One−Step Synthesis of Fe−Based Metal–Organic Framework (MOF) Nanosheet Array as Efficient Cathode for Hybrid Supercapacitors
Abstract
1. Introduction
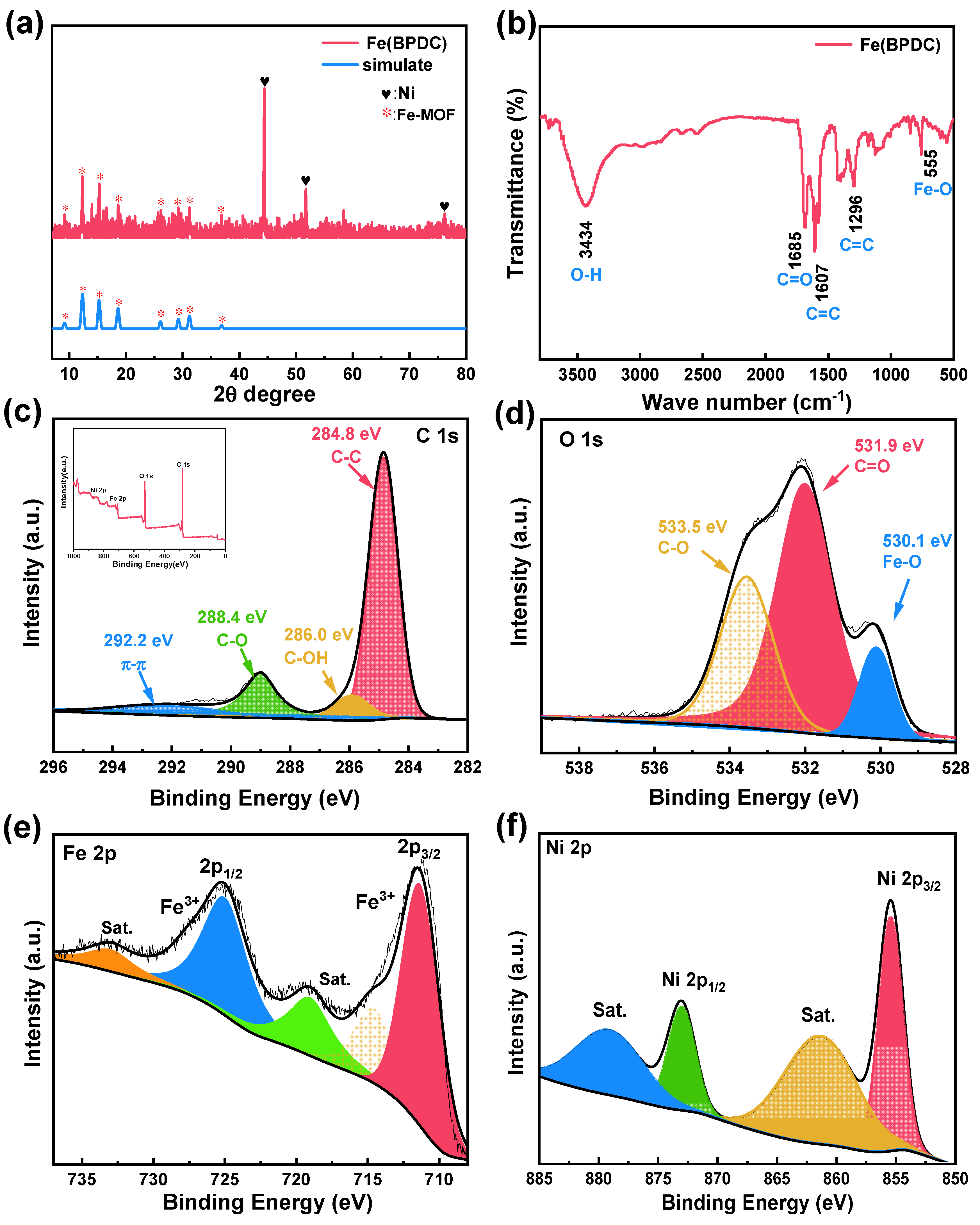
2. Results and Discussion
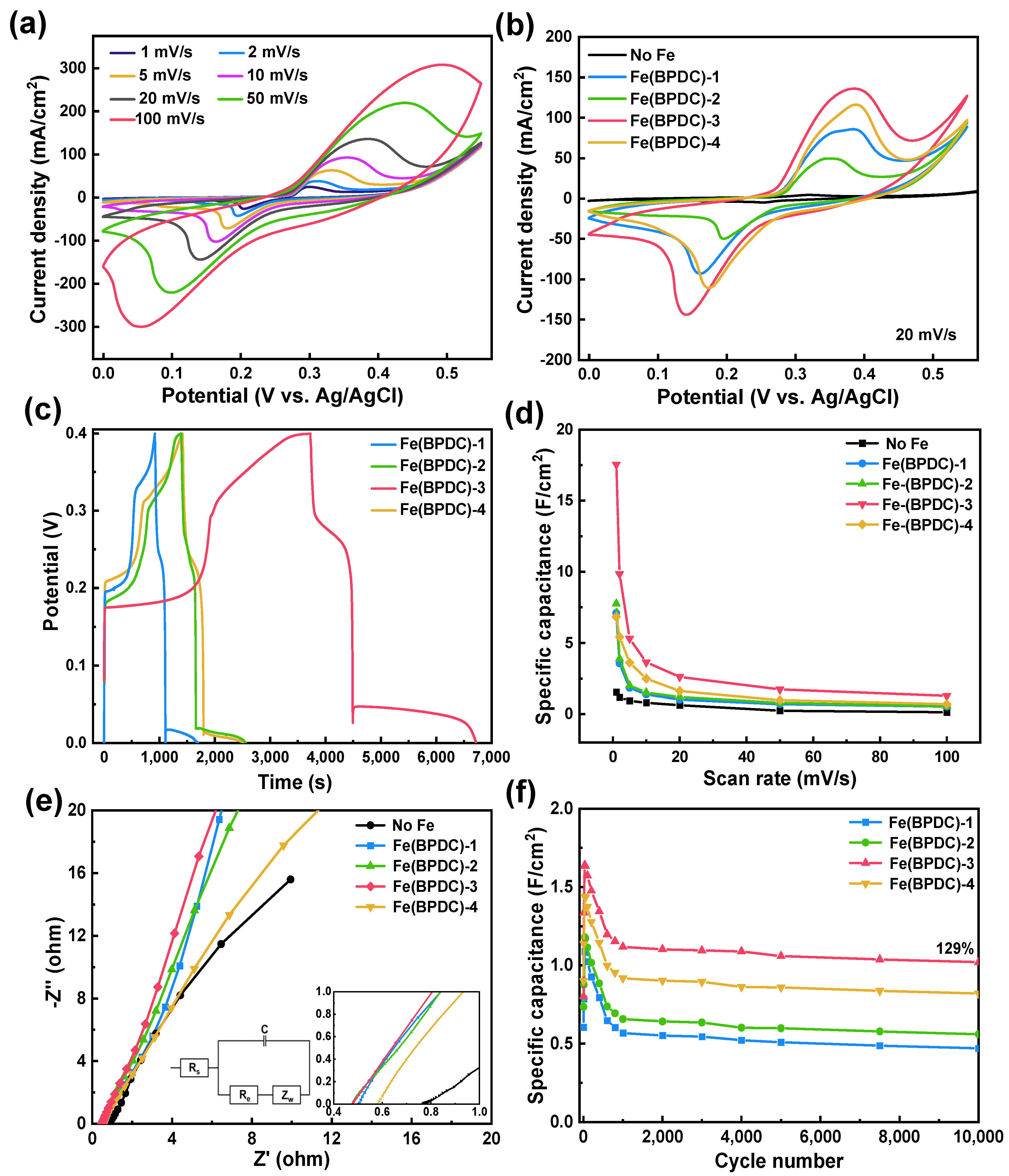
2.1. Electrochemical Performances
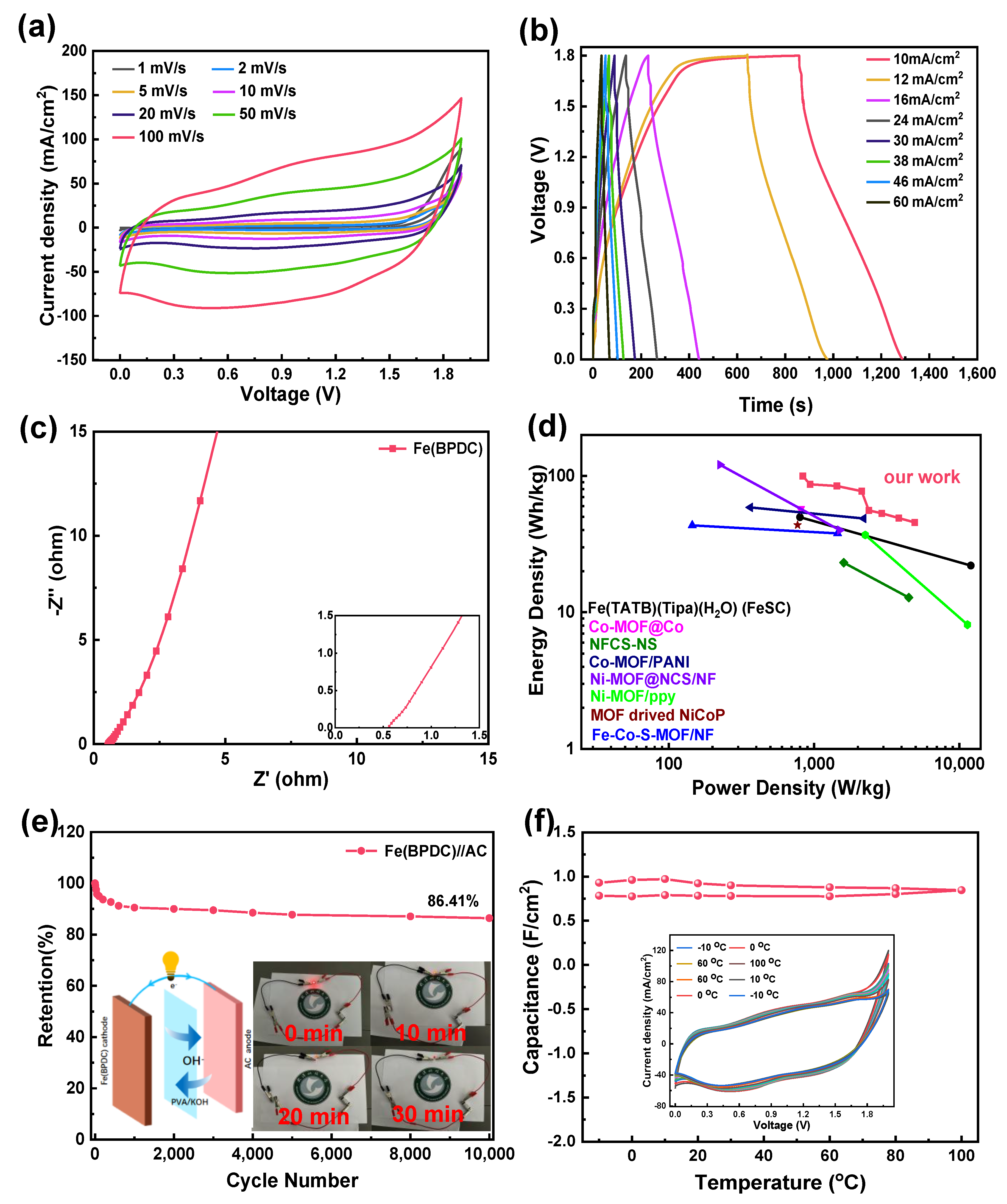
2.2. Fabrication of Hybrid Supercapacitor
3. Materials and Methods
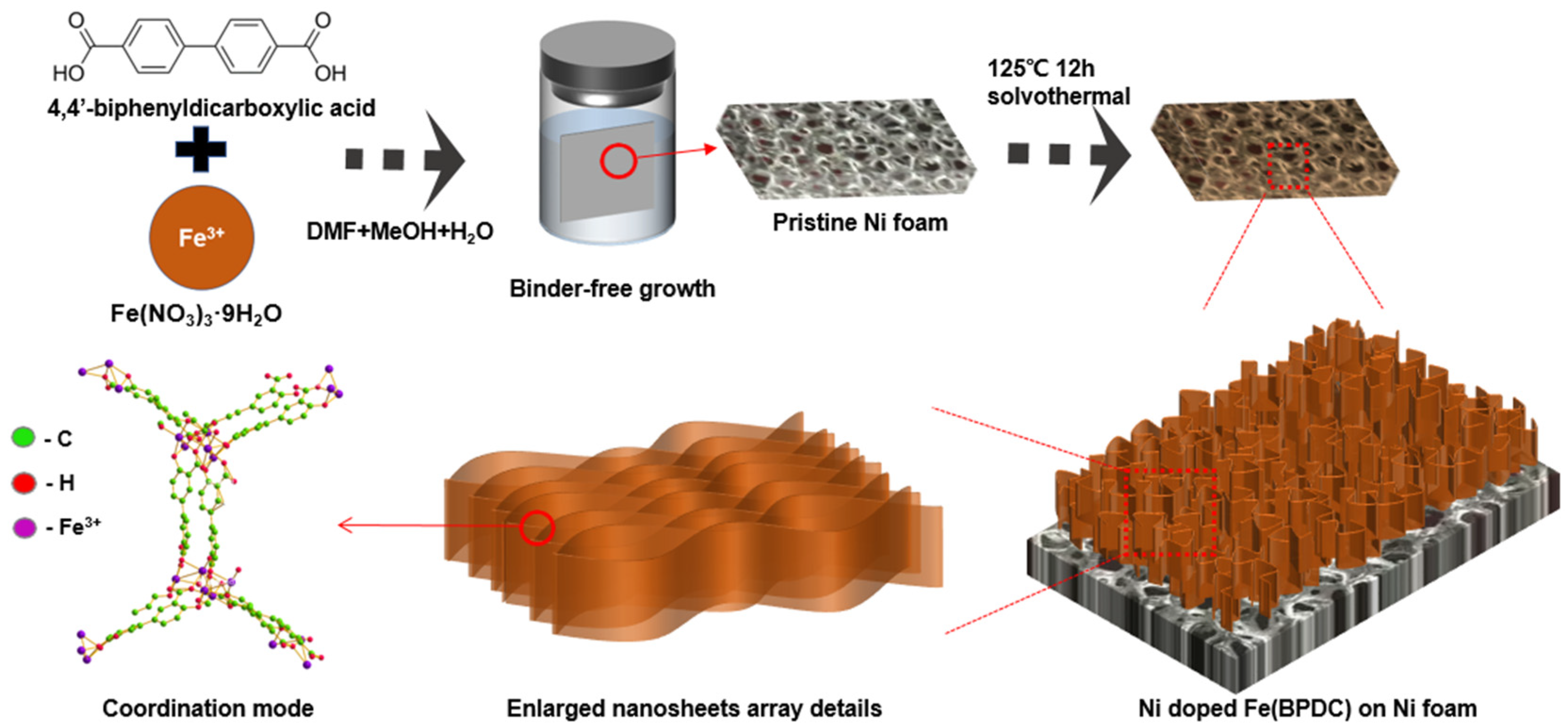
3.1. Synthesis of Fe(BPDC) Nanosheet Arrays Cathode
3.2. Fabrication of Fe(BPDC)//AC Hybrid Supercapacitors
3.3. Morphology and Structure Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, X.F.; Li, G.; Tong, Y. A review of negative electrode materials for electrochemical supercapacitors. Sci. China Technol. Sci. 2015, 58, 1799–1808. [Google Scholar] [CrossRef]
- Mustafa, E.S.; Frede, B.; Ariya, S. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar]
- Wang, H.-Y.; Li, B.; Teng, J.-X.; Zhu, H.-L.; Qi, Y.-X.; Yin, L.-W.; Li, H.; Lun, N.; Bai, Y.-J. N–doped carbon–coated TiN exhibiting excellent electrochemical performance for supercapacitors. Electrochim. Acta 2017, 257, 56–63. [Google Scholar] [CrossRef]
- Li, B.; Pang, H.; Xue, H. Fe–based phosphate nanostructures for supercapacitors. Chin. Chem. Lett. 2020, 32, 885–889. [Google Scholar] [CrossRef]
- Wu, Y.; Ran, F. Vanadium nitride quantum dot/nitrogen–doped microporous carbon nanofibers electrode for high–performance supercapacitors. J. Power Sources 2017, 344, 1–10. [Google Scholar] [CrossRef]
- Nithya, V. A review on holey graphene electrode for supercapacitor. J. Energy Storage 2021, 44, 103380. [Google Scholar] [CrossRef]
- Kumar, R.D.; Nagarani, S.; Sethuraman, V.; Andra, S.; Dhinakaran, V. Investigations of conducting polymers, carbon materials, oxide and sulfide materials for supercapacitor applications: A review. Chem. Pap. 2022, 76, 3371–3385. [Google Scholar] [CrossRef]
- Mani, M.P.; Ponnarasi, K.; Rajendran, A.; Venkatachalam, V.; Thamizharasan, K.; Jothibas, M. Electrochemical Behavior of an Advanced FeCo2O4 Electrode for Supercapacitor Applications. J. Electron. Mater. 2020, 49, 5964–5969. [Google Scholar] [CrossRef]
- Ravikant, A.; Meenakshi, S.; Siddharth, S.; Ashwani, K.; Gaurav, M.; Rabah, B.; Ramesh, C. Metal nitrides as efficient electrode material for supercapacitors: A review. J. Energy Storage 2022, 56, 105912. [Google Scholar]
- Chatterjee, D.P.; Arun, K.N. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880. [Google Scholar]
- Zan, G.T.; Wu, T.; Zhang, Z.L.; Li, J.; Zhou, J.C.; Zhu, F.; Chen, H.X.; Wen, M.; Yang, X.C.; Peng, X.J.; et al. Bi–oinspired Nanocomposites with Self–Adaptive Stress Dispersion for Super–Foldable Electrodes. Adv. Sci. 2021, 9, 2103714. [Google Scholar] [CrossRef] [PubMed]
- Zan, G.; Wu, T.; Dong, W.; Zhou, J.; Tu, T.; Xu, R.; Chen, Y.; Wang, Y.; Wu, Q. Two–Level Biomimetic Designs Enable Intelligent Stress Dispersion for Super–Foldable C/NiS Nanofiber Free–Standing Electrode. Adv. Fiber Mater. 2022, 4, 1177–1190. [Google Scholar] [CrossRef]
- Rasmita, B.; Nafiseh, M.; Kam, T.L.; Mamata, M. Effect of synthesis parameters on tuning of phase and shape of hierarchical iron oxides and selective application as supercapacitor. Ionics 2019, 25, 1793–1803. [Google Scholar]
- O’keeffe, M.; Yaghi, O.M. Deconstructing the Crystal Structures of Metal–Organic Frameworks and Related Materials into Their Underlying Nets. Chem. Rev. 2011, 112, 675–702. [Google Scholar] [CrossRef]
- Jiao, Y.; Pei, J.; Yan, C.S.; Chen, D.H.; Hu, Y.Y.; Chen, G. Layered nickel metal–organicframework for high performance alkaline battery– supercapacitor hybrid deceives. J. Mater. Chem. A 2019, 4, 13344–13351. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal–Organic Framework–Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef]
- Ajdari, F.B.; Kowsari, E.; Shahrak, M.N.; Ehsani, A.; Kiaei, Z.; Torkzaban, H.; Ershadi, M.; Eshkalak, S.K.; Haddadi-Asl, V.; Chinnappan, A.; et al. A review on the field patents and recent developments over the application of metal organic frameworks (MOFs) in supercapacitors. Coord. Chem. Rev. 2020, 422, 213441. [Google Scholar] [CrossRef]
- Jana, S.; Ray, A.; Chandra, A.; Fallah, M.S.E.; Das, S.; Sinha, C. Studies on Magnetic and Dielectric Properties of Antiferromagnetically Coupled Dinuclear Cu(II) in a One–Dimensional Cu(II) Coordination Polymer. ACS Omega 2020, 1, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Tombesi, A. MOFs for Electrochemical Energy Conversion and Storage. Inorganics 2023, 11, 65. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fan, W.; Sun, D. Flexible metal–organic frameworks for gas storage and separation. Dalton Trans. 2022, 51, 4608–4618. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, H.; Mei, H.; Sun, D. Recent progress in metal–organic framework–based supercapacitor electrode materials. Coord. Chem. Rev. 2020, 420, 213438. [Google Scholar] [CrossRef]
- Rajak, R.; Saraf, M.; Mohammad, A.; Mobin, S.M. Design and construction of a ferrocene based inclined polycatenated Co–MOF for supercapacitor and dye adsorption applications. J. Mater. Chem. A 2017, 5, 17998–18011. [Google Scholar] [CrossRef]
- Yan, J.; Liu, T.; Liu, X.D.; Yan, Y.H.; Huang, Y. Metal–organic framework–based materials for flexible supercapacitor application. Coord. Chem. Rev. 2022, 452, 214300. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, C.; Ma, L.; Fu, L.; Li, G.; Hu, Q.; Liu, Q. MOF–derived Fe2O3 decorated with MnO2 nanosheet arrays as anode for high energy density hybrid supercapacitor. Chem. Eng. J. 2021, 417, 129243. [Google Scholar] [CrossRef]
- Ke, F.-S.; Wu, Y.-S.; Deng, H. Metal–organic frameworks for lithium ion batteries and supercapacitors. J. Solid. State Chem. 2015, 223, 109–121. [Google Scholar] [CrossRef]
- Xia, W.; Qu, C.; Liang, Z.; Zhao, B.; Dai, S.; Qiu, B.; Jiao, Y.; Zhang, Q.; Huang, X.; Guo, W.; et al. High–Performance Energy Storage and Conversion Materials Derived from a Single Metal–Organic Framework/Graphene Aerogel Composite. Nano Lett. 2017, 17, 2788–2795. [Google Scholar] [CrossRef]
- Li, M.C.; Wang, W.X.; Yang, M.Y.; Lv, F.C.; Cao, L.J.; Tang, Y.G.; Sun, R.; Lu, Z.G. Large–scale fabrication of porous carbon–decorated iron oxide microcuboids from Fe–MOF as high–performance anode materials for lithium–ion batteries. RSC Adv. 2015, 5, 7356–7362. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q.; Guo, X.; Zhang, S.; Li, W.; Pang, H. Metal organic frameworks and their composites for supercapacitor application. J. Energy Storage 2022, 56, 105819. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, D.; Chu, L.; Wang, J. Enhancement of ionizing radiation–induced catalytic degradation of antibiotics using Fe/C nanomaterials derived from Fe–based MOFs. J. Hazard. Mater. 2020, 389, 122148. [Google Scholar] [CrossRef]
- Raza, N.; Kumar, T.; Singh, V.; Kiml, K.H. Recent advances in bimetallic metal–organic framework as a potentialcandidate for supercapacitor electrode material. Coord. Chem. Rev. 2021, 430, 213660. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yoon, S.J.; Shrestha, N.K.; Lee, S.-H.; Ahn, H.; Han, S.-H. Unusual energy storage and charge retention in Co–based metal–organic–frameworks. Microporous Mesoporous Mater. 2012, 153, 163–165. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, P.; Zheng, C.; Qiu, H.; Wei, M. Metal–organic frameworks: A new promising class of materials for a high performance supercapacitor electrode. J. Mater. Chem. A 2014, 2, 16640–16644. [Google Scholar] [CrossRef]
- Lin, X.; Lai, S.; Fang, G.; Li, X. Nickel(II) Cluster–Based Pillar–Layered Metal–Organic Frameworks for High–Performance Supercapacitors. Inorg. Chem. 2022, 61, 17278–17288. [Google Scholar] [CrossRef]
- Zheng, D.; Wen, H.; Sun, X.; Guan, X.; Zhang, J.; Tian, W.; Feng, H.; Wang, H.; Yao, Y. Ultrathin Mn Doped Ni–MOF Nanosheet Array for Highly Capacitive and Stable Asymmetric Supercapacitor. Chem. A Eur. J. 2020, 26, 17149–17155. [Google Scholar] [CrossRef]
- Gong, Y.; Li, J.; Jiang, P.G.; Li, Q.F.; Lin, J.H. Novel metal(II) coordination polymers based on N,N′–bis–(4–pyridyl)phthalamide as supercapacitor electrode materials in an aqueous electrolyte. Dalton T. 2013, 5, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, C.; Xiong, P.; Li, Y.; Wei, M. Zn–doped Ni–MOF material with a high supercapacitive performance. J. Mater. Chem. A 2014, 2, 19005–19010. [Google Scholar] [CrossRef]
- Li, Z.H.; Tan, M.J.; Zheng, Y.H.; Luo, Y.Y.; Jing, Q.S.; Jiang, J.K.; Li, M.J. Application of Conductive Metal Organic Frameworks in Supercapacitors. J. Inorg. Mater. 2020, 7, 770–780. [Google Scholar]
- Hangarter, C.M.; Dyatkin, B.; Laskoski, M.; Palenik, M.C.; Miller, J.B.; Klug, C.A. A Combined Theoretical and Experimental Characterization of a Zirconium MOF with Potential Application to Supercapacitors. Appl. Magn. Reson. 2022, 53, 915–930. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, C.; Liu, Y.; Li, D.; Chen, W.; Ma, Y.; Wang, C.; Zhang, J. Electrochemical performance and transformation of Co–MOF/reduced graphene oxide composite. Mater. Lett. 2017, 193, 216–219. [Google Scholar] [CrossRef]
- Campagnol, N.; Romero-Vara, R.; Deleu, W. A hybrid supercapacitor based on porous carbon and the metal–organic frame–work MIL–100(Fe). ChemElectroChem 2014, 1, 1182–1188. [Google Scholar] [CrossRef]
- Wang, K.B.; Wang, Z.K.; Wang, X.; Zhou, X.Q.; Tao, Y.H.; Wu, H. Flexible long–chain–linker constructed Ni–based metal–organic frameworks with 1D helical channel and their pseudo–capacitor behavior studies. J. Power Sources 2018, 377, 44–51. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Kim, J.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for metal–organic framework–derived na–noporous carbons toward supercapacitor applications. Acc. Chem. Res. 2016, 12, 2796–2806. [Google Scholar]
- Anaraki, M.A.; Safarifard, A. Fe3O4@MOFs magnetic nanocomposites: Synthesis and applications. Eur. J. Inorg. Chem. 2020, 20, 1916–1937. [Google Scholar] [CrossRef]
- Thakur, B.; Karve, V.V.; Sun, D.T.; Semrau, A.L.; Weiß, L.J.K.; Grob, L.; Fischer, R.A.; Queen, W.L.; Wolfrum, B. An Investi–gation into the Intrinsic Peroxidase–Like Activity of Fe–MOFs and Fe–MOFs/Polymer Composites. Adv. Mater. Technol. 2021, 6, 2001048. [Google Scholar] [CrossRef]
- Bi, S.; Banda, H.; Chen, M.; Niu, L.; Wu, T.; Wang, J.; Wang, R.; Feng, J.; Chen, T.; Dincă, M.; et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes. Nat. Mater. 2020, 19, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.y.; Zhang, X.y.; Wei, L.; Guo, X. MOF–derived porous hollow α–Fe2O3 microboxes modified by silver nanoclusters for enhanced pseudocapacitive storage. Appl. Surf. Sci. 2019, 463, 616–625. [Google Scholar] [CrossRef]
- Sui, Y.; Zhang, D.; Han, Y.; Sun, Z.; Qi, J.; Wei, F.; He, Y.; Meng, Q. Effects of Carbonization Temperature on Nature of Nanostructured Electrode Materials Derived from Fe–MOF for Supercapacitors. Electron. Mater. Lett. 2018, 14, 548–555. [Google Scholar] [CrossRef]
- Bara, D.; Wilson, C.; Mörtel, M.; Khusniyarov, M.M.; Ling, S.L.; Slater, B.; Sproules, S.; Forgan, R.S. Kinetic Control of Inter–penetration in Fe–Biphenyl–4,4#–Dicarboxylate Metal–Organic Frameworks by Coordination and Oxidation Modulation. JACS 2019, 20, 8346–8357. [Google Scholar]
- Pan, L.; Nancy, C.; Huang, X.J.; Li, J. Reactions and Reactivity of Co–bpdc Coordination Polymers (bpdc) 4,4–biphenyldicarboxylate). Inorg. Chem. 2000, 39, 5333–5340. [Google Scholar] [CrossRef]
- Wang, S.E.; Wang, S.S.; Guo, X.; Wang, Z.K.; Mao, F.F.; Su, L.H.; Wu, H.; Wang, K.B.; Zhang, Q.C. An asymmetric superca–pacitor with an interpenetrating crystalline Fe–MOF as the positive electrode and its congenetic derivative as the negative electrode. Inorg. Chem. Front. 2021, 8, 4878. [Google Scholar]
- Jiang, S.; Li, S.; Xu, Y.; Liu, Z.; Weng, S.; Lin, M.; Xu, Y.; Jiao, Y.; Chen, J. An iron based organic framework coated with nickel hydroxide for energy storage, conversion and detection. J. Colloid. Interface Sci. 2021, 600, 150–160. [Google Scholar] [CrossRef]
- Kavian, S.; Hajati, S.; Moradi, M. High–rate supercapacitor based on NiCo–MOF–derived porous NiCoP for efficient energy storage. J. Mater. Sci. Mater. Electron. 2021, 32, 13117–13128. [Google Scholar] [CrossRef]
- Le, K.; Gao, M.J.; Liu, W.; Liu, J.R.; Wang, Z.; Wang, F.L.; Murugadoss, V.; Wu, S.D.; Ding, T.; Guo, Z.H. MOF–derived hier–archical core–shell hollow iron–cobalt sulfides nanoarrays on Ni foam with enhanced electrochemical properties for high energy density asymmetric supercapacitors. Electrochim. Acta 2019, 323, 134826. [Google Scholar] [CrossRef]
- Lee, C.S.; Moon, J.Y.; Park, J.T.; Kim, J.H. Highly Interconnected Nanorods and Nanosheets Based on a Hierarchically Layered Metal Organic Framework for a flexible, high–performance energy storage device. ACS Sustain. Chem. Eng. 2020, 9, 3773–3785. [Google Scholar] [CrossRef]
- Pan, Y.; Shi, C.J.; Chen, Y.J.; Li, D.; Tian, Z.; Guo, L.; Wang, Y.Z. Fishbone–like Ni3S2/Co3S4 integrated with nickel MOF nanosheets for hybrid supercapacitors. Appl. Surf. Sci. 2021, 566, 150744. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, Y.; Zhu, Z.; Han, S.; Zhao, C.; Chen, G. One–pot construction of highly oriented Co–MOF nanoneedle arrays on Co foam for high–performance supercapacitor. Nanotechnology 2021, 32, 395606. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R.; Fari, R.; Afzal, A.M. Co–MOF/polyaniline–based electrode material for high performance asymmetric supercapacitor devices. Electrochim. Acta 2020, 346, 136039. [Google Scholar] [CrossRef]
- Alitabar, K.; Zardkhoshoui, A.M.; Davarani, S.S.H. One–step synthesis of porous Ni–Co–Fe–S nanosheet arrays as an efficient battery–type electrode material for hybrid supercapacitors. Batter. Supercaps 2020, 3, 1311–1320. [Google Scholar] [CrossRef]
- Wang, B.L.; Li, W.; Liu, Z.L.; Duan, Y.J.; Zhao, B.; Wang, Y.; Liu, J.H. Incorporating Ni–MOF structure with polypyrrole: En–hanced capacitive behavior as electrode material for supercapacitor. RSC Adv. 2020, 10, 12129. [Google Scholar] [CrossRef]

| HSC | Voltage Range (V) | Energy Density (Wh/kg) | Power Density (W/kg) | Number of Cycles | Capacitance Retention (%) | Year [Reference] |
|---|---|---|---|---|---|---|
| Fe–ASC | 0–1.7 | 22 | 12,000 | 6000 | 90.6 | 2021 [50] |
| Fe– MOF@Ni(OH)2–20/NF//AC/NF | 0–1.6 | 67.1 | 800 | 4000 | 100 | 2021 [51] |
| NiCoP//AC | 0–1.6 | 58.8 | 781.3 | 10,000 | 96.2 | 2021 [52] |
| Fe–Co–S/NF //rGO | 0–1.6 | 43.6 | 770 | 5000 | 89.6 | 2019 [53] |
| Ni–MOF@NCS/NF//AC | 0–1.7 | 58.8 | 363.7 | 10,000 | 96.2 | 2021 [55] |
| AC//Co–MOF@Co | 0–1.7 | 43.4 | 145.1 | 10,000 | 87.5 | 2021 [56] |
| AC//MOF/PANI | 0–1.6 | 23.11 | 600 | 3000 | 146 | 2020 [57] |
| NFCS–NS//AC | 0–1.6 | 57.3 | 807.04 | 10,000 | 90.8 | 2020 [58] |
| Ni–MOF–ppy//AC | 0–1.5 | 40.1 | 1500.6 | 10,000 | 80 | 2020 [59] |
| Fe(BPDC)//AC | 0–1.9 | 45.64 99.85 | 4919.55 835.1 | 10,000 | 86.41 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yang, L.; Li, R.; Zhou, Y. One−Step Synthesis of Fe−Based Metal–Organic Framework (MOF) Nanosheet Array as Efficient Cathode for Hybrid Supercapacitors. Inorganics 2023, 11, 169. https://doi.org/10.3390/inorganics11040169
Zhao J, Yang L, Li R, Zhou Y. One−Step Synthesis of Fe−Based Metal–Organic Framework (MOF) Nanosheet Array as Efficient Cathode for Hybrid Supercapacitors. Inorganics. 2023; 11(4):169. https://doi.org/10.3390/inorganics11040169
Chicago/Turabian StyleZhao, Jicheng, Liu Yang, Ruizhi Li, and Yingke Zhou. 2023. "One−Step Synthesis of Fe−Based Metal–Organic Framework (MOF) Nanosheet Array as Efficient Cathode for Hybrid Supercapacitors" Inorganics 11, no. 4: 169. https://doi.org/10.3390/inorganics11040169
APA StyleZhao, J., Yang, L., Li, R., & Zhou, Y. (2023). One−Step Synthesis of Fe−Based Metal–Organic Framework (MOF) Nanosheet Array as Efficient Cathode for Hybrid Supercapacitors. Inorganics, 11(4), 169. https://doi.org/10.3390/inorganics11040169







