Elimination of Arsenic Using Sorbents Derived from Chitosan and Iron Oxides, Applying Factorial Designs
Abstract
:1. Introduction
1.1. Use and Pollution
1.2. Arsenic as a Regional Problem
2. Results
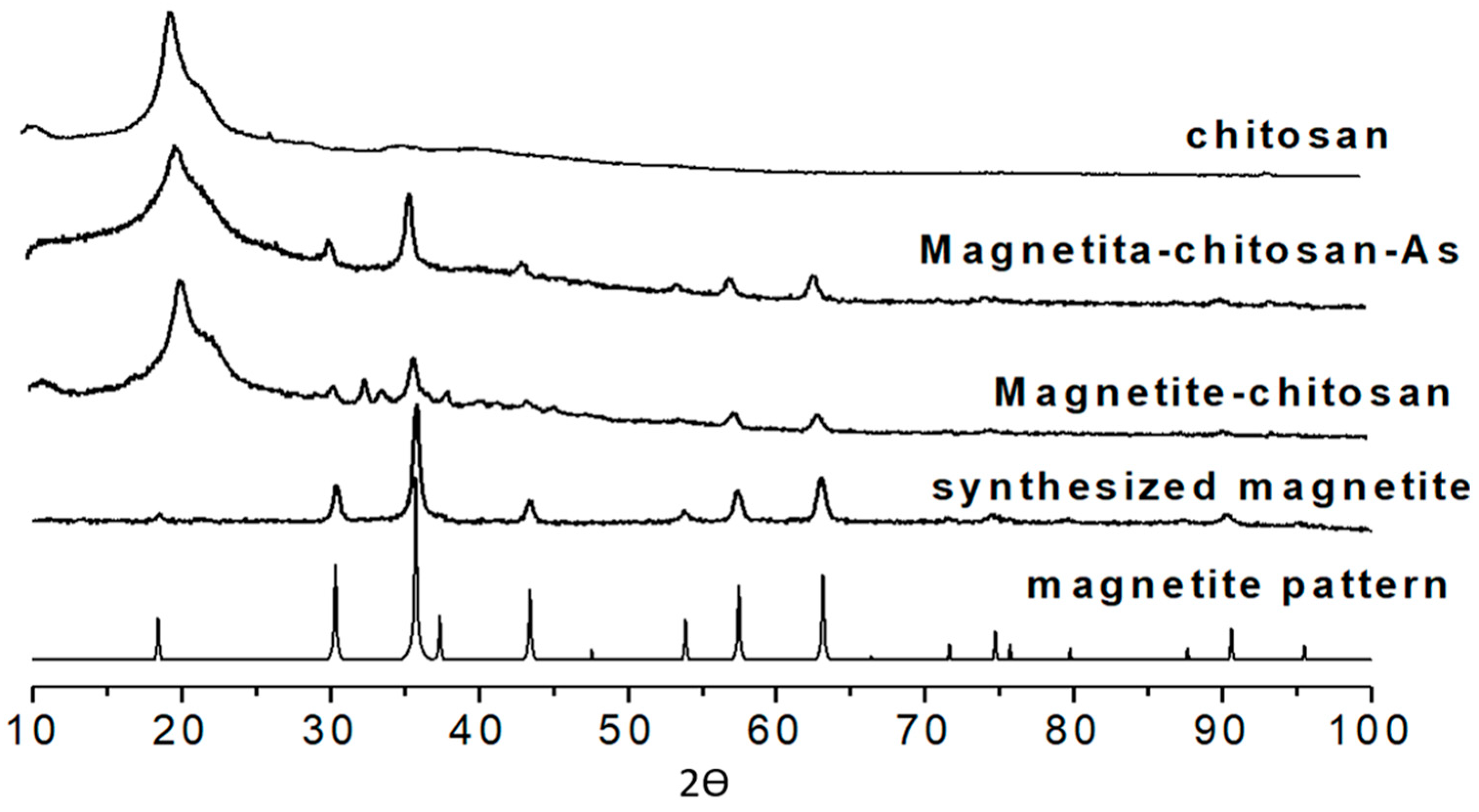
2.1. FT-IR and XRD Spectroscopy
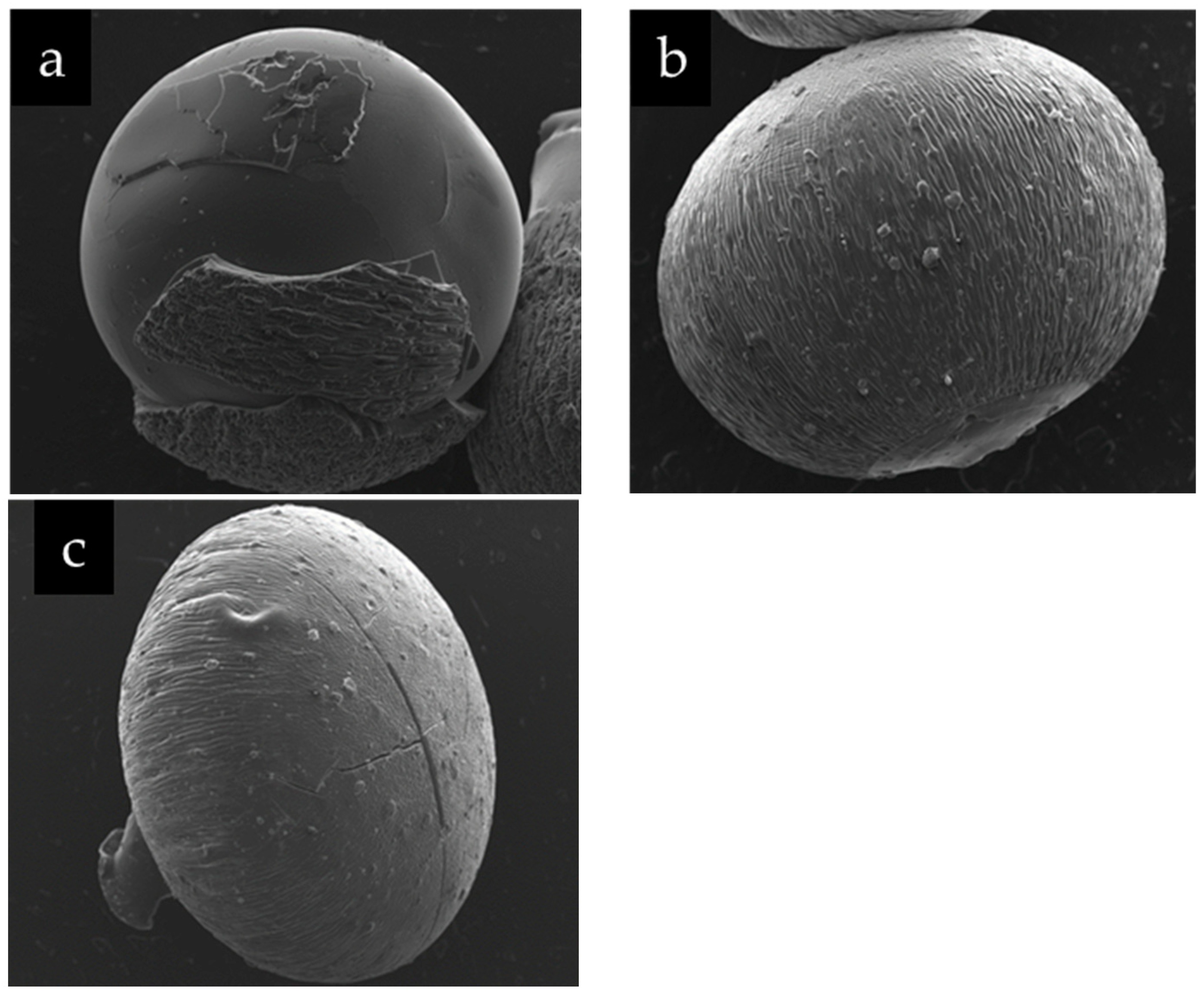
2.2. Scanning Electron Microscope (SEM)
2.3. pH Value at Zero Charge Point (pHpzc)
2.4. As(V) Detection and Quantification
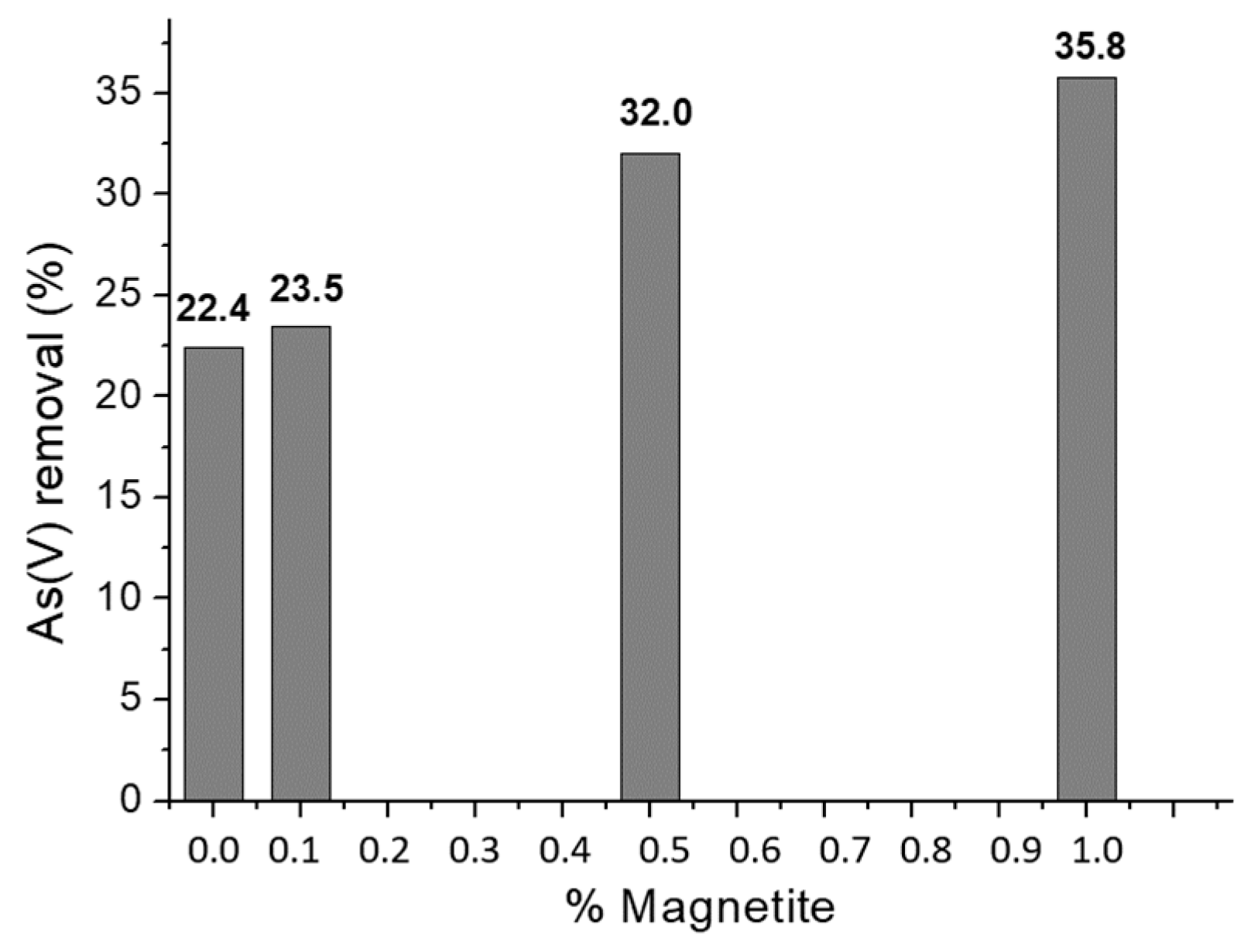
2.5. Effect of the Amount of Magnetite on As(V) Sorption
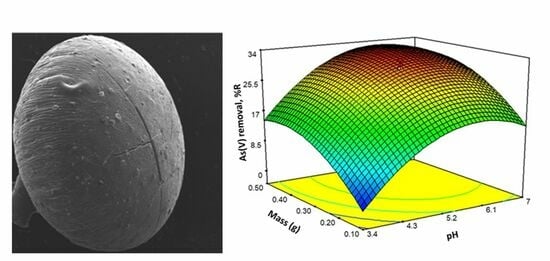
2.6. Experimental Design and Optimization of the Sorption Process
2.7. Kinetic Studies
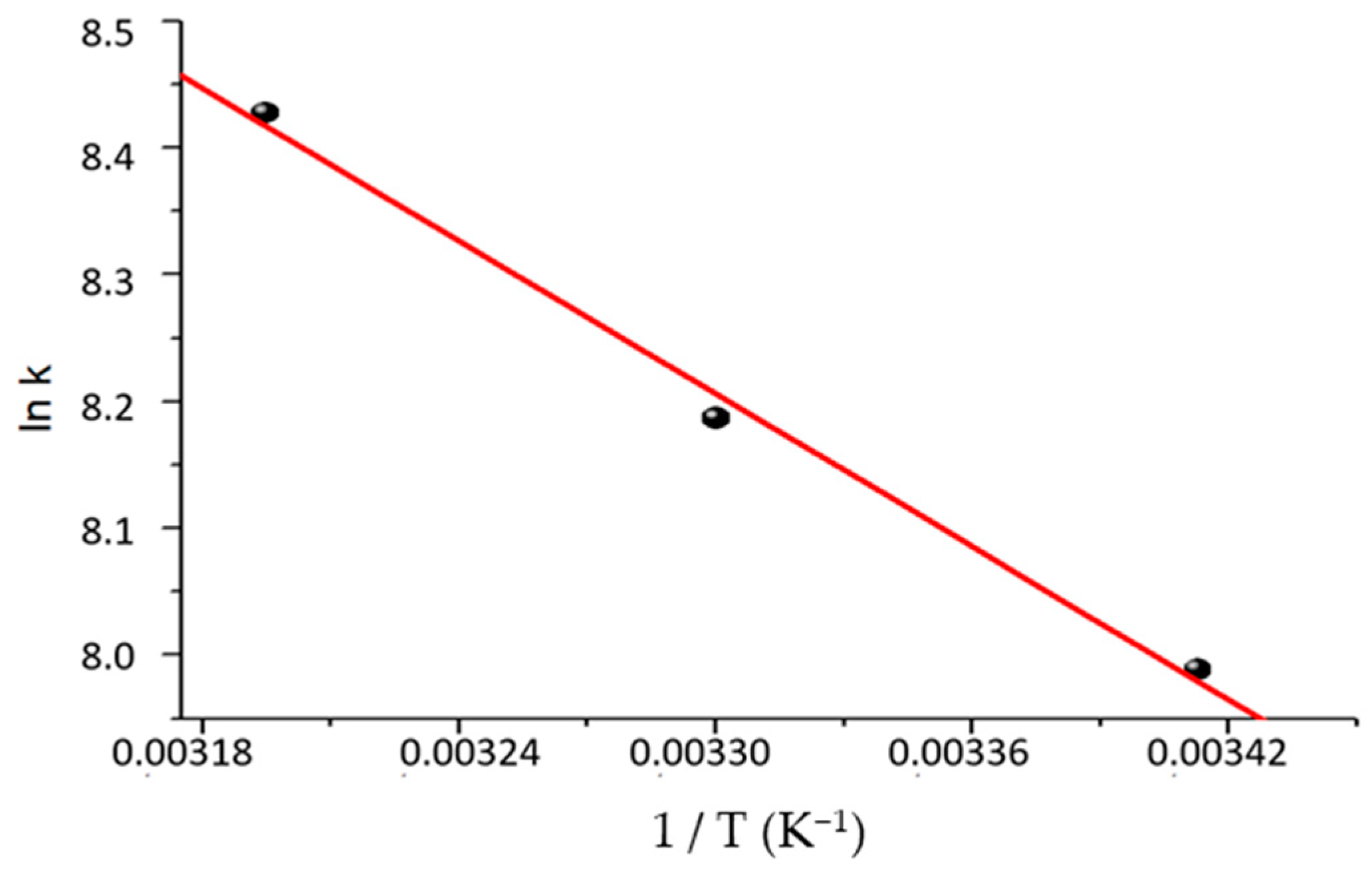
2.8. Thermodynamic Study
2.9. Desorption Studies
3. Materials and Methods
3.1. Obtaining Organic–Inorganic Hybrid Sorbents
3.1.1. Synthesis of Magnetite
3.1.2. Synthesis of Magnetite–Chitosan Spheres
3.2. Sorbent Characterization
3.2.1. FT-IR and XRD Spectroscopy
3.2.2. Scanning Electron Microscope
3.2.3. Determination of the pHpzc
3.3. As(V) Detection and Quantification
3.4. Effect of the Amount of Magnetite on the Sorption of As(V)
3.5. Sorption Process Optimization
3.6. Sorption Kinetic Studies
3.7. Determination of Activation Energy
3.8. Sorption Isotherms
3.8.1. Modified Langmuir Model
3.8.2. Freundlich Model
3.8.3. Dubinin Radushkevich Model
3.9. Desorption Studies
4. Conclusions
- A chitosan magnetite hybrid sorbent has been synthesized for the remediation of As and characterized using XRD, FT-IR and SEM spectroscopies. The XRD and FT-IR spectroscopy showed the characteristic bands of the components of the hybrid sorbent: magnetite and chitosan. The presence of As on the sorbent is observed in the FT-IR spectrum (521 cm−1) and in the broadening of the bands in XRD.
- The hybrid sorbent has sufficient stability to be used in batches and an adequate retention capacity comparable with similar materials. The pH and mass of magnetite were optimized through experimental designs. The qmax value obtained using equilibrium studies suggests that the sorption process is favorable, in agreement with the thermodynamics parameters.
- The sorption end activation energy indicates that the sorption mechanism is chemical ion exchange.
- The value of qmax increases with increasing temperature according to the endothermic nature of the sorption process.
- The hybrid sorbent has sufficient stability to be used in batches in three sorption/desorption cycles without modifying its structure and integrity, yielding similar removal percentages.
- From an environmental point of view, we highlight the use of environmentally friendly sorbents for As(V) sorption and an inexpensive and straightforward spectrophotometric method with an appropriate sensitivity, limit detection and quantification.
- This work can be described as a starting point for removing arsenic, combining the elimination process of the contaminant with a simple analytical methodology for As determination that can be applied to assist the country’s most deprived areas. This is the primary purpose of this research.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- International Agency for Research on Cancer (IARC). Some Drinking-Water Disinfectants and Contaminants, Including Arsenic. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 84; World Health Organization: Lyon, France, 2004; Volume 84, pp. 1–477. [Google Scholar]
- Risk Information System Division. United States Environmental Protection Agency (USEPA). Arsenic, Inorganic (CASRN 7440-38-2). Integrated Risk Information System (IRIS) U.S. Environmental Protection Agency Chemical, Assessment Summary, National Center for Environmental Assessment. 1998. Available online: http://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0278_summary.pdf (accessed on 25 October 2023).
- Argos, M.; Kalra, T.; Pierce, B.L.; Chen, Y.; Parvez, F.; Islam, T.; Ahmed, A.; Hasan, R.; Hasan, K.; Sarwar, G.; et al. A prospective study of arsenic exposure from drinking water and incidence of skin lesions in Bangladesh. Am. J. Epidemiol. 2011, 174, 185–194. [Google Scholar] [CrossRef]
- Marshall, G.; Ferreccio, C.; Yuan, Y.; Bates, M.N.; Steinmaus, C.; Selvin, S.; Liaw, J.; Smith, A.H. Fifty-Year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J. Natl. Cancer Inst. 2007, 99, 920–928. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Claesson, M.; Bundschuh, J.; Sracek, O.; Fagerberg, J.; Jacks, G.; Martin, R.A.; Storniolo, A.d.R.; Thir, J.M. Distribution and mobility of arsenic in the Río Dulce alluvial aquifers in Santiago del Estero Province, Argentina. Sci. Total Environ. 2006, 358, 97–120. [Google Scholar] [CrossRef]
- Dubey, B.; Solo-Gabriele, H.M.; Townsend, T.G. Quantities of arsenic-treated wood in demolition debris generated by Hurricane Katrina. Environ. Sci. Technol. 2007, 41, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Umans, J.G.; Howard, B.V.; Goessler, W.; Francesconi, K.A.; Crainiceanu, C.M.; Silbergeld, E.K.; Guallar, E. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: The strong heart study. Environ. Health Perspect. 2009, 117, 1428–1433. [Google Scholar] [CrossRef]
- Agency for Toxic Substance and Disease Registry (ATSDR). Toxicological Profile for Arsenic U.S. Department of Health and Humans Services; Public Health Humans Services, Centers for Diseases Control: Washington, DC, USA, 2007. [Google Scholar]
- Litter, M.I. Actualización. La problemática del arsénico en la Argentina: El HACER. Rev. Soc. Argent. Endocrinol. Ginecol. Reprod. 2010, XVII, 5–10. [Google Scholar]
- Carrera, A.P.; Cayla, C.; Fabre, J.; Cirelli, A.F. Tecnologías Económicas para el Abatimiento de Arsénico en Aguas January 2010. Available online: https://www.researchgate.net/publication/292257365_Tecnologias_economicas_para_el_abatimiento_de_arsenico_en_aguas/citations (accessed on 25 October 2023).
- Bardach, A.E.; Ciapponi, A.; Soto, N.; Chaparro, M.R.; Calderon, M.; Briatore, A.; Cadoppi, N.; Tassara, R.; Litter, M.I. Epidemiology of chronic disease related to arsenic in Argentina: A systematic review. Sci. Total Environ. 2015, 538, 802–816. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; p. 564. [Google Scholar]
- Ning, R.Y. Arsenic removal by reverse osmosis. Desalination 2002, 143, 237–241. [Google Scholar] [CrossRef]
- Madhura, L.; Kanchi, S.; Sabela, M.I.; Singh, S.; Bisetty, K.; Inamuddin. Membrane technology for water purification. Environ. Chem. Lett. 2018, 16, 343–365. [Google Scholar] [CrossRef]
- Gabris, M.A.; Rezania, S.; Rafieizonooz, M.; Khankhaje, E.; Devanesan, S.; AlSalhi, M.S.; Aljaafreh, M.J.; Shadravan, A. Chitosan magnetic graphene grafted polyaniline doped with cobalt oxide for removal of Arsenic(V) from water. Environ. Res. 2022, 207, 112209. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, N.; Li, P.-Y.; Liu, B.; Lai, H.-J.; Jin, T. One-Step Preparation of Chitosan-Based Magnetic Adsorbent and Its Application to the Adsorption of Inorganic Arsenic in Water. Molecules 2021, 26, 1785. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Sun, S.; Xu, K.; Zhao, W.; Hao, R.; Zhang, J.; Li, D. Adsorption of As(V) by magnetic alginate-chitosan porous beads based on iron sludge. J. Clean. Prod. 2022, 358, 2022. [Google Scholar] [CrossRef]
- Batistelli, M.; Mora, B.P.; Mangiameli, F.; Mamana, N.; Lopez, G.; Goddio, M.F.; Bellú, S.; González, J.C. A continuous method for arsenic removal from groundwater using hybrid biopolymer-iron-nanoaggregates: Improvement through factorial designs. J. Chem. Technol. Biotechnol. 2021, 96, 923–929. [Google Scholar] [CrossRef]
- Litter, M.I.; Ingallinella, A.M.; Olmos, V.; Savio, M.; Difeo, G.; Botto, L.; Torres, E.M.F.; Taylor, S.; Frangie, S.; Herkovits, J.; et al. Arsenic in Argentina: Occurrence, human health, legislation and determination. Sci. Total Environ. 2019, 676, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Lugo, V.; Barrera-Díaz, C.; Ureña-Núñez, F.; Bilyeu, B.; Hernández, I. Biosorption of Cr(III) and Fe(III) in single and binary systems onto pretreated orange peel. J. Environ. Manag. 2012, 112, 120–127. [Google Scholar] [CrossRef]
- Yasmeen, S.; Kabiraz, M.; Saha, B.; Qadir; Gafur; Masum, S. Chromium (VI) Ions Removal from Tannery Effluent using Chitosan-Microcrystalline Cellulose Composite as Adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Castaldi, P.; Silvetti, M.; Enzo, S.; Melis, P. Study of sorption processes and FT-IR analysis of arsenate sorbed onto red muds (a bauxite ore processing waste). J. Hazard. Mater. 2010, 175, 172–178. [Google Scholar] [CrossRef]
- Palacios, J.G.; SilvaYumi, M. Síntesis de Nanopartículas de Magnetita Recubiertas de Quitosano para la Adsorción de Cromo Hexavalentel; Escuela Superior Politécnica de Chimborazo: Riobamba, Ecuador, 2023. [Google Scholar]
- Raz, M.; Moztarzadeh, F.; Hamedani, A.; Ashuri, M.; Tahriri, M. Controlled Synthesis, Characterization and Magnetic Properties of Magnetite (Fe3O4) Nanoparticles without Surfactant under N 2 Gas at Room Temperature. Key Eng. Mater. 2012, 493–494, 746–751. [Google Scholar] [CrossRef]
- JCPDS file, PDF No. 65-310.
- de Moraes, M.A.; Cocenza, D.S.; Vasconcellos, F.d.C.; Fraceto, L.F.; Beppu, M.M. Chitosan and alginate biopolymer membranes for remediation of contaminated water with herbicides. J. Environ. Manag. 2013, 131, 222–227. [Google Scholar] [CrossRef]
- Kloster, G.A.; Londoño, O.M.; Pirota, K.R.; Mosiewicki, M.A.; Marcovich, N.E. Design of super-paramagnetic bilayer films based on chitosan and sodium alginate. Carbohydr. Polym. Technol. Appl. 2021, 2, 100083. [Google Scholar] [CrossRef]
- Singh, J.; Srivastava, M.; Dutta, J.; Dutta, P. Preparation and properties of hybrid monodispersed magnetic α-Fe2O3 based chitosan nanocomposite film for industrial and biomedical applications. Int. J. Biol. Macromol. 2011, 48, 170–176. [Google Scholar] [CrossRef]
- Kloster, G.A.; Muraca, D.; Londoño, O.M.; Knobel, M.; Marcovich, N.E.; Mosiewicki, M.A. Structural analysis of magnetic nanocomposites based on chitosan. Polym. Test. 2018, 72, 202–213. [Google Scholar] [CrossRef]
- Currie, L.A. Detection and quantification limits: Origins and historical overview. Anal. Chim. Acta 1999, 391, 127–134. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A. Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Desalin. Water Treat. 2012, 39, 149–157. [Google Scholar] [CrossRef]
- Bertoni, F.A.; Medeot, A.C.; González, J.C.; Sala, L.F.; Bellú, S.E. Application of green seaweed biomass for MoVI sorption from contaminated waters. Kinetic, thermodynamic and continuous sorption studies. J. Colloid Interface Sci. 2015, 446, 122–132. [Google Scholar] [CrossRef]
- Mora, B.E.P.; E Bellú, S.; Mangiameli, M.F.; I García, S.; González, J.C. Optimization of continuous arsenic biosorption present in natural contaminated groundwater. J. Chem. Technol. Biotechnol. 2019, 94, 547–555. [Google Scholar] [CrossRef]
- Zur Lagergren, S. Theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Castellan, G.W. Fisicoquímica; Addison-Wesley Iberoamericana: San Juan, Puerto Rico, 1998. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die, adsorption in losungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the Characteristic Curve of Activated Charcoal Proceedings of the Academy of Sciences. Phys. Chem. Sect. USSR 1947, 55, 331–333. [Google Scholar]
- Azizian, S.; Eris, S.; Wilson, L.D. Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem. Phys. 2018, 513, 99–104. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Introduction to Adsorption Phenomena. In Adsorption, in MWH’s Water Treatment: Principles and Design; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Abe, M.; Kawashima, K.; Kozawa, K.; Sakai, H.; Kaneko, K. Amination of activated carbon and adsorption characteristics of its aminated surface. Langmuir 2000, 16, 5059–5063. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Xing, B. Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ. Sci. Technol. 2006, 40, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]


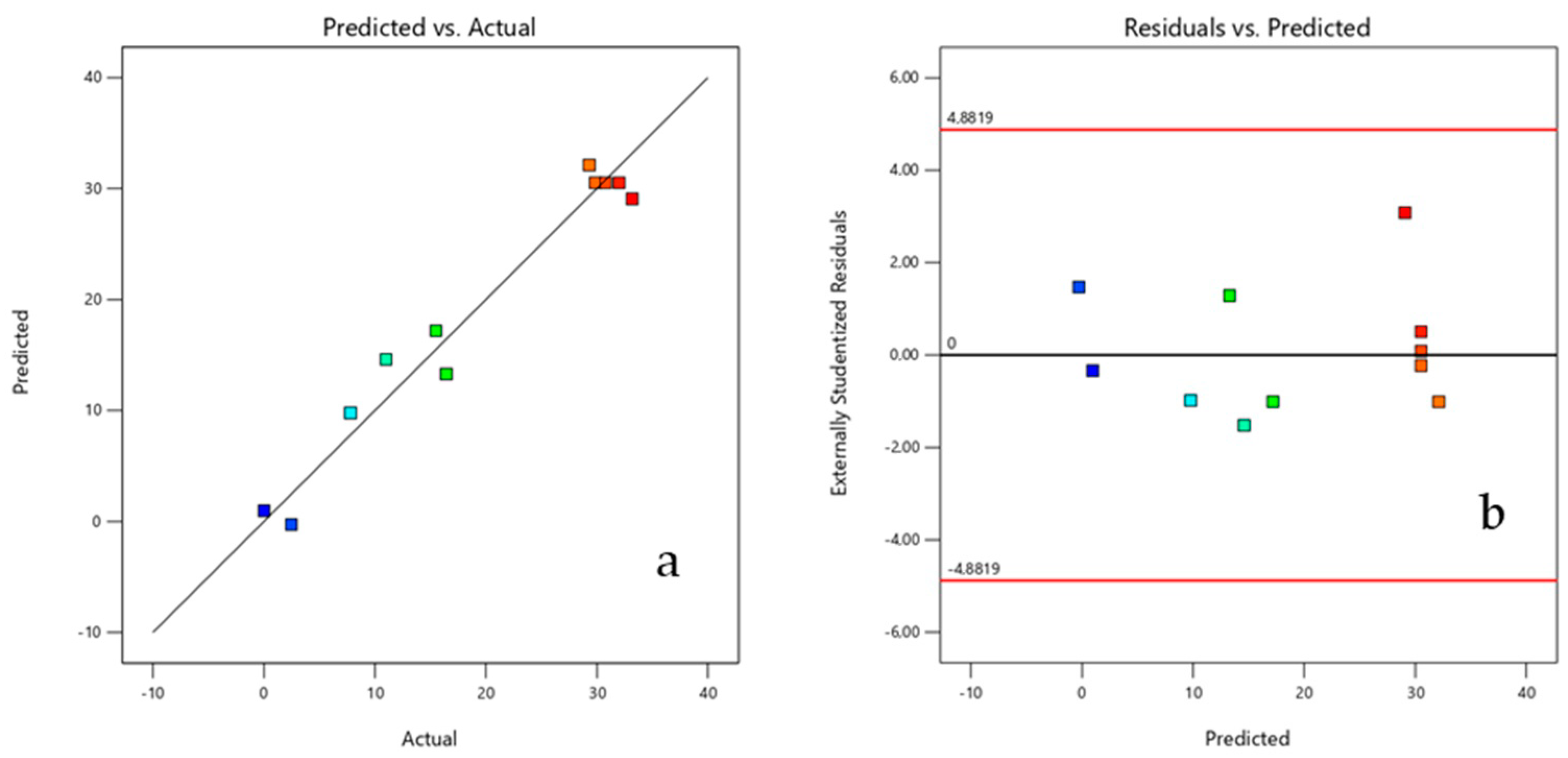
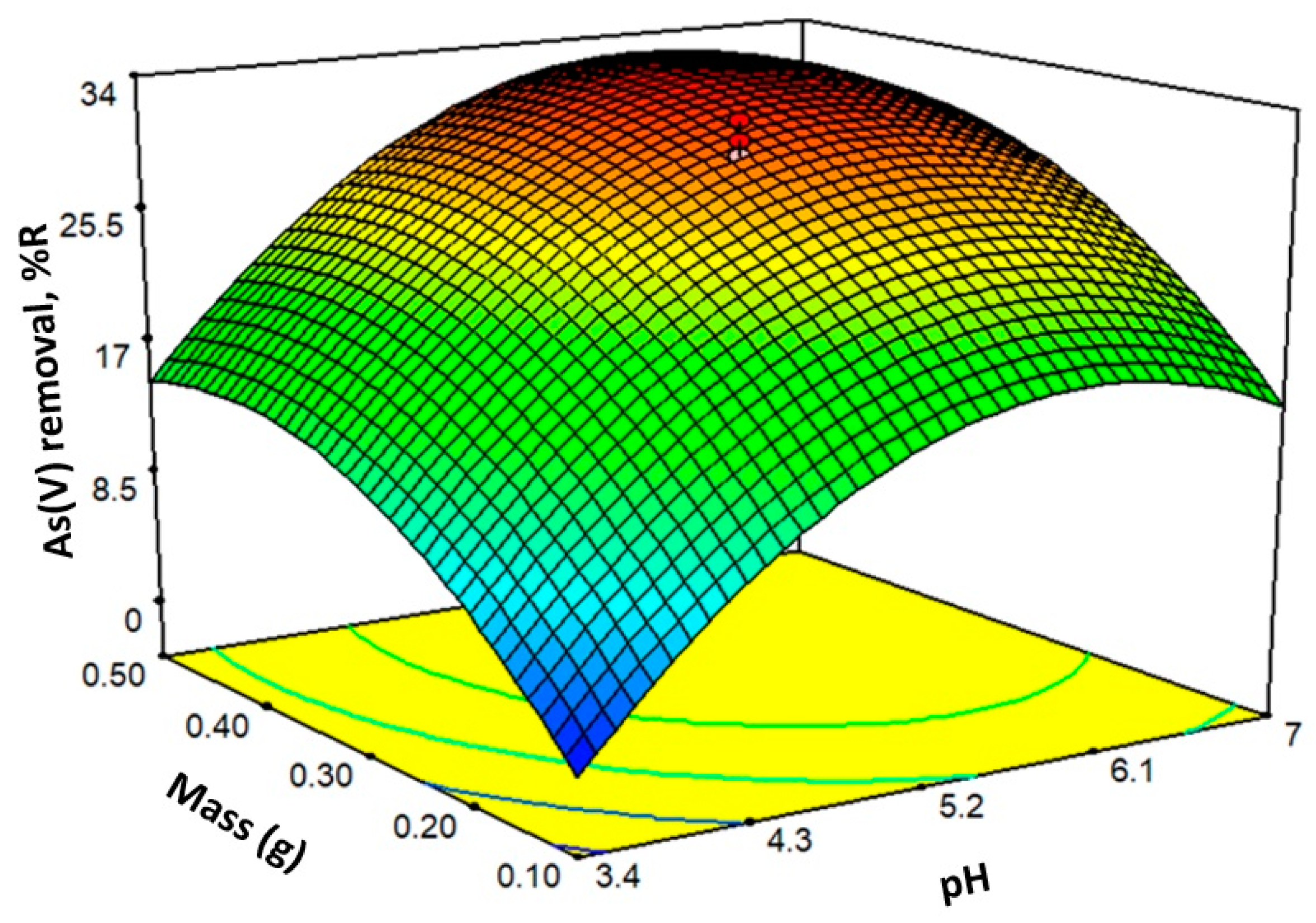
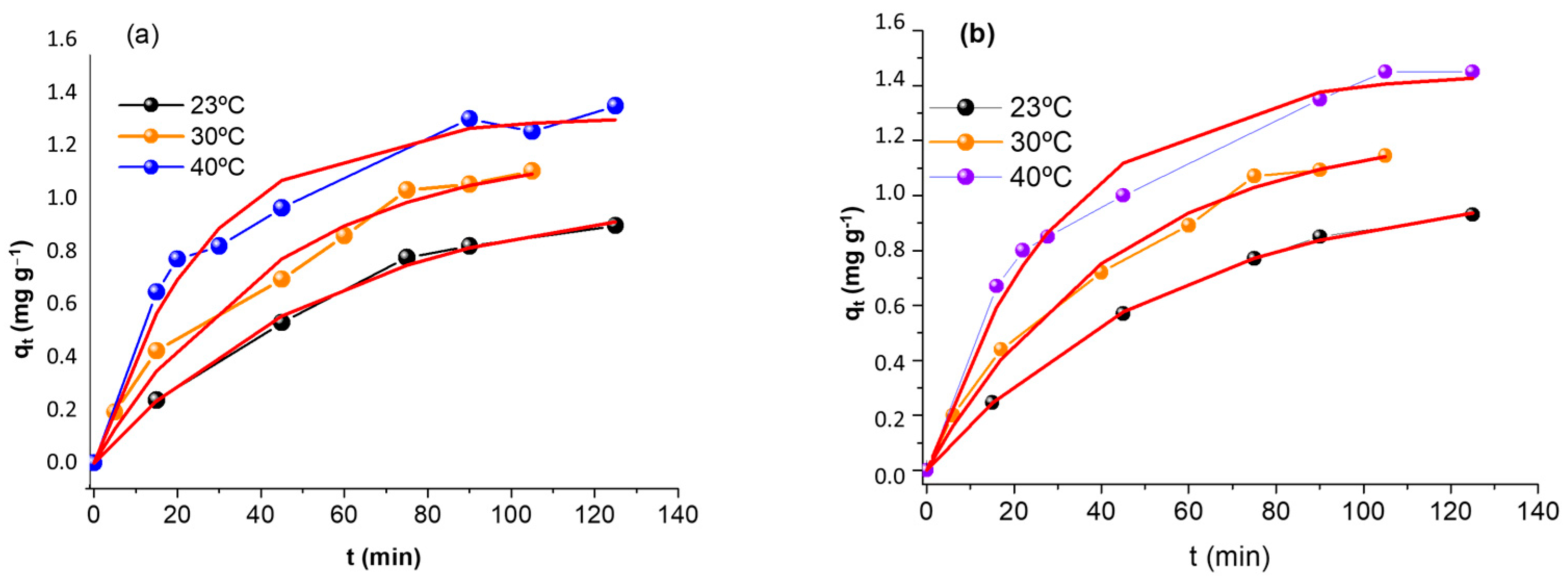
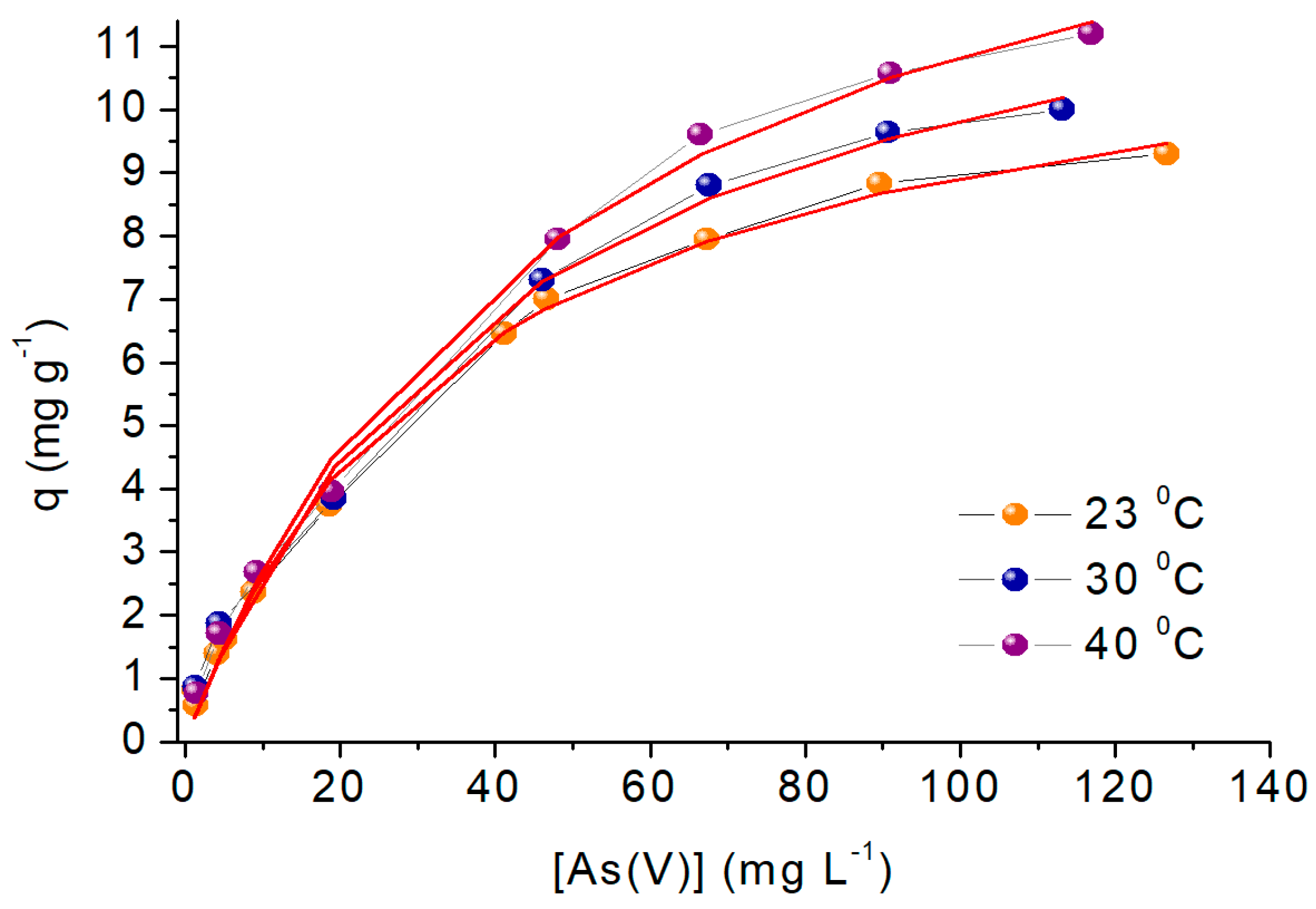
| Run | pH | Mass (g) | R (%) |
|---|---|---|---|
| 1 | 5.40 | 0.30 | 32.00 |
| 2 | 5.40 | 0.30 | 30.79 |
| 3 | 2.60 | 0.30 | 2.47 |
| 4 | 3.40 | 0.10 | 0.01 |
| 5 | 5.40 | 0.58 | 33.18 |
| 6 | 7.40 | 0.50 | 29.31 |
| 7 | 5.40 | 0.02 | 7.80 |
| 8 | 8.20 | 0.30 | 15.50 |
| 9 | 3.40 | 0.50 | 11.00 |
| 10 | 5.40 | 0.30 | 29.86 |
| 11 | 7.40 | 0.10 | 16.43 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Reduced quadratic model | 1509.95 | 4 | 377.49 | 34.55 | 0.0003 | significant |
| A-pH | 1034.32 | 1 | 1034.32 | 94.67 | <0.0001 | |
| B-mass | 365.46 | 1 | 365.46 | 33.45 | 0.0012 | |
| A2 | 669.07 | 1 | 669.07 | 61.24 | 0.0002 | |
| B2 | 169.64 | 1 | 169.64 | 15.53 | 0.0076 | |
| Residual | 65.55 | 6 | 10.93 | |||
| Lack of Fit | 63.25 | 4 | 15.81 | 13.73 | 0.0690 | not significant |
| Pure Error | 2.30 | 2 | 1.15 | |||
| Cor Total | 1575.51 | 10 |
| Std. Dev. | 3.31 | R2 | 0.9584 |
|---|---|---|---|
| Mean | 18.94 | Adjusted R2 | 0.9307 |
| C.V.% | 17.45 | Predicted R2 | 0.7988 |
| Adeq. Precision | 14.543 |
| Model | Parameters | 23 °C | 30 °C | 40 °C |
|---|---|---|---|---|
| pseudo-first order | k1 | 0.0176 ± 0.0026 | 0.0228 ± 0.0025 | 0.0327 ± 0.0033 |
| %E | 14.7 | 11.0 | 10.1 | |
| qt %E | 1.05 ± 0.03 2.6% | 1.25 ± 0.06 4.4% | 1.45 ± 0.06 3.8% | |
| Chi2 | 0.0001 | 0.00127 | 0.0045 | |
| R2 | 0.9996 | 0.9943 | 0.9841 | |
| pseudo-second order | k2 | 0.0093 ± 0.0013 | 0.0120 ± 0.0016 | 0.0186 ± 0.0020 |
| %E | 14.0 | 14.4 | 10.7 | |
| qt %E | 1.50 ± 0.07 8.4% | 1.73 ± 0.09 5.4% | 1.82 ± 0.060 3.3% | |
| Chi2 | 0.0002 | 0.0009 | 0.0021 | |
| R2 | 0.9986 | 0.9960 | 0.9926 |
| Model | 23 °C | 30 °C | 40 °C |
|---|---|---|---|
| Langmuir | |||
| qmax(mg g−1) | 12.2 ± 1.3 | 14.0 ± 1.1 | 15.7 ± 0.6 |
| KLM | 3012 ± 275 | 3593 ± 690 | 4570 ± 424 |
| R2 | 0.9957 | 0.9897 | 0.9972 |
| Χ2 | 0.0558 | 0.1785 | 0.0577 |
| Freundlich | |||
| KF | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.2 |
| n | 2.1 ± 0.2 | 2.0 ± 0.2 | 1.8 ± 0.2 |
| R2 | 0.9629 | 0.9785 | 0.9722 |
| Χ2 | 0.4631 | 0.3462 | 0.5342 |
| Dubinin Radushkevich | |||
| qmax(mg g−1) | 32.2 ± 3.7 | 36.2 ± 5.2 | 46.7 ± 6.7 |
| β(mol2J−2) × 10−9 | 5.16 ± 0.50 | 5.20 ± 0.5 | 5.40 ± 0.46 |
| E(kJ mol−1) | 9.77 ± 0.08 | 8.80 ± 0.05 | 9.60 ± 0.05 |
| R2 | 0.9795 | 0.9854 | 0.9839 |
| T(K) | KLM (Dimensionless) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) |
|---|---|---|---|---|
| 296 | 3012 | −19.7 | 16.7 | 123.3 |
| 303 | 3593 | −20.5 | ||
| 313 | 4570 | −21.9 |
| Desorbent | Concentration (M) | Contact Time (h) | D (%) |
|---|---|---|---|
| Na2SO4 | 1 | 4 | 6.68 |
| NaCl | 0.5 | 4 | 7.14 |
| NaOH | 1 | 4 | 50.44 |
| NaOH | 1 | 20 | 84.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batistelli, M.; Bultri, J.; Hernandez Trespalacios, M.; Mangiameli, M.F.; Gribaudo, L.; Bellú, S.; Frascaroli, M.I.; González, J.C. Elimination of Arsenic Using Sorbents Derived from Chitosan and Iron Oxides, Applying Factorial Designs. Inorganics 2023, 11, 428. https://doi.org/10.3390/inorganics11110428
Batistelli M, Bultri J, Hernandez Trespalacios M, Mangiameli MF, Gribaudo L, Bellú S, Frascaroli MI, González JC. Elimination of Arsenic Using Sorbents Derived from Chitosan and Iron Oxides, Applying Factorial Designs. Inorganics. 2023; 11(11):428. https://doi.org/10.3390/inorganics11110428
Chicago/Turabian StyleBatistelli, Marianela, Julián Bultri, Mayra Hernandez Trespalacios, María Florencia Mangiameli, Lina Gribaudo, Sebastián Bellú, María Inés Frascaroli, and Juan Carlos González. 2023. "Elimination of Arsenic Using Sorbents Derived from Chitosan and Iron Oxides, Applying Factorial Designs" Inorganics 11, no. 11: 428. https://doi.org/10.3390/inorganics11110428
APA StyleBatistelli, M., Bultri, J., Hernandez Trespalacios, M., Mangiameli, M. F., Gribaudo, L., Bellú, S., Frascaroli, M. I., & González, J. C. (2023). Elimination of Arsenic Using Sorbents Derived from Chitosan and Iron Oxides, Applying Factorial Designs. Inorganics, 11(11), 428. https://doi.org/10.3390/inorganics11110428