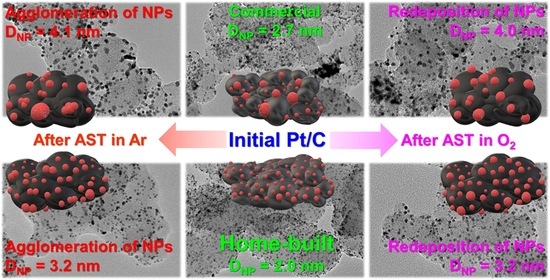
Effect of AST Atmosphere on Pt/C Electrocatalyst Degradation
Abstract
1. Introduction
2. Results and Discussion
2.1. Initial Morphology, Structure, and Electrochemical Parameters of Pt/C Catalysts
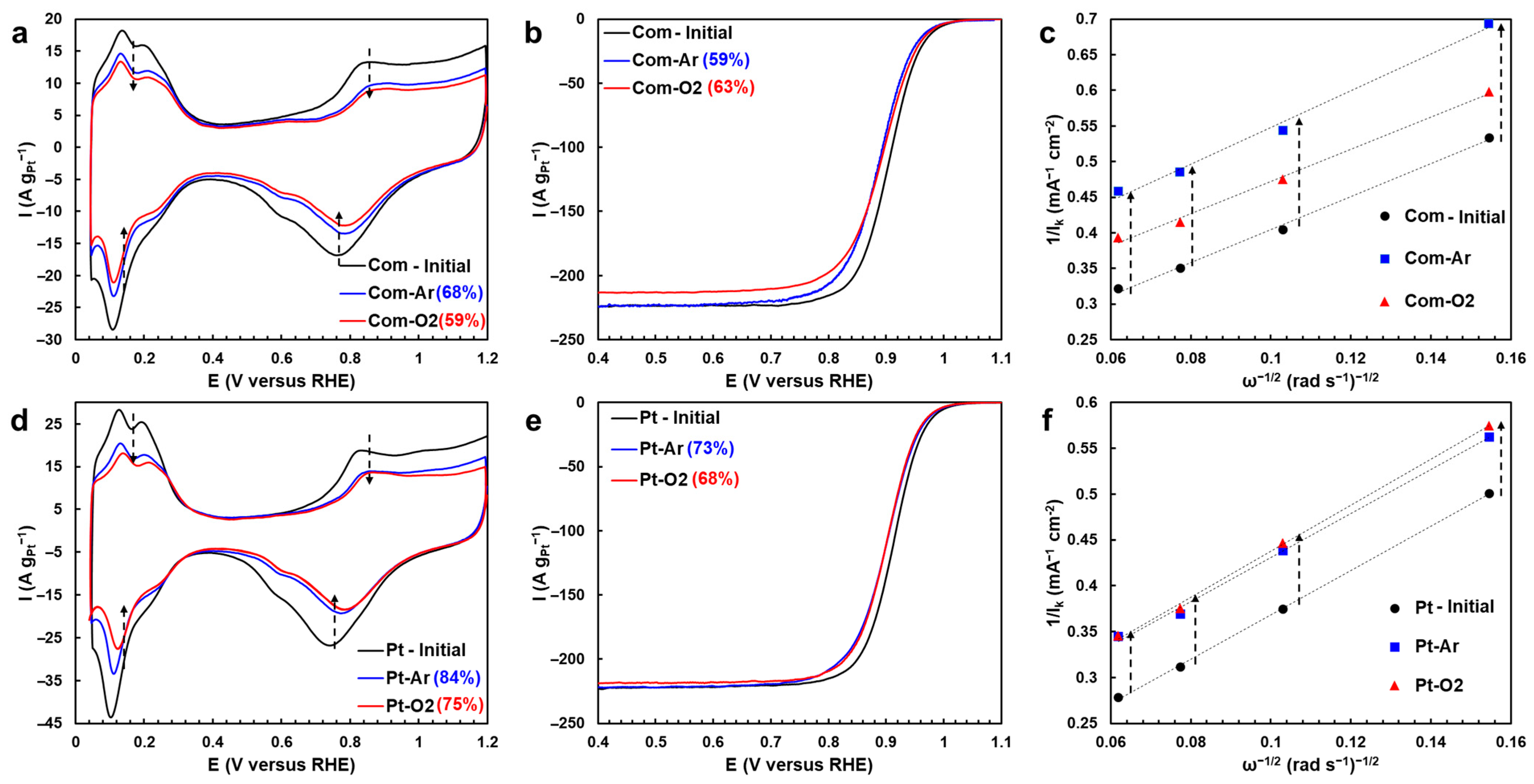
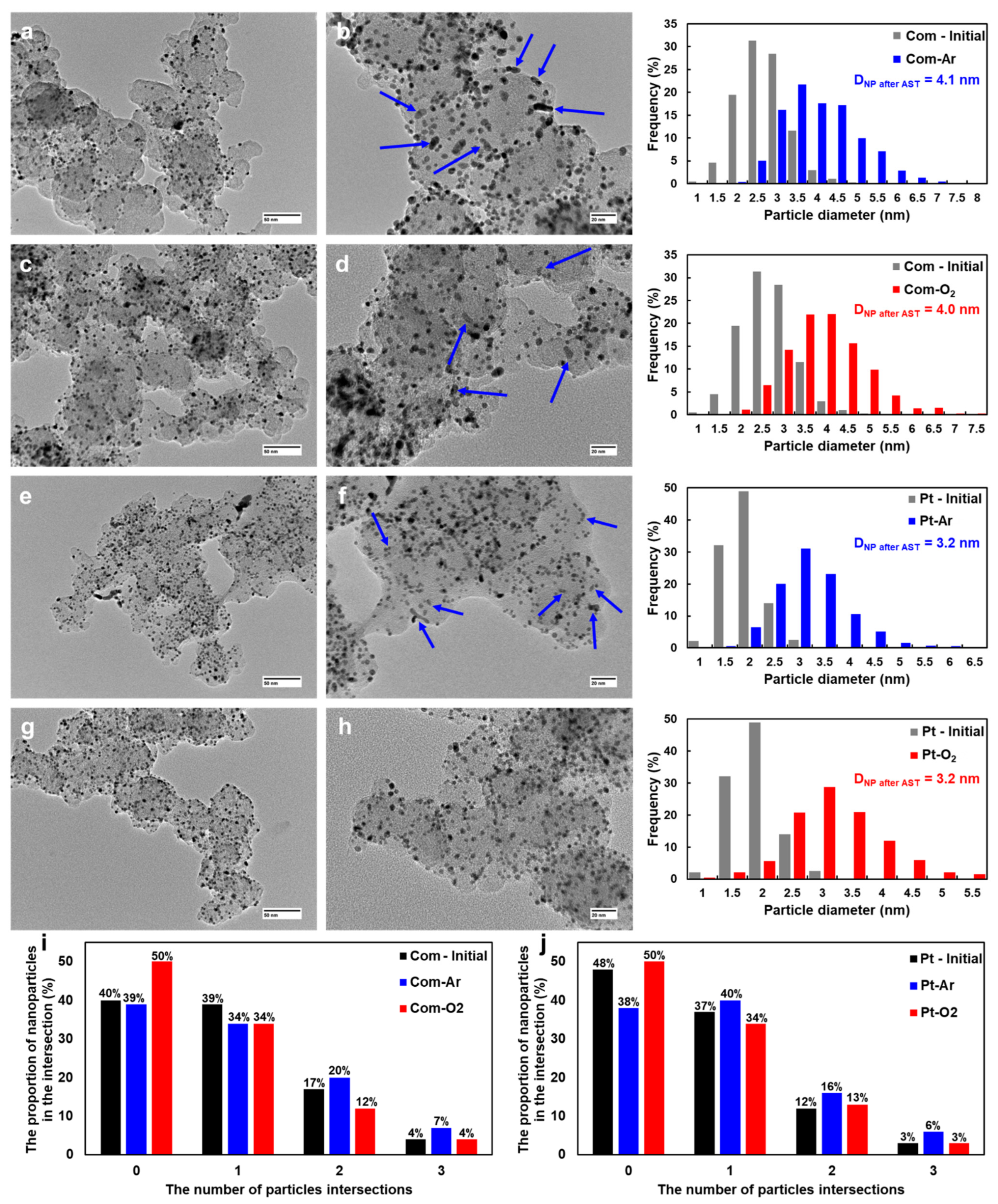
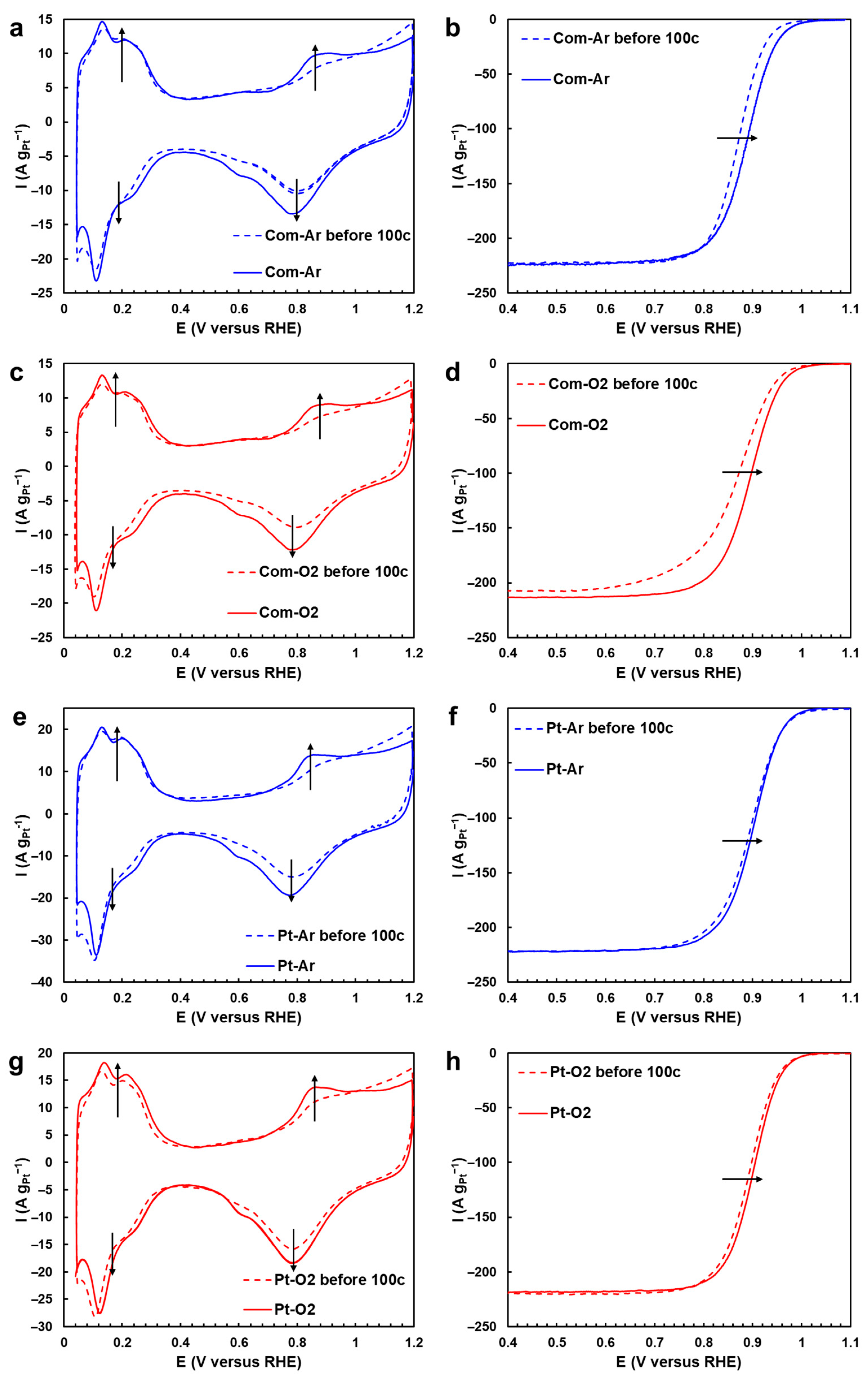
2.2. Changes in Morphology, Structure, and Electrochemical Parameters of Pt/C Samples after AST in Ar and O2
3. Materials and Methods
3.1. Preparation of Pt/C Catalysts
3.2. Composition and Structural and Morphological Characteristics of Pt/C Catalysts
3.2.1. Thermogravimetry
3.2.2. X-ray Examination
3.2.3. Transmission Electron Microscopy
3.3. Study of Electrochemical Characteristics
3.3.1. Preparation of Catalytic Suspension
3.3.2. ESA Determination
3.3.3. ORR Activity Determination
3.4. AST Protocols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uchida, M. PEFC catalyst layers: Effect of support microstructure on both distributions of Pt and ionomer and cell performance and durability. Curr. Opin. Electrochem. 2020, 21, 209–218. [Google Scholar] [CrossRef]
- Wang, F.C.; Peng, C.H. The development of an exchangeable PEMFC power module for electric vehicles. Int. J. Hydrogen Energy 2014, 39, 3855–3867. [Google Scholar] [CrossRef]
- He, W.; Xiang, Y.; Xin, M.; Qiu, L.; Dong, W.; Zhao, W.; Diao, Y.; Zheng, A.; Xu, G. Investigation of multiple commercial electrocatalysts and electrocatalyst degradation for fuel cells in real vehicles. RSC Adv. 2022, 12, 32374–32382. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Groom, D.J.; Wang, X.; Yang, Z.; Gummalla, M.; Ball, S.C.; Myers, D.J.; Ferreira, P.J. Degradation Mechanisms of Platinum Nanoparticle Catalysts in Proton Exchange Membrane Fuel Cells: The Role of Particle Size. Chem. Mater. 2014, 26, 5540–5548. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Ferreira, P.; la O’, G.; Morgan, D.; Gasteiger, H.A.; Makharia, R. Coarsening of Pt Nanoparticles in Proton Exchange Membrane Fuel Cells upon Potential Cycling. ECS Trans. 2006, 1, 185–195. [Google Scholar] [CrossRef]
- Ferreira, P.J.; la O’, G.J.; Shao-Horn, Y.; Morgan, D.; Makharia, R.; Kocha, S.; Gasteiger, H.A. Instability of Pt∕C Electrocatalysts in Proton Exchange Membrane Fuel Cells: A Mechanistic Investigation. J. Electrochem. Soc. 2005, 152, A2256–A2271. [Google Scholar] [CrossRef]
- Marcu, A.; Toth, G.; Kundu, S.; Colmenares, L.; Behm, R. Ex situ testing method to characterize cathode catalysts degradation under simulated start-up/shut-down conditions—A contribution to polymer electrolyte membrane fuel cell benchmarking. J. Power Sources 2012, 215, 266–273. [Google Scholar] [CrossRef]
- Peng, J.; Tao, P.; Song, C.; Shang, W.; Deng, T.; Wu, J. Structural evolution of Pt-based oxygen reduction reaction electrocatalysts. Chin. J. Catal. 2021, 43, 47–58. [Google Scholar] [CrossRef]
- Arán-Ais, R.M.; Yu, Y.; Hovden, R.; Solla-Gullón, J.; Herrero, E.; Feliu, J.M.; Abruña, H.D. Identical Location Transmission Electron Microscopy Imaging of Site-Selective Pt Nanocatalysts: Electrochemical Activation and Surface Disordering. J. Am. Chem. Soc. 2015, 137, 14992–14998. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Topalov, A.A.; Kostka, A.; Schüth, F.; Mayrhofer, K.J.J. Degradation Mechanisms of Pt/C Fuel Cell Catalysts under Simulated Start–Stop Conditions. ACS Catal. 2012, 2, 832–843. [Google Scholar] [CrossRef]
- Angel, G.M.A.; Mansor, N.; Jervis, R.; Rana, Z.; Gibbs, C.; Seel, A.; Kilpatrick, A.F.R.; Shearing, P.R.; Howard, C.A.; Brett, D.J.L.; et al. Realising the electrochemical stability of graphene: Scalable synthesis of an ultra-durable platinum catalyst for the oxygen reduction reaction. Nanoscale 2020, 12, 16113–16122. [Google Scholar] [CrossRef]
- Spasov, D.D.; Ivanova, N.A.; Pushkarev, A.S.; Pushkareva, I.V.; Presnyakova, N.N.; Chumakov, R.G.; Presnyakov, M.Y.; Grigoriev, S.A.; Fateev, V.N. On the Influence of Composition and Structure of Carbon-Supported Pt-SnO2 Hetero-Clusters onto Their Electrocatalytic Activity and Durability in PEMFC. Catalysts 2019, 9, 803. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Bethune, K.; Rubio, M.A.; Rocheleau, R. Study of low concentration CO poisoning of Pt anode in a proton exchange membrane fuel cell using spatial electrochemical impedance spectroscopy. J. Power Sources 2014, 269, 344–362. [Google Scholar] [CrossRef]
- Reshetenko, T.V. Impacts of operating conditions on the recovery of proton exchange membrane fuel cells exposed to sulfur dioxide in an air stream. J. Power Sources 2023, 559, 232676. [Google Scholar] [CrossRef]
- Cherevko, S.; Keeley, G.P.; Geiger, S.; Zeradjanin, A.R.; Hodnik, N.; Kulyk, N.; Mayrhofer, K.J.J. Dissolution of Platinum in the Operational Range of Fuel Cells. Chemelectrochem 2015, 2, 1471–1478. [Google Scholar] [CrossRef]
- Pavlišič, A.; Jovanovič, P.; Šelih, V.S.; Šala, M.; Hodnik, N.; Gaberšček, M. Platinum Dissolution and Redeposition from Pt/C Fuel Cell Electrocatalyst at Potential Cycling. J. Electrochem. Soc. 2018, 165, F3161–F3165. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Gao, L.; Zeng, G.; Li, M.; Huang, H. Stabilizing Pt-Based Electrocatalysts for Oxygen Reduction Reaction: Fundamental Understanding and Design Strategies. Adv. Mater. 2021, 33, e2006494. [Google Scholar] [CrossRef] [PubMed]
- Speder, J.; Zana, A.; Spanos, I.; Kirkensgaard, J.J.; Mortensen, K.; Arenz, M. On the influence of the Pt to carbon ratio on the degradation of high surface area carbon supported PEM fuel cell electrocatalysts. Electrochem. Commun. 2013, 34, 153–156. [Google Scholar] [CrossRef]
- Jovanovič, P.; Pavlišič, A.; Šelih, V.S.; Šala, M.; Hodnik, N.; Bele, M.; Hočevar, S.; Gaberšček, M. New Insight into Platinum Dissolution from Nanoparticulate Platinum-Based Electrocatalysts Using Highly Sensitive In Situ Concentration Measurements. Chemcatchem 2014, 6, 449–453. [Google Scholar] [CrossRef]
- Nagai, T.; Jahn, C.; Jia, H. Improved Accelerated Stress Tests for ORR Catalysts Using a Rotating Disk Electrode. J. Electrochem. Soc. 2019, 166, F3111–F3115. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Wang, K.; Wu, G.-P.; Arges, C. Extended-Surface Thin Film Platinum Electrocatalysts with Tunable Nanostructured Morphologies. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Yano, H.; Watanabe, M.; Iiyama, A.; Uchida, H. Particle-size effect of Pt cathode catalysts on durability in fuel cells. Nano Energy 2016, 29, 323–333. [Google Scholar] [CrossRef]
- Liu, D.; Gao, S.; Xu, J.; Zhang, X.; Yang, Z.; Yang, T.; Wang, B.; Yang, S.; Liang, C.; Kong, C. Boron induced strong metal-support interaction for high sintering resistance of Pt-based catalysts toward oxygen reduction reaction. Appl. Surf. Sci. 2022, 604, 154466. [Google Scholar] [CrossRef]
- Takimoto, D.; Toma, S.; Suda, Y.; Shirokura, T.; Tokura, Y.; Fukuda, K.; Matsumoto, M.; Imai, H.; Sugimoto, W. Platinum nanosheets synthesized via topotactic reduction of single-layer platinum oxide nanosheets for electrocatalysis. Nat. Commun. 2023, 14, 1–9. [Google Scholar] [CrossRef]
- Cheng, T.; Tan, X.; Chen, L.; Zhao, X.; Kotegawa, F.; Huang, J.; Liu, Y.; Jiang, H.; Harada, M.; Wang, Y. A Robust Electrocatalyst for Oxygen Reduction Reaction Assembled with Pt Nanoclusters and a Melem-Modified Carbon Support. Energy Technol. 2022, 10, 2200680. [Google Scholar] [CrossRef]
- Rotating Disk-Electrode Aqueous Electrolyte Accelerated Stress Tests for PGM Electrocatalyst/Support Durability Evaluation DOE Durability Working Group 10/4/2011 2011. Available online: https://www.energy.gov/eere/fuelcells/articles/rotating-disk-electrode-aqueous-electrolyte-accelerated-stress-tests-pgm (accessed on 29 January 2023).
- Hodnik, N.; Jozinović, B.; Zorko, M.; Gaberšček, M. Stability of Commercial Pt/C Low Temperature Fuel Cell Catalyst: Electro-chemical IL-SEM Study. Acta Chim. Slov. 2014, 61, 280–283. [Google Scholar]
- Zhao, Z.; Dubau, L.; Maillard, F. Evidences of the migration of Pt crystallites on high surface area carbon supports in the presence of reducing molecules. J. Power Sources 2012, 217, 449–458. [Google Scholar] [CrossRef]
- Paperzh, K.O.; Alekseenko, A.A.; Volochaev, V.A.; Pankov, I.V.; Safronenko, O.A.; Guterman, V.E. Stability and activity of platinum nanoparticles in the oxygen electroreduction reaction: Is size or uniformity of primary importance? Beilstein J. Nanotechnol. 2021, 12, 593–606. [Google Scholar] [CrossRef]
- Paperzh, K.; Alekseenko, A.; Danilenko, M.; Pankov, I.; Guterman, V.E. Advanced Methods of Controlling the Morphology, Activity, and Durability of Pt/C Electrocatalysts. ACS Appl. Energy Mater. 2022, 5, 9530–9541. [Google Scholar] [CrossRef]
- Yamashita, Y.; Miyahara, R.; Sakamoto, K. Emulsion and Emulsification Technology. Cosmet. Sci. Technol. Theor. Princ. Appl. 2017, 489–506. [Google Scholar]
- Arenz, M.; Quinson, J. Degradation of Metal Clusters and Nanoparticles Under Electrochemical Control. Encycl. Interfacial Chem. Surf. Sci. Electrochem. 2018, 434–441. [Google Scholar] [CrossRef]
- Xu, S.; Kim, Y.; Park, J.; Higgins, D.; Shen, S.-J.; Schindler, P.; Thian, D.; Provine, J.; Torgersen, J.; Graf, T.; et al. Extending the limits of Pt/C catalysts with passivation-gas-incorporated atomic layer deposition. Nat. Catal. 2018, 1, 624–630. [Google Scholar] [CrossRef]
- Sharma, R.; Gyergyek, S.; Chamier, J.; Morgen, P.; Andersen, S.M. Pt/C Electrocatalyst Durability Enhancement by Inhibition of Pt Nanoparticle Growth Through Microwave Pretreatment of Carbon Support. Chemelectrochem 2021, 8, 1183–1195. [Google Scholar] [CrossRef]
- Shokhen, V.; Strandberg, L.; Skoglundh, M.; Wickman, B. Impact of Accelerated Stress Tests on the Cathodic Catalytic Layer in a Proton Exchange Membrane (PEM) Fuel Cell Studied by Identical Location Scanning Electron Microscopy. ACS Appl. Energy Mater. 2022, 5, 11200–11212. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wang, Y.; Ding, W.; Wu, R.; Li, L.; Qi, X.; Wei, Z. Study of the degradation mechanisms of carbon-supported platinum fuel cells catalyst via different accelerated stress test. J. Power Sources 2015, 273, 62–69. [Google Scholar] [CrossRef]
- Paperzh, K.; Alekseenko, A.; Safronenko, O.; Nikulin, A.; Pankov, I.; Guterman, V. UV radiation effect on the microstructure and performance of electrocatalysts based on small Pt nanoparticles synthesized in the liquid phase. Colloid Interface Sci. Commun. 2021, 45, 100517. [Google Scholar] [CrossRef]
- Đukić, T.; Moriau, L.J.; Pavko, L.; Kostelec, M.; Prokop, M.; Ruiz-Zepeda, F.; Šala, M.; Dražić, G.; Gatalo, M.; Hodnik, N. Understanding the Crucial Significance of the Temperature and Potential Window on the Stability of Carbon Supported Pt-Alloy Nanoparticles as Oxygen Reduction Reaction Electrocatalysts. ACS Catal. 2021, 12, 101–115. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2016, 5, 1808–1825. [Google Scholar] [CrossRef]
- Paperzh, K.O.; Pavlets, A.S.; Alekseenko, A.A.; Pankov, I.V.; Guterman, V.E. The integrated study of the morphology and the electrochemical behavior of Pt-based ORR electrocatalysts during the stress testing. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Logeshwaran, N.; Panneerselvam, I.R.; Ramakrishnan, S.; Kumar, R.S.; Kim, A.R.; Wang, Y.; Yoo, D.J. Quasihexagonal Platinum Nanodendrites Decorated over CoS2-N-Doped Reduced Graphene Oxide for Electro-Oxidation of C1-, C2-, and C3-Type Alcohols. Adv. Sci. 2022, 9, 2105344. [Google Scholar] [CrossRef]
- Shinozaki, K.; Zack, J.W.; Richards, R.; Pivovar, B.S.; Kocha, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique: I. Impact of Impurities, Measurement Protocols and Applied Corrections. J. Electrochem. Soc. 2015, 162, F1144–F1158. [Google Scholar] [CrossRef]
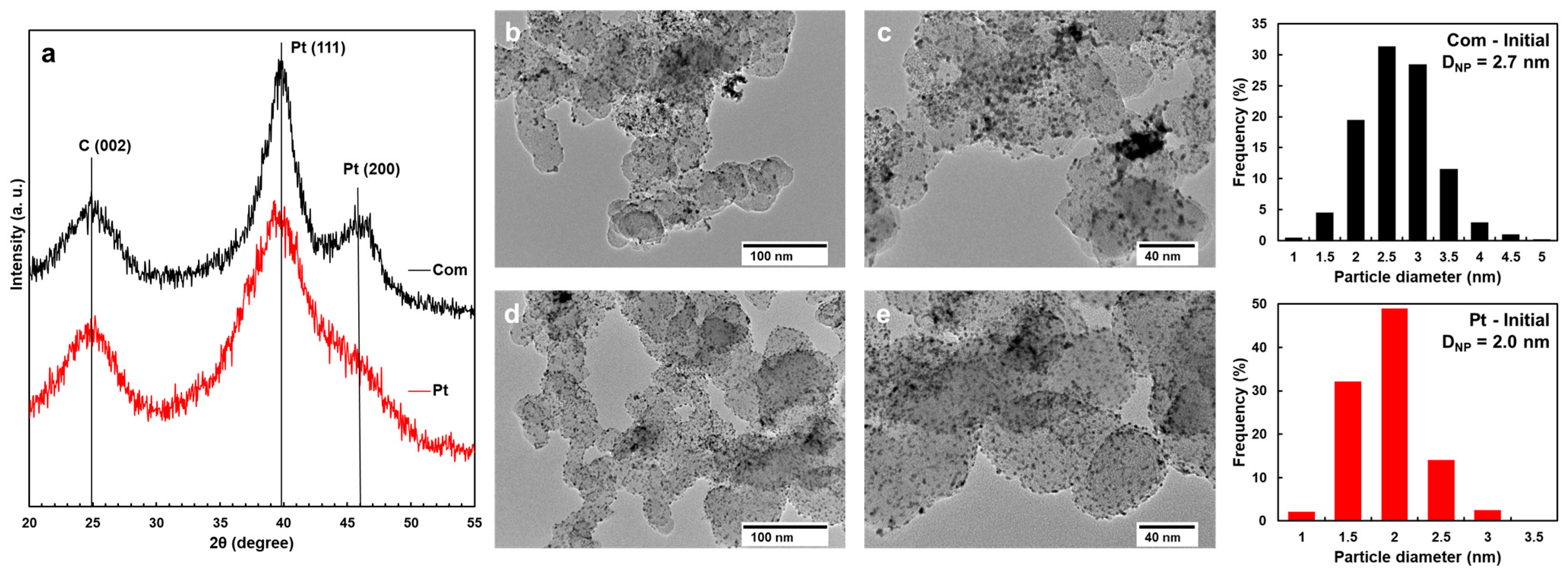
| Sample | ω(Pt), % | DAv, nm (XRD) | DNP, nm (TEM) | Specific Number of NPs, N, 1015 m−2 | Average Inter-Particle Distance, ʎ, nm | ESA, m2 gPt−1 | Imass, A gPt−1 |
|---|---|---|---|---|---|---|---|
| Com | 20 | 2.5 | 2.7 | 4.5 | 12.2 | 82 | 257 |
| Pt | 20 | 1.3 | 2.0 | 11.1 | 7.5 | 112 | 326 |
| Initial/after AST Protocols | DNP, nm | ESAafter AST, m2 gPt−1 | Imassafter AST, A gPt−1 | Specific Number of NPs, N, 1015 m−2 * | Average Inter-Particle Distance, ʎ, nm * |
|---|---|---|---|---|---|
| Com-Ar | 4.1 | 56 | 152 | 1.3 | 23.7 |
| Com-O2 | 4.0 | 48 | 163 | 1.4 | 22.8 |
| Pt-Ar | 3.2 | 84 | 239 | 2.7 | 16.0 |
| Pt-O2 | 3.2 | 75 | 223 | 2.7 | 16.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paperzh, K.; Moguchikh, E.; Pankov, I.; Belenov, S.; Alekseenko, A. Effect of AST Atmosphere on Pt/C Electrocatalyst Degradation. Inorganics 2023, 11, 237. https://doi.org/10.3390/inorganics11060237
Paperzh K, Moguchikh E, Pankov I, Belenov S, Alekseenko A. Effect of AST Atmosphere on Pt/C Electrocatalyst Degradation. Inorganics. 2023; 11(6):237. https://doi.org/10.3390/inorganics11060237
Chicago/Turabian StylePaperzh, Kirill, Elizaveta Moguchikh, Ilya Pankov, Sergey Belenov, and Anastasia Alekseenko. 2023. "Effect of AST Atmosphere on Pt/C Electrocatalyst Degradation" Inorganics 11, no. 6: 237. https://doi.org/10.3390/inorganics11060237
APA StylePaperzh, K., Moguchikh, E., Pankov, I., Belenov, S., & Alekseenko, A. (2023). Effect of AST Atmosphere on Pt/C Electrocatalyst Degradation. Inorganics, 11(6), 237. https://doi.org/10.3390/inorganics11060237