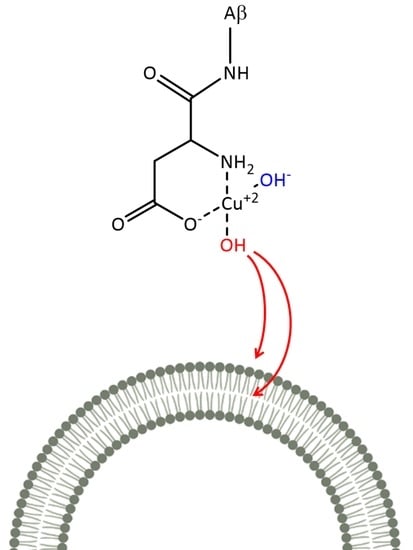
Oxidation of Phospholipids by OH Radical Coordinated to Copper Amyloid-β Peptide—A Density Functional Theory Modeling †
Abstract
1. Introduction
2. Results
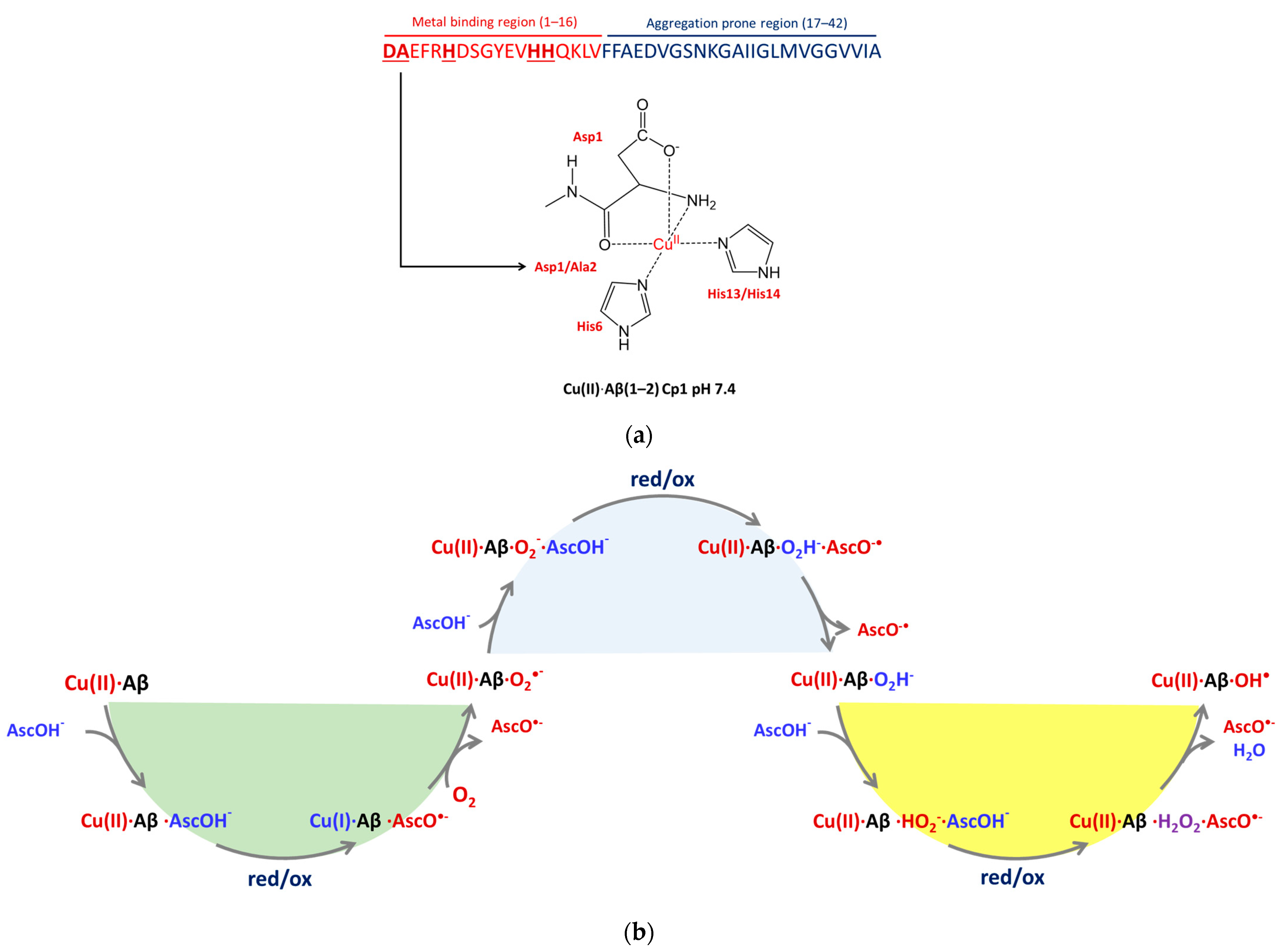
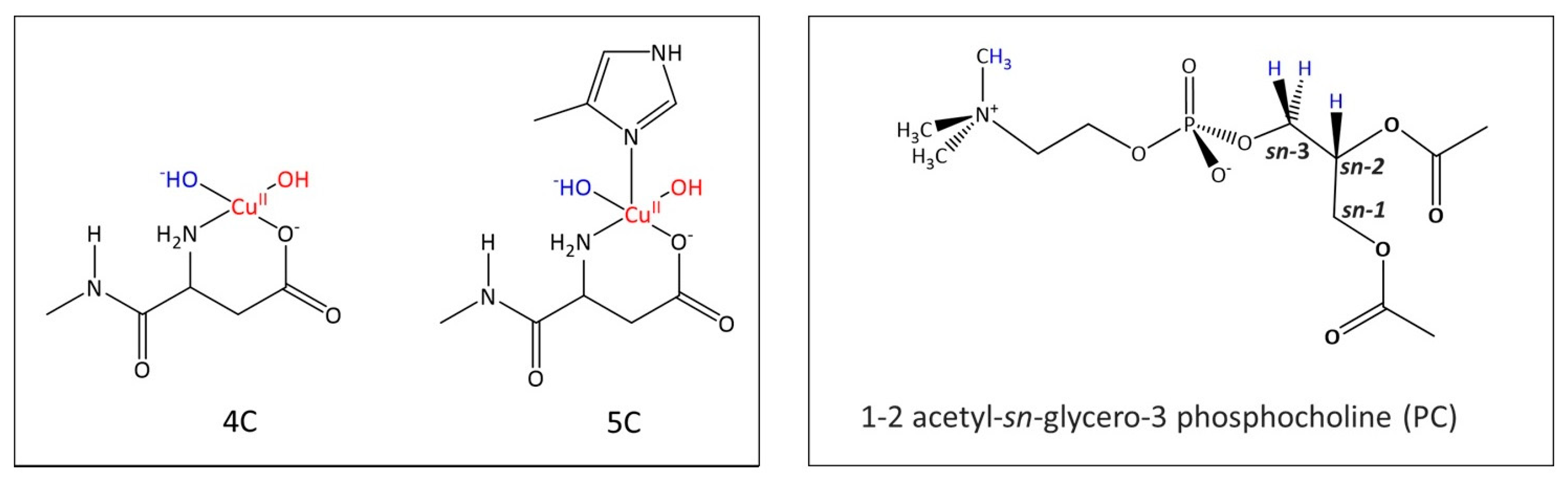
2.1. The Electronic Structure of Cu(II)-Aβ-OH
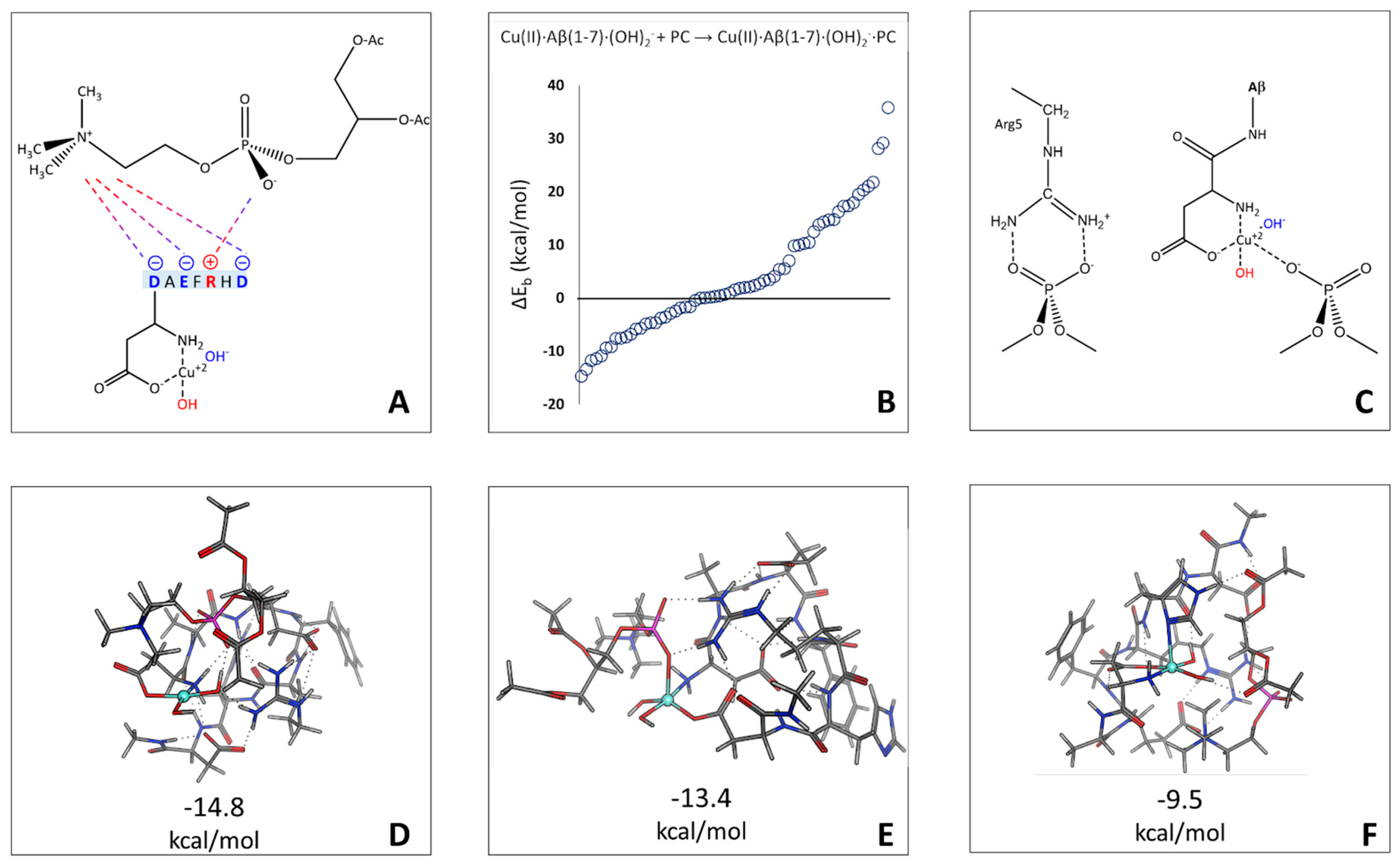
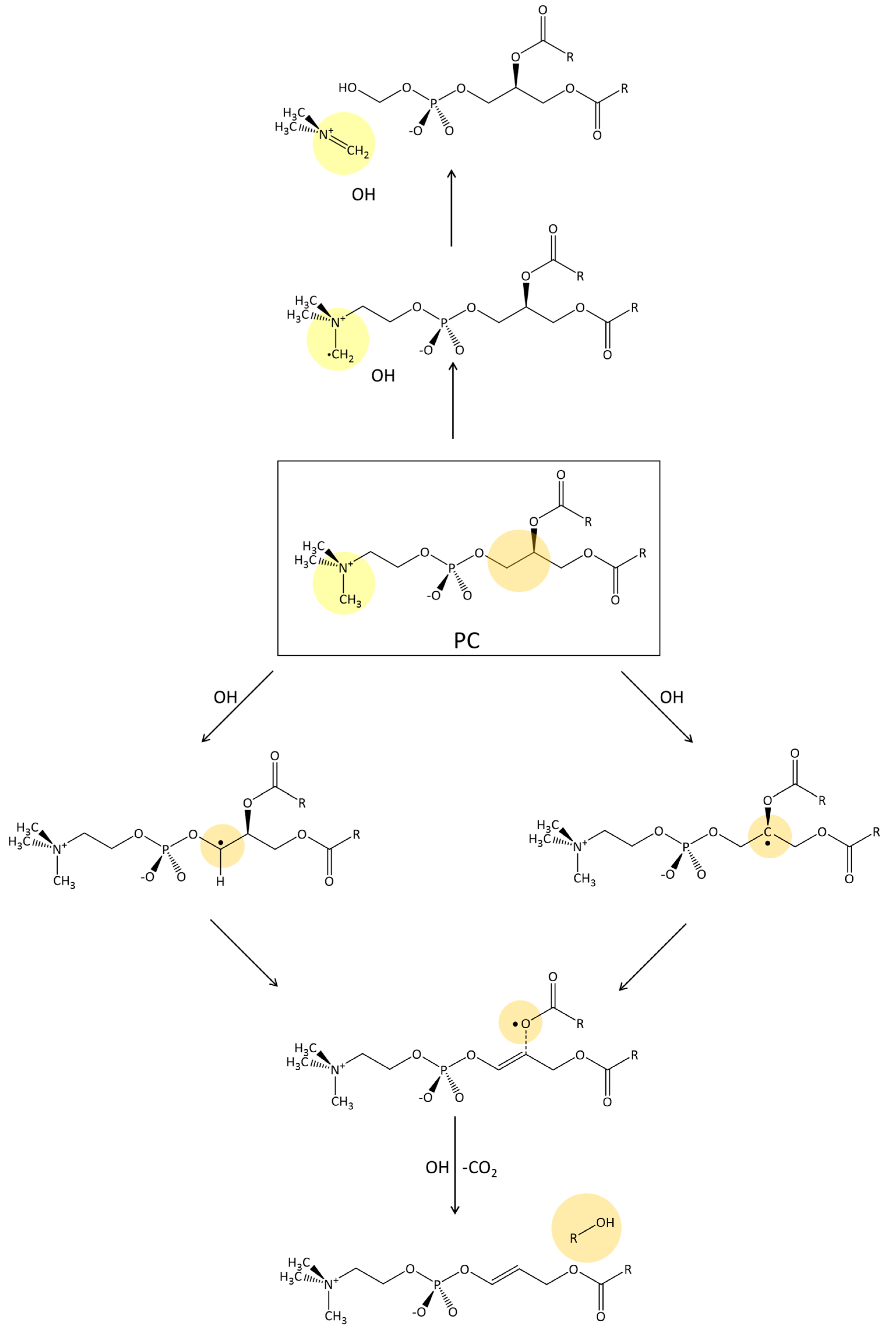
2.2. Cu(II)-Aβ-OH Propagation Mechanism
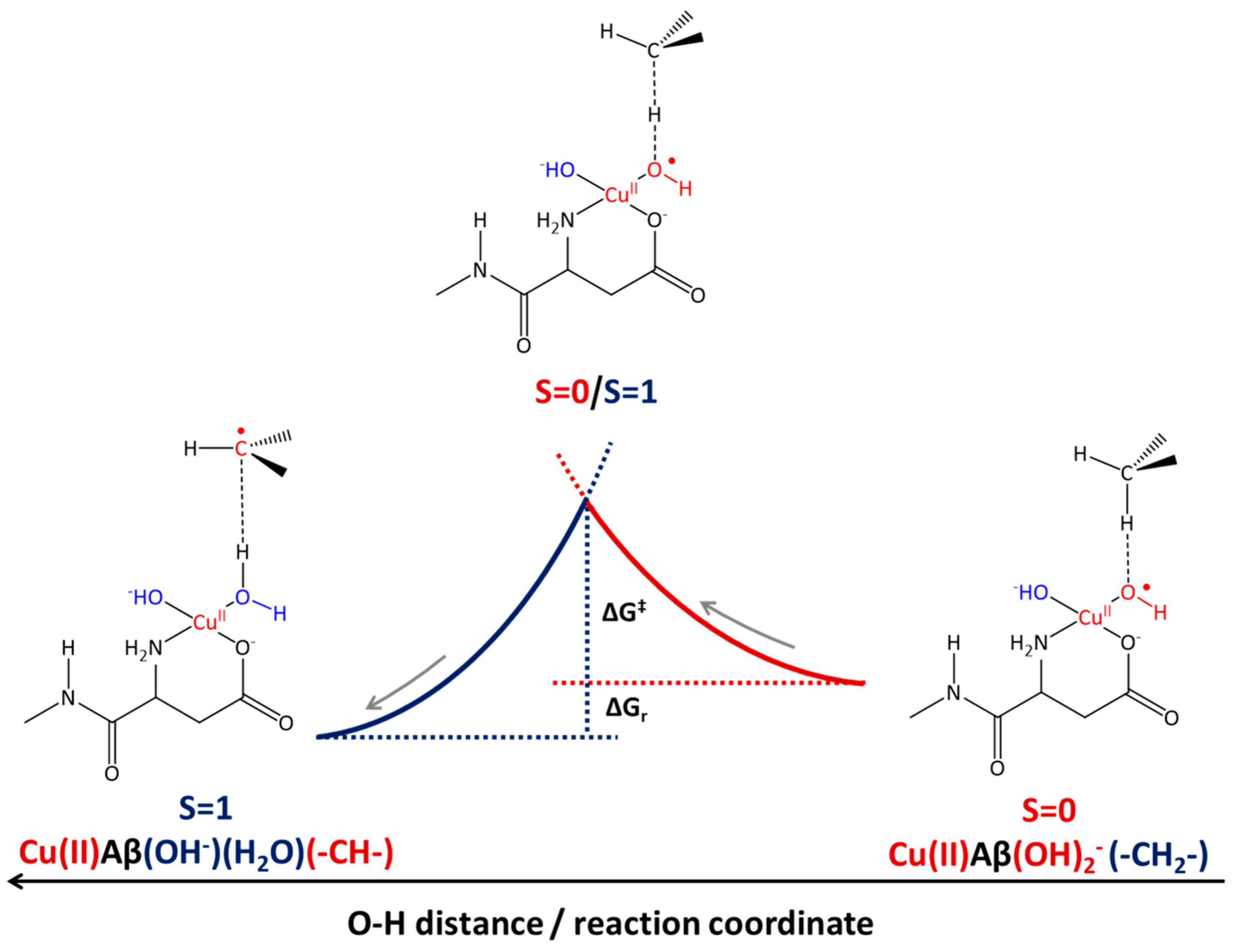
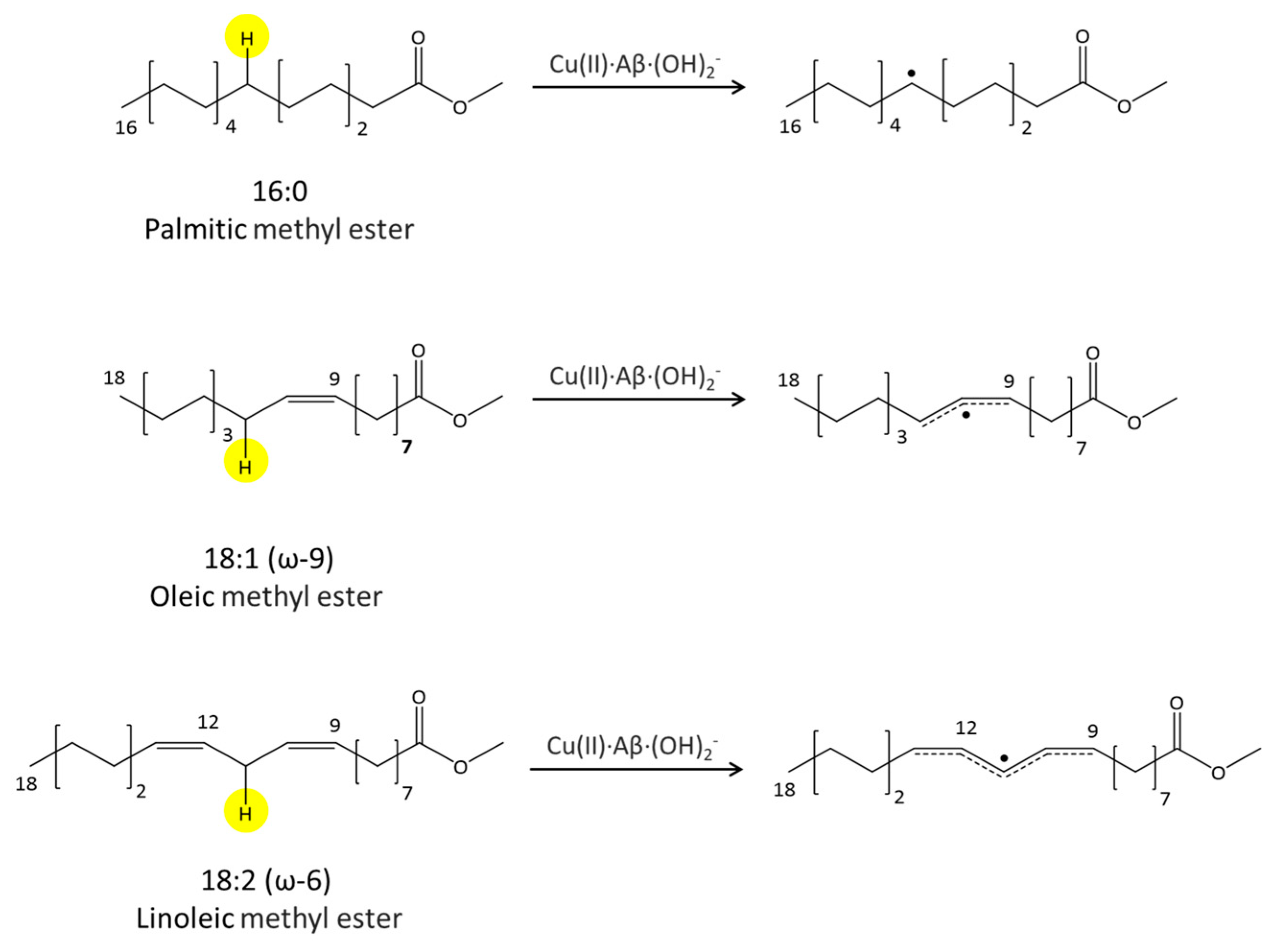
2.3. Phosphatidylcholine Moiety, Structure, and Reactivity toward Cu(II)-Aβ-OH
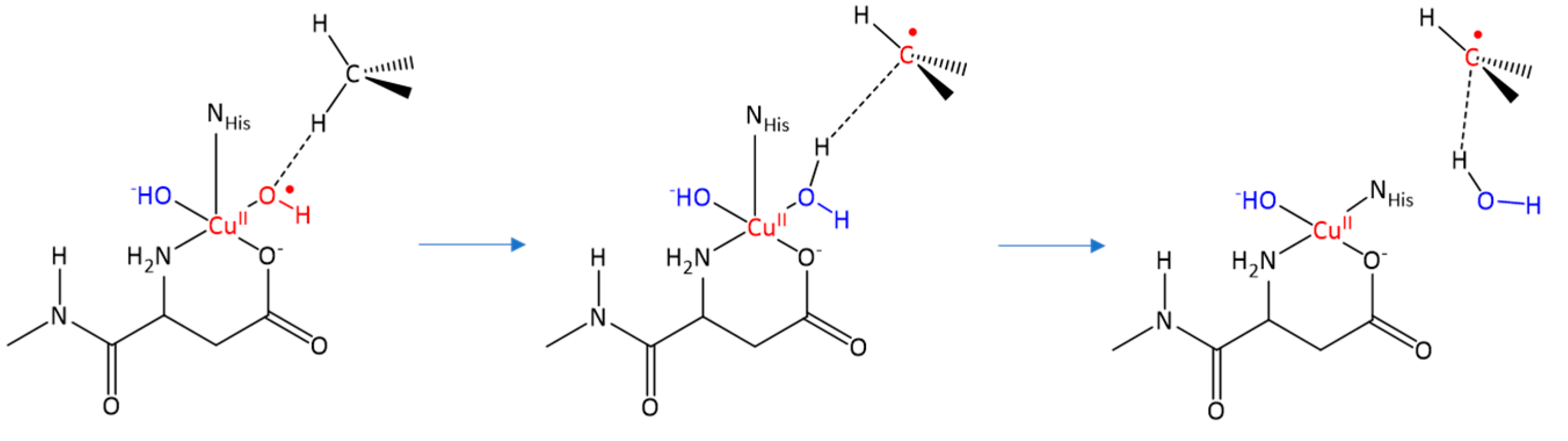 where after OH propagation, the H2O ligand leaves the Cu(II) coordination sphere with the formation of a four-coordinated form. Here, the activation barrier does not change significantly (+0.8 kcal/mol), but the ΔGr value decreases by 8.1 kcal/mol.
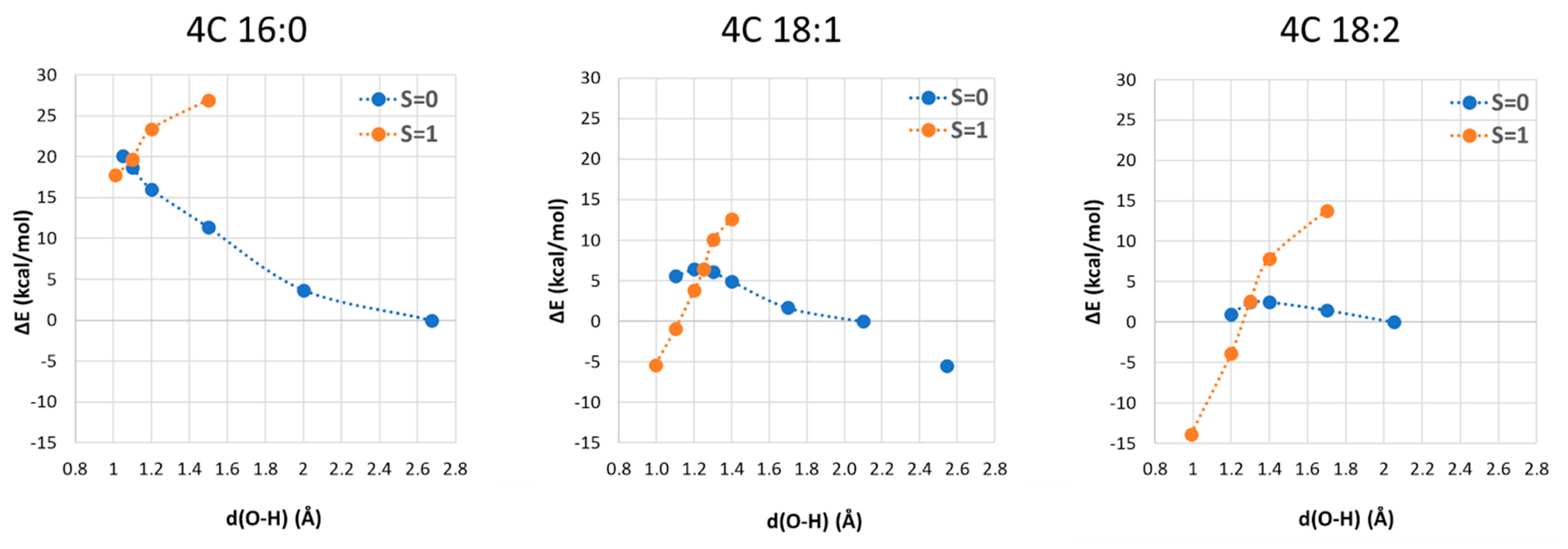
where after OH propagation, the H2O ligand leaves the Cu(II) coordination sphere with the formation of a four-coordinated form. Here, the activation barrier does not change significantly (+0.8 kcal/mol), but the ΔGr value decreases by 8.1 kcal/mol. 2.4. Fatty Acid Aliphatic Chains, Structure, and Reactivity toward Cu(II)-Aβ-OH
3. Discussion and Conclusions
4. Computational Details
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2022 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Makin, S. The Amyloid Hypothesis on Trial. Nature 2018, 559, S4–S7. [Google Scholar] [CrossRef]
- Doig, A.J.; del Castillo-Frias, M.P.; Berthoumieu, O.; Tarus, B.; Nasica-Labouze, J.; Sterpone, F.; Nguyen, P.H.; Hooper, N.M.; Faller, P.; Derreumaux, P. Why Is Research on Amyloid Failing to Give New Drugs for Alzheimers Disease? ACS Chem. Neurosci. 2017, 8, 1435–1437. [Google Scholar] [CrossRef]
- Lei, P.; Ayton, S.; Bush, A.I. The Essential Elements of Alzheimer’s Disease. J. Biol. Chem. 2021, 296, 100105. [Google Scholar] [CrossRef]
- Ayton, S.; Bush, A.I. β-Amyloid: The Known Unknowns. Ageing Res. Rev. 2021, 65, 101212. [Google Scholar] [CrossRef]
- Abelein, A.; Abrahams, J.P.; Danielsson, J.; Gräslund, A.; Jarvet, J.; Luo, J.; Tiiman, A.; Wärmländer, S.K.T.S. The Hairpin Conformation of the Amyloid β Peptide Is an Important Structural Motif along the Aggregation Pathway. J. Biol. Inorg. Chem. 2014, 19, 623–634. [Google Scholar] [CrossRef]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimers. Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef]
- Michaels, T.C.T.; Šarić, A.; Curk, S.; Bernfur, K.; Arosio, P.; Meisl, G.; Dear, A.J.; Cohen, S.I.A.; Dobson, C.M.; Vendruscolo, M.; et al. Author Correction: Dynamics of Oligomer Populations Formed during the Aggregation of Alzheimer’s Aβ42 Peptide. Nat. Chem. 2020, 12, 497. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Ramamoorthy, A.; Sahoo, B.R.; Zheng, J.; Faller, P.; Straub, J.E.; Dominguez, L.; Shea, J.-E.; Dokholyan, N.V.; De Simone, A.; et al. Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimer’s Disease, Parkinson’s Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis. Chem. Rev. 2021, 121, 2545–2647. [Google Scholar] [CrossRef]
- Squitti, R.; Faller, P.; Hureau, C.; Granzotto, A.; White, A.R.; Kepp, K.P. Copper Imbalance in Alzheimer’s Disease and Its Link with the Amyloid Hypothesis: Towards a Combined Clinical, Chemical, and Genetic Etiology. J. Alzheimers. Dis. 2021, 83, 23–41. [Google Scholar] [CrossRef]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, Iron and Zinc in Alzheimer’s Disease Senile Plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Gonzalez, P.; Santoro, A.; Alies, B.; Faller, P.; Hureau, C. Cu and Zn Coordination to Amyloid Peptides: From Fascinating Chemistry to Debated Pathological Relevance. Coord. Chem. Rev. 2018, 375, 38–55. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Cerrada, E.; Conte-Daban, A.; Testemale, D.; Faller, P.; Laguna, M.; Hureau, C. Copper(I) Targeting in the Alzheimer’s Disease Context: A First Example Using the Biocompatible PTA Ligand. Metallomics 2015, 7, 1229–1232. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C. A Bioinorganic View of Alzheimer’s Disease: When Misplaced Metal Ions (Re)direct the Electrons to the Wrong Target. Chem. Eur. J. 2012, 18, 15910–15920. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; González-Acedo, A.; Rivas-Domínguez, A.; García-Morales, V.; García-Cozar, F.J.; Ramos-Rodríguez, J.J.; Melguizo-Rodríguez, L. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef]
- Dong, J.; Atwood, C.S.; Anderson, V.E.; Siedlak, S.L.; Smith, M.A.; Perry, G.; Carey, P.R. Metal Binding and Oxidation of Amyloid-Beta within Isolated Senile Plaque Cores: Raman Microscopic Evidence. Biochemistry 2003, 42, 2768–2773. [Google Scholar] [CrossRef]
- Greenough, M.A.; Camakaris, J.; Bush, A.I. Metal Dyshomeostasis and Oxidative Stress in Alzheimer’s Disease. Neurochem. Int. 2013, 62, 540–555. [Google Scholar] [CrossRef]
- Kepp, K.P.; Squitti, R. Copper Imbalance in Alzheimer’s Disease: Convergence of the Chemistry and the Clinic. Coord. Chem. Rev. 2019, 397, 168–187. [Google Scholar] [CrossRef]
- Bradley, M.A.; Xiong-Fister, S.; Markesbery, W.R.; Lovell, M.A. Elevated 4-Hydroxyhexenal in Alzheimer’s Disease (AD) Progression. Neurobiol. Aging 2012, 33, 1034–1044. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Reed, T.; Perluigi, M.; De Marco, C.; Coccia, R.; Cini, C.; Sultana, R. Elevated Protein-Bound Levels of the Lipid Peroxidation Product, 4-Hydroxy-2-Nonenal, in Brain from Persons with Mild Cognitive Impairment. Neurosci. Lett. 2006, 397, 170–173. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Furlan, S.; Hureau, C.; Faller, P.; La Penna, G. Modeling Copper Binding to the Amyloid-β Peptide at Different pH: Toward a Molecular Mechanism for Cu Reduction. J. Phys. Chem. B 2012, 116, 11899–11910. [Google Scholar] [CrossRef]
- Singh, S.K.; Balendra, V.; Obaid, A.A.; Esposto, J.; Tikhonova, M.A.; Gautam, N.K.; Poeggeler, B. Copper-Mediated β-Amyloid Toxicity and Its Chelation Therapy in Alzheimer’s Disease. Metallomics 2022, 14, mfac018. [Google Scholar] [CrossRef]
- Mutter, S.T.; Turner, M.; Deeth, R.J.; Platts, J.A. Metal Binding to Amyloid-β: A Ligand Field Molecular Dynamics Study. ACS Chem. Neurosci. 2018, 9, 2795–2806. [Google Scholar] [CrossRef]
- Rauk, A. The Chemistry of Alzheimer’s Disease. Chem. Soc. Rev. 2009, 38, 2698–2715. [Google Scholar] [CrossRef]
- Cheignon, C.; Collin, F.; Faller, P.; Hureau, C. Is Ascorbate Dr Jekyll or Mr Hyde in the Cu(Aβ) Mediated Oxidative Stress Linked to Alzheimer’s Disease? Dalton Trans. 2016, 45, 12627–12631. [Google Scholar] [CrossRef]
- Cheignon, C.; Jones, M.; Atrián-Blasco, E.; Kieffer, I.; Faller, P.; Collin, F.; Hureau, C. Identification of Key Structural Features of the Elusive Cu–Aβ Complex That Generates ROS in Alzheimer’s Disease. Chem. Sci. 2017, 8, 5107–5118. [Google Scholar] [CrossRef]
- Reybier, K.; Ayala, S.; Alies, B.; Rodrigues, J.V.; Bustos Rodriguez, S.; La Penna, G.; Collin, F.; Gomes, C.M.; Hureau, C.; Faller, P. Free Superoxide Is an Intermediate in the Production of H2O2 by Copper(I)-Aβ Peptide and O2. Angew. Chem. Int. Ed. Engl. 2016, 55, 1085–1089. [Google Scholar] [CrossRef]
- Cassagnes, L.-E.; Hervé, V.; Nepveu, F.; Hureau, C.; Faller, P.; Collin, F. The Catalytically Active Copper-Amyloid-Beta State: Coordination Site Responsible for Reactive Oxygen Species Production. Angew. Chem. Int. Ed. Engl. 2013, 52, 11110–11113. [Google Scholar] [CrossRef]
- La Penna, G.; Hureau, C.; Andreussi, O.; Faller, P. Identifying, By First-Principles Simulations, Cu[Amyloid-β] Species Making Fenton-Type Reactions in Alzheimer’s Disease. J. Phys. Chem. B 2013, 117, 16455–16467. [Google Scholar] [CrossRef]
- Bradley-Whitman, M.A.; Timmons, M.D.; Beckett, T.L.; Murphy, M.P.; Lynn, B.C.; Lovell, M.A. Nucleic Acid Oxidation: An Early Feature of Alzheimer’s Disease. J. Neurochem. 2014, 128, 294–304. [Google Scholar] [CrossRef]
- Sultana, R.; Allan Butterfield, D. Oxidative Modification of Brain Proteins in Alzheimer’s Disease: Perspective on Future Studies Based on Results of Redox Proteomics Studies. J. Alzheimer’s Dis. 2012, 33, S243–S251. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Lauderback, C.M. Lipid Peroxidation and Protein Oxidation in Alzheimer’s Disease Brain: Potential Causes and Consequences Involving Amyloid β-Peptide-Associated Free Radical Oxidative Stress. Free. Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Allan Butterfield, D.; Reed, T.; Newman, S.F.; Sultana, R. Roles of Amyloid β-Peptide-Associated Oxidative Stress and Brain Protein Modifications in the Pathogenesis of Alzheimer’s Disease and Mild Cognitive Impairment. Free Radic. Biol. Med. 2007, 43, 658–677. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Tirle, T.; López-Nogueroles, M.; Vento, M.; Baquero, M.; Cháfer-Pericás, C. Oxidative Damage of DNA as Early Marker of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 6136. [Google Scholar] [CrossRef]
- Nasica-Labouze, J.; Nguyen, P.H.; Sterpone, F.; Berthoumieu, O.; Buchete, N.-V.; Coté, S.; De Simone, A.; Doig, A.J.; Faller, P.; Garcia, A.; et al. Amyloid β Protein and Alzheimer’s Disease: When Computer Simulations Complement Experimental Studies. Chem. Rev. 2015, 115, 3518–3563. [Google Scholar] [CrossRef]
- Strodel, B.; Coskuner-Weber, O. Transition Metal Ion Interactions with Disordered Amyloid-β Peptides in the Pathogenesis of Alzheimer’s Disease: Insights from Computational Chemistry Studies. J. Chem. Inf. Model. 2019, 59, 1782–1805. [Google Scholar] [CrossRef]
- Giacovazzi, R.; Ciofini, I.; Rao, L.; Amatore, C.; Adamo, C. Copper–amyloid-β Complex May Catalyze Peroxynitrite Production in Brain: Evidence from Molecular Modeling. Phys. Chem. Chem. Phys. 2014, 16, 10169–10174. [Google Scholar] [CrossRef]
- Prosdocimi, T.; De Gioia, L.; Zampella, G.; Bertini, L. On the Generation of OH(·) Radical Species from H2O2 by Cu(I) Amyloid Beta Peptide Model Complexes: A DFT Investigation. J. Biol. Inorg. Chem. 2016, 21, 197–212. [Google Scholar] [CrossRef]
- Arrigoni, F.; Prosdocimi, T.; Mollica, L.; De Gioia, L.; Zampella, G.; Bertini, L. Copper Reduction and Dioxygen Activation in Cu-Amyloid Beta Peptide Complexes: Insight from Molecular Modelling. Metallomics 2018, 10, 1618–1630. [Google Scholar] [CrossRef]
- Arrigoni, F.; Di Carlo, C.; Rovetta, A.; De Gioia, L.; Zampella, G.; Bertini, L. Superoxide Reduction by Cu-Amyloid Beta Peptide Complexes: A Density Functional Theory Study. Eur. J. Inorg. Chem. 2022, 2022, e202200245. [Google Scholar] [CrossRef]
- Arrigoni, F.; Rizza, F.; Tisi, R.; De Gioia, L.; Zampella, G.; Bertini, L. On the Propagation of the OH Radical Produced by Cu-Amyloid Beta Peptide Model Complexes. Insight Mol. Modelling Met. 2020, 12, 1765–1780. [Google Scholar]
- Maciel, E.; da Silva, R.N.; Simões, C.; Domingues, P.; Domingues, M.R.M. Structural Characterization of Oxidized Glycerophosphatidylserine: Evidence of Polar Head Oxidation. J. Am. Soc. Mass. Spectrom. 2011, 22, 1804–1814. [Google Scholar] [CrossRef]
- Yusupov, M.; Wende, K.; Kupsch, S.; Neyts, E.C.; Reuter, S.; Bogaerts, A. Effect of Head Group and Lipid Tail Oxidation in the Cell Membrane Revealed through Integrated Simulations and Experiments. Sci. Rep. 2017, 7, 5761. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef]
- Solís-Calero, C.; Ortega-Castro, J.; Frau, J.; Muñoz, F. Nonenzymatic Reactions above Phospholipid Surfaces of Biological Membranes: Reactivity of Phospholipids and Their Oxidation Derivatives. Oxid. Med. Cell. Longev. 2015, 2015, 319505. [Google Scholar] [CrossRef] [PubMed]
- Martín, V.; Fabelo, N.; Santpere, G.; Puig, B.; Marín, R.; Ferrer, I.; Díaz, M. Lipid Alterations in Lipid Rafts from Alzheimer’s Disease Human Brain Cortex. J. Alzheimers. Dis. 2010, 19, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Gehman, J.D.; O’Brien, C.C.; Shabanpoor, F.; Wade, J.D.; Separovic, F. Metal Effects on the Membrane Interactions of Amyloid-β Peptides. Eur. Biophys. J. 2008, 37, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, C.; Klimov, D.K. Alzheimer’s Aβ10–40 Peptide Binds and Penetrates DMPC Bilayer: An Isobaric–Isothermal Replica Exchange Molecular Dynamics Study. J. Phys. Chem. B. 2014, 118, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, M.F.M.; Kotler, S.A.; Brender, J.R.; Chen, J.; Lee, D.-K.; Ramamoorthy, A. Two-Step Mechanism of Membrane Disruption by Aβ through Membrane Fragmentation and Pore Formation. Biophys. J. 2012, 103, 702–710. [Google Scholar] [CrossRef]
- Jang, H.; Zheng, J.; Nussinov, R. Models of β-Amyloid Ion Channels in the Membrane Suggest That Channel Formation in the Bilayer Is a Dynamic Process. Biophys. J. 2007, 93, 1938–1949. [Google Scholar] [CrossRef]
- Sepehri, A.; Lazaridis, T. Putative Structures of Membrane-Embedded Amyloid β Oligomers. ACS Chem. Neurosci. 2023, 14, 99–110. [Google Scholar] [CrossRef]
- Supnet, C.; Bezprozvanny, I. The Dysregulation of Intracellular Calcium in Alzheimer Disease. Cell. Calcium 2010, 47, 183–189. [Google Scholar] [CrossRef]
- LaFerla, F.M. Calcium Dyshomeostasis and Intracellular Signalling in Alzheimer’s Disease. Nat. Rev. Neurosci. 2002, 3, 862–872. [Google Scholar] [CrossRef]
- Kawahara, M.; Negishi-Kato, M.; Sadakane, Y. Calcium Dyshomeostasis and Neurotoxicity of Alzheimer’s β-Amyloid Protein. Expert. Rev. Neurother. 2009, 9, 681–693. [Google Scholar] [CrossRef]
- Chami, M. Calcium Signalling in Alzheimer’s Disease: From Pathophysiological Regulation to Therapeutic Approaches. Cells 2021, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Yusupov, M.; Bogaerts, A.; Cordeiro, R.M. Molecular Dynamics Simulations of Mechanical Stress on Oxidized Membranes. Biophys. Chem. 2019, 254, 106266. [Google Scholar] [CrossRef] [PubMed]
- Haluska, C.K.; Baptista, M.S.; Fernandes, A.U.; Schroder, A.P.; Marques, C.M.; Itri, R. Photo-Activated Phase Separation in Giant Vesicles Made from Different Lipid Mixtures. Biochim. Biophys. Acta 2012, 1818, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-Functional Approximation for the Correlation Energy of the Inhomogeneous Electron Gas. Phys. Rev. B. Condens. Matter 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary Basis Sets for Main Row Atoms and Transition Metals and Their Use to Approximate Coulomb Potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic Structure Calculations on Workstation Computers: The Program System Turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets of Triple Zeta Valence Quality for Atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Bertini, L.; Bruschi, M.; Romaniello, M.; Zampella, G.; Tiberti, M.; Barbieri, V.; Greco, C.; La Mendola, D.; Bonomo, R.P.; Fantucci, P.; et al. Copper Coordination to the Putative Cell Binding Site of Angiogenin: A DFT Investigation. In Highlights in Theoretical Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; pp. 255–269. [Google Scholar]
- Klamt, A.; Schüürmann, G. COSMO: A New Approach to Dielectric Screening in Solvents with Explicit Expressions for the Screening Energy and Its Gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]


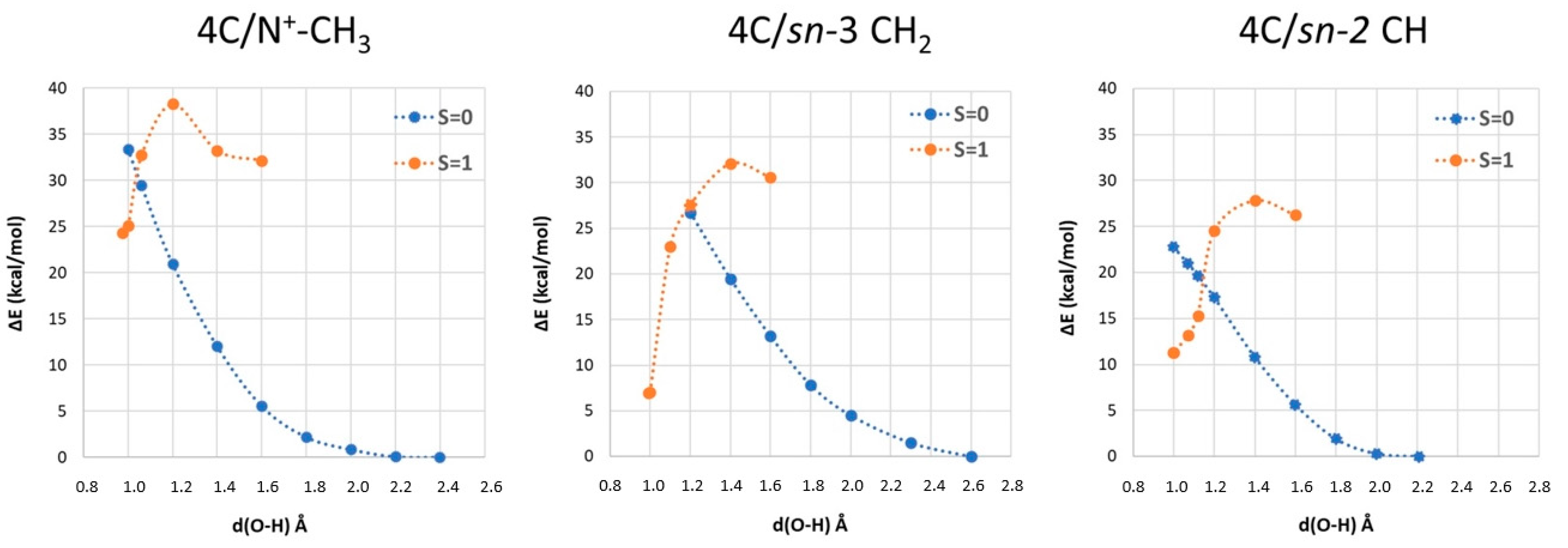
| Cu(II) Coordination | Attack Site | ΔGb | ΔG‡ | ΔGr |
|---|---|---|---|---|
| 4C | N-CH3 | −2.9 | 26.9 | 22.8 |
| CH2 | 6.8 | 23.6 | 5.3 | |
| CH | 3.7 | 15.1 | 6.9 | |
| 5C | CH | 0 | 15.9 | −1.2 |
| 4C | 16:0 | 8.5 | 16.6 | 16.2 |
| 18:1 | 6.3 | 11.7 | −1.4 | |
| 18:2 | 4.5 | 2.7 | −15.4 | |
| 5C | 16:0 | 11.4 | 10.6 | 11.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rovetta, A.; Carosella, L.; Arrigoni, F.; Vertemara, J.; De Gioia, L.; Zampella, G.; Bertini, L. Oxidation of Phospholipids by OH Radical Coordinated to Copper Amyloid-β Peptide—A Density Functional Theory Modeling. Inorganics 2023, 11, 227. https://doi.org/10.3390/inorganics11060227
Rovetta A, Carosella L, Arrigoni F, Vertemara J, De Gioia L, Zampella G, Bertini L. Oxidation of Phospholipids by OH Radical Coordinated to Copper Amyloid-β Peptide—A Density Functional Theory Modeling. Inorganics. 2023; 11(6):227. https://doi.org/10.3390/inorganics11060227
Chicago/Turabian StyleRovetta, Alberto, Laura Carosella, Federica Arrigoni, Jacopo Vertemara, Luca De Gioia, Giuseppe Zampella, and Luca Bertini. 2023. "Oxidation of Phospholipids by OH Radical Coordinated to Copper Amyloid-β Peptide—A Density Functional Theory Modeling" Inorganics 11, no. 6: 227. https://doi.org/10.3390/inorganics11060227
APA StyleRovetta, A., Carosella, L., Arrigoni, F., Vertemara, J., De Gioia, L., Zampella, G., & Bertini, L. (2023). Oxidation of Phospholipids by OH Radical Coordinated to Copper Amyloid-β Peptide—A Density Functional Theory Modeling. Inorganics, 11(6), 227. https://doi.org/10.3390/inorganics11060227