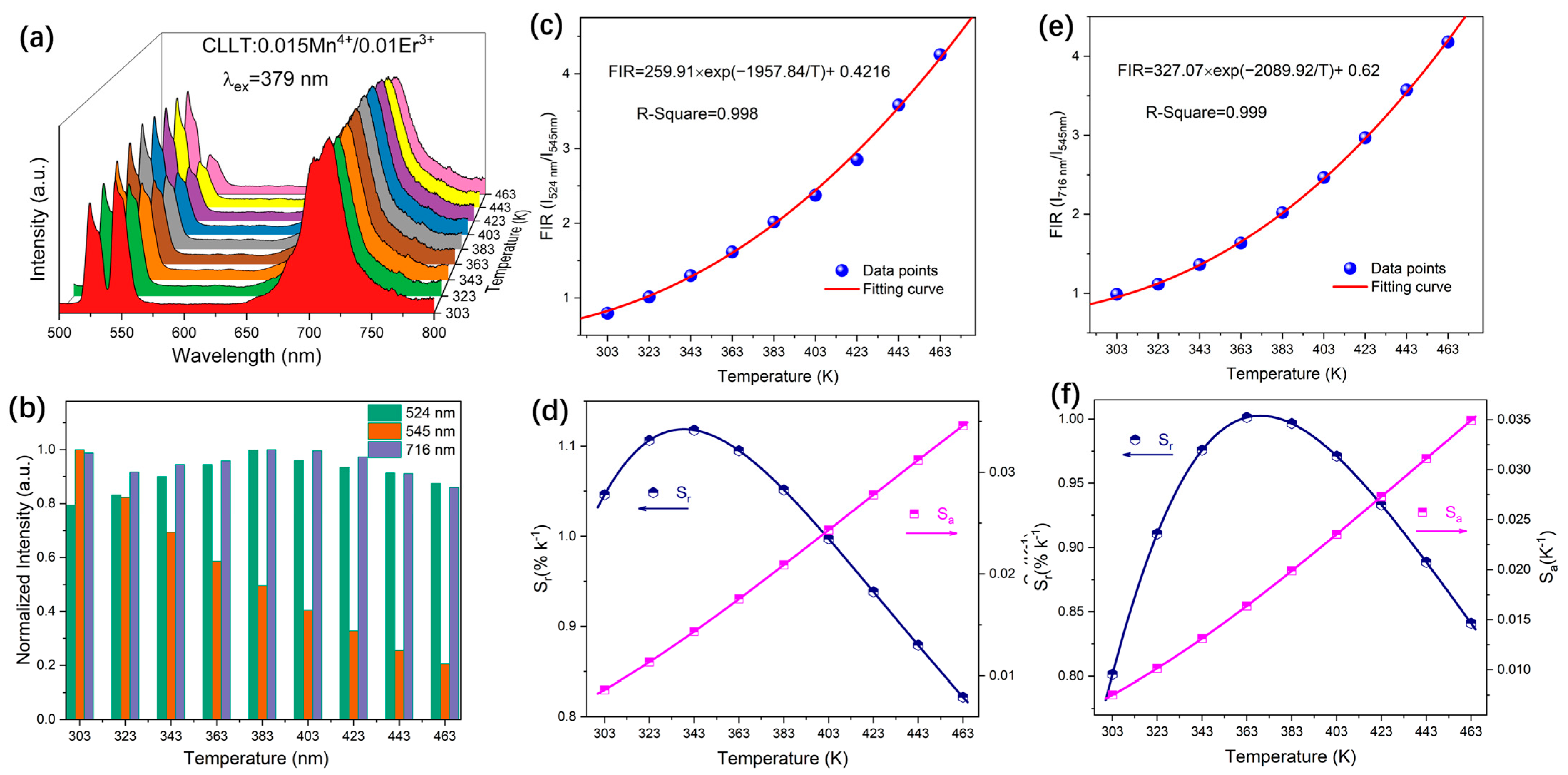
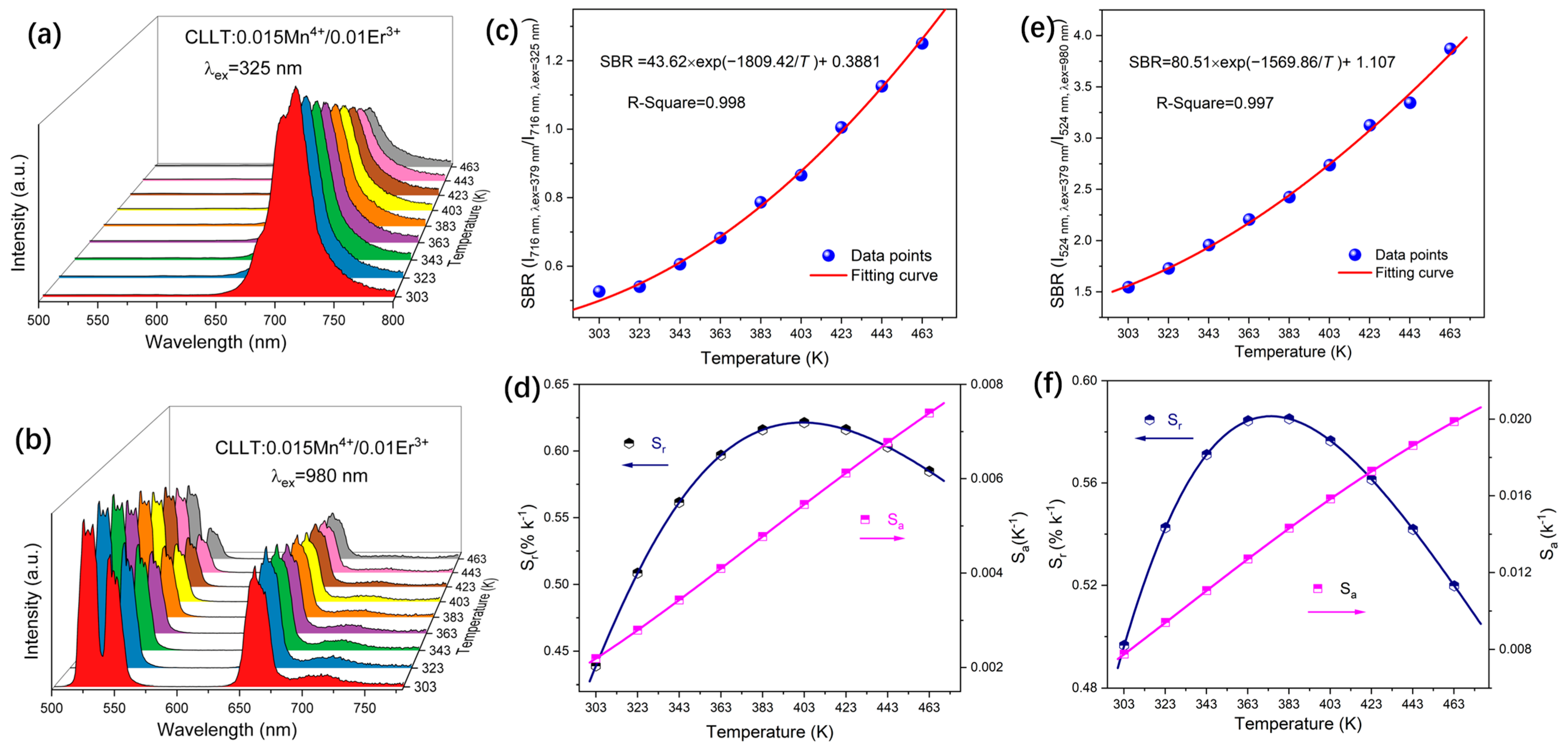
2.4. Temperature Sensing Properties
2.4.1. Dual Emission FIR Method
2.4.2. Dual Excitation SBR Method
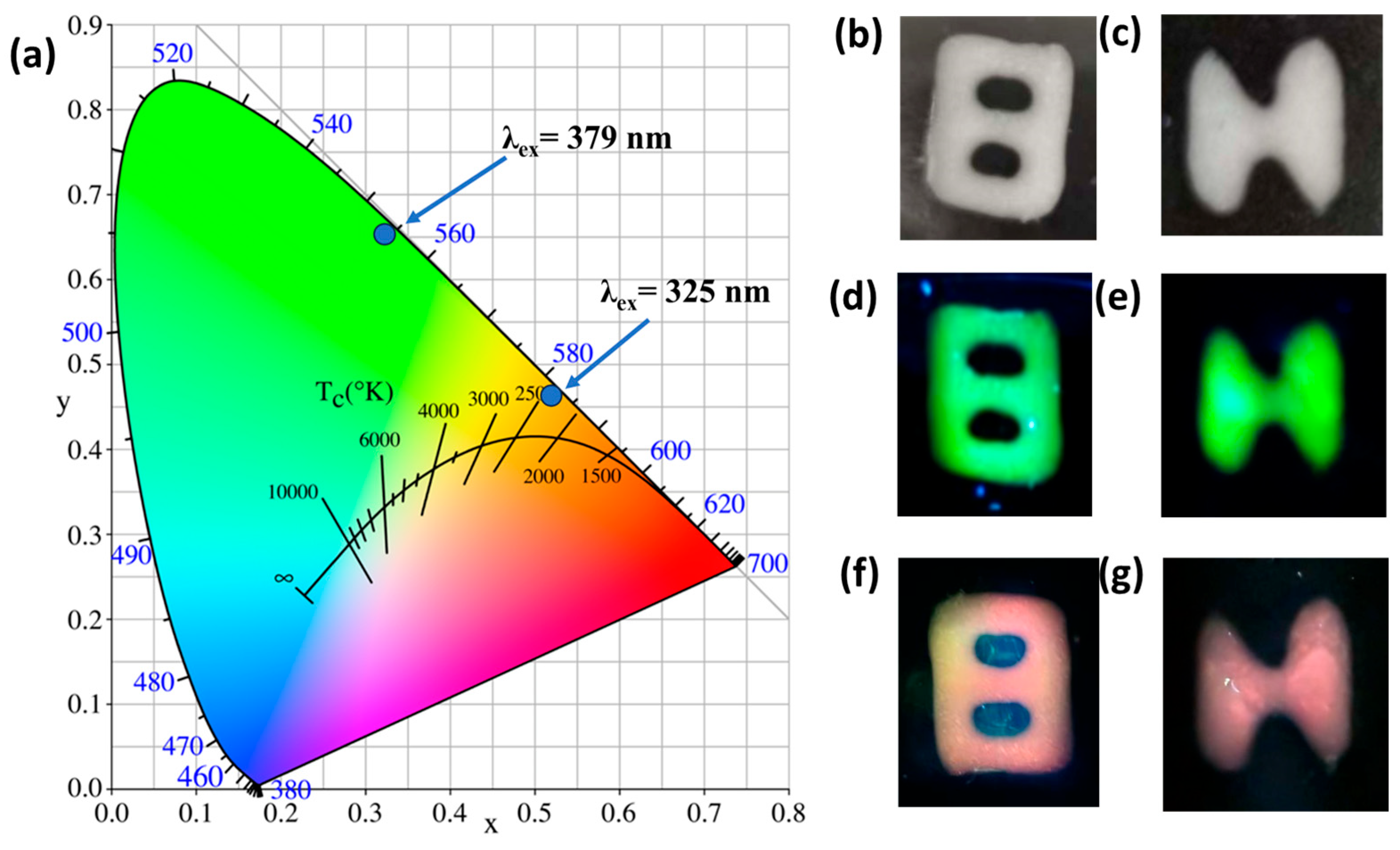
2.5. Application in Optical Anti-Counterfeiting
3. Experimental Section
3.1. Synthetic Method
3.2. Characterization
3.3. Density Functional Theory Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.-L.; Zhao, D.; Zhang, R.-J.; Yao, Q.-X.; Jia, L.; Zong, Q. A New Luminescent Material LaTiSbO6:Mn4+/Er3+ with High Optical Temperature Sensing Sensitivity Based on Fluorescence Intensity Ratio. J. Mater. Chem. C 2025, 13, 7741–7749. [Google Scholar] [CrossRef]
- Pu, L.; Li, P.; Zhao, J.; Wang, Y.; Guo, D.; Li, L.; Wang, Z.; Suo, H. Eu3+-Activated Single-Band Ratiometric Nanothermometry by Lattice Negative Thermal Expansion. Laser Photonics Rev. 2023, 17, 2200884. [Google Scholar] [CrossRef]
- Xing, J.; Luo, F.; Qin, Y.; Chen, X.; Liang, Y.; Gao, Z.; Shang, F.; Xu, H.; Chen, G. Multi-Ratio Optical Thermometry and Energy Storage Characteristics of Yb3+/Er3+/Tm3+ Doped BaNb2O6 Transparent Glass-Ceramics. J. Mater. Sci. Technol. 2023, 138, 138–148. [Google Scholar] [CrossRef]
- Tcelykh, L.; Latipov, E.; Lepnev, L.; Anosov, A.; Kozhevnikova, V.; Kuzmina, N.; Utochnikova, V.V. Highly Sensitive and Highly Emissive Luminescent Thermometers for Elevated Temperatures Based on Lanthanide-Doped Polymers. Inorganics 2023, 11, 189. [Google Scholar] [CrossRef]
- Periša, J.; Milićević, B.; Ćirić, A.; Zeković, I.; Đorđević, V.; Antić, Ž.; Dramićanin, M.D. Advancing Luminescence Thermometry: Employing Multiple Fluorescence Intensity Ratios of YAG:Er3+/Mn4+ Nanocrystals for Supersensitive Temperature Sensing. Ceram. Int. 2025, 51, 23224–23232. [Google Scholar] [CrossRef]
- Liu, J.; Xin, Y.; Qu, S.; Yang, C.; Song, J.; Li, W.; Guo, N. Design of Dual-Mode Optical Thermometry Based on Thermally Activated Efficient Energy Transfer in LaNbO4/Ln3+ (Eu3+/Sm3+/Pr3+) Phosphors. ACS Appl. Mater. Interfaces 2024, 16, 57421–57427. [Google Scholar] [CrossRef] [PubMed]
- Anu; Seema; Kumari, S.; Rohilla, P.; Barik, S.; Ruchi, K.; Kumar, K.; Rao, A.S.; Prasad, A. Comprehensive Luminescence Analysis of YTaO4:Er3+/Yb3+/Tm3+ Co-Doped Phosphor for Optical Thermometry and Color Tunable Device Applications. J. Alloys Compd. 2025, 1023, 180108. [Google Scholar] [CrossRef]
- Fu, M.; Fan, Z.; Qiao, P.; Hu, L.; Liu, S.; Deng, D.; Xu, S.; Ma, H. Dual-Mode Optical Thermometers via the Thermochromic Bi3+, Eu3+ Co-Doped La2LiSbO6 Phosphors for Real-Time Chip Temperature Monitoring. Ceram. Int. 2024, 50, 26454–26463. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Yang, N.; Ye, Z.; Cao, R.; Shi, J.; Wang, J. A Four-Mode Optical Thermometer Designed in Double Perovskite Ca2GdNbO6:Bi3+,Eu3+. J. Alloys Compd. 2024, 1002, 175220. [Google Scholar] [CrossRef]
- Yan, B.; Wei, Y.; Wang, W.; Fu, M.; Li, G. Red-Tunable LuAG Garnet Phosphors via Eu3+ → Mn4+ Energy Transfer for Optical Thermometry Sensor Application. Inorg. Chem. Front. 2021, 8, 746–757. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Y.; Zhang, Y.; Hu, J. Achieving High Thermal Sensitivity from Ratiometric CaGdAlO4:Mn4+,Tb3+ Thermometers. Dalton Trans. 2021, 50, 13447–13458. [Google Scholar] [CrossRef]
- Trejgis, K.; Bednarkiewicz, A.; Marciniak, L. Engineering Excited State Absorption Based Nanothermometry for Temperature Sensing and Imaging. Nanoscale 2020, 12, 4667–4675. [Google Scholar] [CrossRef]
- Tian, X.; Yang, L.; Wen, J.; Ji, C.; Huang, Z.; Zhu, L.; Luo, F.; Zhong, H.; Peng, H.; Lin, H.-T. Energy Transfer and a Novel SBR Thermometry of SrY2O4:Sm3+/Eu3+ Phosphor Based on Redshift of Charge Transfer Band Edge. Ceram. Int. 2024, 50, 36849–36863. [Google Scholar] [CrossRef]
- Pankratov, V.; Popov, A.I.; Chernov, S.A.; Zharkouskaya, A.; Feldmann, C. Mechanism for Energy Transfer Processes between Ce3+ and Tb3+ in LaPO4:Ce,Tb Nanocrystals by Time-Resolved Luminescence Spectroscopy. Phys. Status Solidi B 2010, 247, 2252–2257. [Google Scholar] [CrossRef]
- Piotrowski, W.; Dalipi, L.; Szukiewicz, R.; Fond, B.; Dramicanin, M.; Marciniak, L. The Role of Cr3+ and Cr4+ in Emission Brightness Enhancement and Sensitivity Improvement of NIR-Emitting Nd3+/Er3+ Ratiometric Luminescent Thermometers. J. Mater. Chem. C 2021, 9, 12671–12680. [Google Scholar] [CrossRef]
- Su, S.; Hu, C.; Ding, S.; Sun, Y.; Sun, L.; Zou, Y.; Liu, R.; Lei, Z.; Teng, B.; Zhong, D. Achieving Broadband NIR Emission in Fe3+-activated ALaBB′O6 (a = Ba, Sr, ca; B–B′ = Li–Te, Mg–Sb) Phosphors via Multi-site Ionic Co-substitutions. Adv. Opt. Mater. 2024, 12, 2302383. [Google Scholar] [CrossRef]
- Su, S.; Sun, Y.; Liu, G.; Gong, K.; Wang, M.; Ding, S.; Dong, H.; Wang, W.; Teng, B.; Hu, C. CaLaLiTeO6:Mn4+, Tm3+ Phosphors with Multiband near-Infrared Emission towards Multifunctional Applications. J. Lumin. 2024, 267, 120394. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, T.; Li, H.; Xiang, Y.; Song, R.; Zhang, H.; Wang, B. Improving the up/down-Conversion Luminescence via Cationic Substitution and Dual-Functional Temperature Sensing Properties of Er3+ Doped Double Perovskites. Chem. Eng. J. 2023, 471, 144550. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, T.; Li, H.; Xiang, Y.; Song, R.; Zhang, H. Cationic Composition Engineering in Double Perovskite XLaLiTeO6:Eu3+ (X = Ba, Sr, ca, and Mg) toward Efficient and Thermally Stable Red Luminescence for Domestic White-LEDs. J. Mater. Chem. C 2023, 11, 11017–11026. [Google Scholar] [CrossRef]
- Dong, H.; Lei, Z.; Su, S.; Yang, W.; Zhang, X.; Teng, W.; Zu, G.; Teng, B.; Zhong, D. Achieving High-Sensitivity Dual-Mode Optical Thermometry via Phonon-Assisted Cross-Relaxation in a Double-Perovskite Structured up-Conversion Phosphor. Inorg. Chem. Front. 2024, 11, 2784–2797. [Google Scholar] [CrossRef]
- Su, S.; Ma, J.; Hu, C.; Zhao, J.; Liu, R.; Dong, H.; Sun, L.; Zou, Y.; Lei, Z.; Teng, B.; et al. An Efficient Double-Perovskite CaLaLiTeO6:Mn4+ Far-Red Phosphor towards Indoor Plant Lighting Application. J. Alloys Compd. 2023, 946, 169436. [Google Scholar] [CrossRef]
- Li, H.; Haider, A.A.; Xie, Z.; Liu, C.; Zhang, H.; Jiang, H.; Li, J.; Zhu, J. Synergetic Contributions of High Quenching Concentration and Tuned Square Antiprism Geometry Boosting Far-Red Emission of Eu3+ with Near-Unit Efficiency. Adv. Sci. 2025, 12, e2415989. [Google Scholar] [CrossRef] [PubMed]
- Brik, M.G.; Srivastava, A.M.; Popov, A.I. A Few Common Misconceptions in the Interpretation of Experimental Spectroscopic Data. Opt. Mater. 2022, 127, 112276. [Google Scholar] [CrossRef]
- Borlido, P.; Schmidt, J.; Huran, A.W.; Tran, F.; Marques, M.A.L.; Botti, S. Exchange-Correlation Functionals for Band Gaps of Solids: Benchmark, Reparametrization and Machine Learning. Npj Comput. Mater. 2020, 6, 96. [Google Scholar] [CrossRef]
- Liao, J.; Wang, M.; Han, Z.; Huang, J.; Gong, G.; Fu, J.; Wen, H. Luminescence Properties and Energy Transfer Mechanism of La2ZnTiO6:Mn4+/Er3+ Far-Red/Green Dual-Emitting Phosphors for Plant Lighting. J. Solid State Chem. 2021, 303, 122470. [Google Scholar] [CrossRef]
- Li, L.; Tong, J.; Sun, F.; Hong, F.; Yu, D.; Chen, Z.; Hao, H.; Lin, H. Controllable Morphology, Excellent Stability and Non-Equivalent Doping-Induced Optical Properties of Mn4+-Activated K2NaScF6 Red Phosphor for High-Quality Warm WLED. J. Lumin. 2024, 275, 120808. [Google Scholar] [CrossRef]
- Cao, R.; Hu, X.; Nie, J.; Lan, B.; Wang, J.; Chen, T.; Chen, Y.; Wang, J. LaBaMg(Ti0.5W0.5)O6:Mn4+ for Plant Growth Lighting: Luminescence Properties and Synthesis. J. Mol. Struct. 2024, 1318, 139405. [Google Scholar] [CrossRef]
- Xia, Z.-R.; Li, R.-Q.; Zheng, Q.-H.; Liu, F.-F.; Zhou, W.-W.; Yuan, K.-X.; Tong, Y.; Zhao, W. Optical Thermometry and Fingerprint Detection in SrLa1−x Eux LiTeO6 Phosphors with Abnormal Thermal Quenching Effect. AIP Adv. 2025, 15, 035021. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, N.; Shao, B.; Li, J.; Ouyang, R.; Miao, Y. Photoluminescence and Optical Temperature Measurement of Mn4+/Er3+ Co-Activated Double Perovskite Phosphor through Site-Advantageous Occupation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 259, 119797. [Google Scholar] [CrossRef]
- Song, R.; Yan, S.; Duan, S.; Yang, X.; Balfour, E.A.; Fu, H. Enhancing Upconversion Luminescence via Intermediate State in Double Perovskite Phosphor: Three-Mode Optical Thermometry with Python-Assisted Validation. Inorg. Chem. Front. 2025, 12, 4691–4702. [Google Scholar] [CrossRef]
- Qi, C.-Y.; Zhao, D.; Zhang, R.-J.; Jia, L.; Zong, Q. Cationic Substitution for Enhancing the Temperature Sensing Sensitivity of Mn4+/Er3+ Co-Doped Double Perovskite. Ceram. Int. 2025, 51, 10316–10324. [Google Scholar] [CrossRef]
- Wang, M.; Ding, S.; Zhang, C.; Ren, H.; Zou, Y.; Tang, X.; Zhang, Q. Pure-Green Upconversion Emission and High-Sensitivity Optical Thermometry of Er3+-Doped Stoichiometric NaYb(MoO4)2. Ceram. Int. 2023, 49, 37661–37669. [Google Scholar] [CrossRef]
- Liu, H.; Jian, X.; Liu, M.; Wang, K.; Bai, G.; Zhang, Y. Investigation on the Upconversion Luminescence and Ratiometric Thermal Sensing of SrWO4:Yb3+/RE3+ (RE = Ho/Er) Phosphors. RSC Adv. 2021, 11, 36689–36697. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, D. Color Tunable Upconversion Luminescence and Optical Thermometry Properties of Mixed Gd2O3:Yb3+/Ho3+/Er3+ Nanoparticles Prepared via Laser Ablation in Liquid. J. Mater. Sci. Mater. Electron. 2020, 31, 9321–9327. [Google Scholar] [CrossRef]
- Liu, L.; Liu, H.; Zou, W.; Ding, S. Er3+ Doping YbTaO4 Phosphors: Upconversion Luminescence and Temperature Sensing Characteristics. Ceram. Int. 2023, 49, 28500–28505. [Google Scholar] [CrossRef]
- Kshetri, Y.K.; Kamiyama, T.; Torii, S.; Jeong, S.H.; Kim, T.-H.; Choi, H.; Zhou, J.; Feng, Y.P.; Lee, S.W. Electronic Structure, Thermodynamic Stability and High-Temperature Sensing Properties of Er-α-SiAlON Ceramics. Sci. Rep. 2020, 10, 4952. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Lei, R.; Wang, J.; Huang, F.; Zhao, S.; Li, B.; Xu, S. Multi-Functionalities of Photoluminescence, X-Ray Excited Luminescence and Optical Temperature Sensing in Er3+ and Er3+/Yb3+ Doped Gd2Zr2O7 Phosphors. J. Alloys Compd. 2022, 905, 164226. [Google Scholar] [CrossRef]
- Xiao, Q.; Yin, X.; Wu, X.; Fan, Y.; Lv, L.; Dong, X.; Zhou, N.; Liu, K.; Luo, X. High-Sensitive Thermometry and Tunable Dual-Color Luminescence Based on Effective Energy Transfer Pathways of Er, Tm System. J. Lumin. 2022, 250, 119057. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Lv, X.; Suo, H.; Cai, C.; Lv, P.; Ma, M.; Shi, X.; Yang, Y.; Marciniak, L.; et al. Persistent Luminescence Ratiometric Thermometry. Chem. Eng. J. 2022, 438, 135573. [Google Scholar] [CrossRef]
- Zhou, R.; Zhao, X.; Pun, E.Y.B.; Lin, H. Temperature Sensitivity Based on Er3+ Fluorescence Fluctuation in Gd2Ti2O7:Er3+-Yb3+ Porous Nanofibers. J. Alloys Compd. 2020, 838, 155554. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, N.; Zhu, M.; Li, J.; Miao, Y.; Shao, B. Photoluminescence and Ratiometric Optical Thermometry in Mn4+/Eu3+ Dual-Doped Phosphor via Site-Favorable Occupation. J. Lumin. 2020, 224, 117311. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, L.; Qiu, X.; Yang, D.; Runowski, M.; Lis, S.; Du, P.; Luo, L. Er3+, Yb3+ Co-Doped Sr3(PO4)2 Phosphors: A Ratiometric Luminescence Thermometer Based on Stark Levels with Tunable Sensitivity. J. Lumin. 2020, 227, 117517. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
| Phosphor | Temperature Range (K) | Max Sa (K−1) | Max Sr (%K−1) | Reference |
|---|---|---|---|---|
| NaYb(MoO4)2: Er3+ | 300–570 | 0.013 | 0.69 | [32] |
| SrWO4: Yb3+/Er3+ | 303–573 | 0.013 | 1.03 | [33] |
| Gd2O3: Yb3+/Ho3+/Er3+ | 303–483 | -- | 0.98 | [34] |
| YbTaO4: Er3+ | 300–570 | 0.0038 | 1.00 | [35] |
| α-SiAlON: Er3+ | 298–1273 | 0.0034 | 0.59 | [36] |
| Gd2Zr2O7: Yb3+/Er3+ | 298–573 | -- | 1.10 | [37] |
| La2Mo2O9: Er3+/Tm3+ | 303–478 | 0.83 | [38] | |
| MgLaLiTeO6: Er3+ | 298–573 | 0.0037 | 1.03 | [18] |
| NaYF4: Er3+ | 303–423 | -- | 1.06 | [39] |
| NaLaMgWO6: Mn4+/Er3+ | 303–523 | 0.0017 | 1.31 | [29] |
| Lu3Al5O12: Eu3+/Mn4+ | 303–358 | 0.07 | 0.7 | [10] |
| Gd2Ti2O7: Er3+/Yb3+ | 303–633 | 0.0038 | 1.12 | [40] |
| NaLaMgWO6: Mn4+/Eu3+ | 303–523 | 0.0030 | 0.86 | [41] |
| Sr3(PO4)2: Er3+/Yb3+ | 303–623 | 0.0070 | 0.88 | [42] |
| CaLaLiTeO6: Mn4+/Er3+ | 303–463 | 0.0348 | 1.12 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.-R.; Li, R.-Q.; Liu, F.-F.; Tong, Y.; Zheng, Q.-H.; Ping, Z.-Y.; Zhao, W.; Zhou, W.-W.; Song, M.-J. Upconversion and Downconversion Luminescence of CaLaLiTeO6:Mn4+/Er3+ Phosphors for Dual-Mode Optical Thermometry and Anti-Counterfeiting Application. Inorganics 2025, 13, 308. https://doi.org/10.3390/inorganics13090308
Xia Z-R, Li R-Q, Liu F-F, Tong Y, Zheng Q-H, Ping Z-Y, Zhao W, Zhou W-W, Song M-J. Upconversion and Downconversion Luminescence of CaLaLiTeO6:Mn4+/Er3+ Phosphors for Dual-Mode Optical Thermometry and Anti-Counterfeiting Application. Inorganics. 2025; 13(9):308. https://doi.org/10.3390/inorganics13090308
Chicago/Turabian StyleXia, Zheng-Rong, Rong-Qing Li, Fang-Fang Liu, Yue Tong, Qing-Hua Zheng, Zhao-Yan Ping, Wang Zhao, Wei-Wei Zhou, and Ming-Jun Song. 2025. "Upconversion and Downconversion Luminescence of CaLaLiTeO6:Mn4+/Er3+ Phosphors for Dual-Mode Optical Thermometry and Anti-Counterfeiting Application" Inorganics 13, no. 9: 308. https://doi.org/10.3390/inorganics13090308
APA StyleXia, Z.-R., Li, R.-Q., Liu, F.-F., Tong, Y., Zheng, Q.-H., Ping, Z.-Y., Zhao, W., Zhou, W.-W., & Song, M.-J. (2025). Upconversion and Downconversion Luminescence of CaLaLiTeO6:Mn4+/Er3+ Phosphors for Dual-Mode Optical Thermometry and Anti-Counterfeiting Application. Inorganics, 13(9), 308. https://doi.org/10.3390/inorganics13090308





