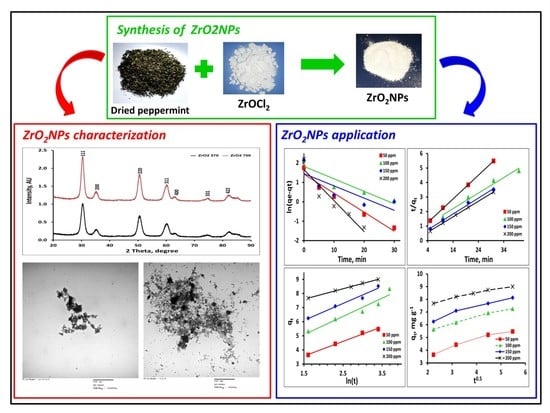
Peppermint-Mediated Green Synthesis of Nano ZrO2 and Its Adsorptive Removal of Cobalt from Water
Abstract
1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. Transmission Electron Microscopy
2.3. BET Surface Area
2.4. Mechanism of ZrO2NPs Green Synthesis
2.5. Adsorption of Co2+ by ZrO2NPs and Optimization of Adsorption Parameters
2.5.1. Effect of pH
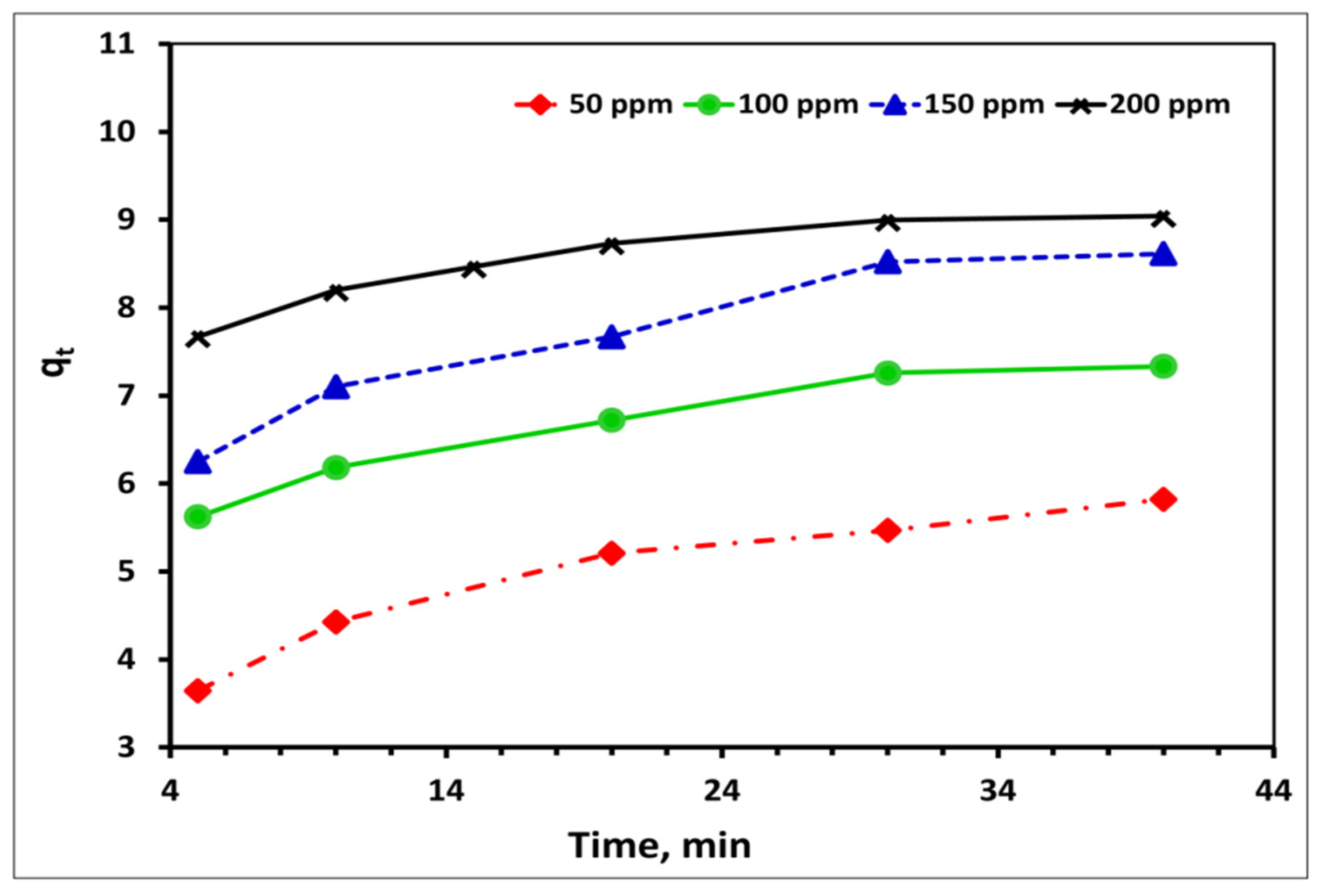
2.5.2. Effect of Contact Time
2.5.3. Effect of Adsorbent Mass
2.5.4. Effect of Initial Co2+ Concentration
2.6. Adsorption Isotherms
2.7. Kinetics of the Adsorption of Co2+ onto ZrO2NPs
3. Materials and Methods
3.1. Synthesis of ZrO2NPs
3.2. Characterization
3.3. Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trejo-Arroyo, D.L.; Acosta, K.E.; Cruz, J.C.; Valenzuela-Muñiz, A.M.; Vega-Azamar, R.E.; Jiménez, L.F. Influence of ZrO2 Nanoparticles on the Microstructural Development of Cement Mortars with Limestone Aggregates. Appl. Sci. 2019, 9, 598. [Google Scholar] [CrossRef]
- Matt, S.B.; Shivanna, M.; Manjunath, S.; Siddalinganahalli, M.; Siddalingappa, D.M. Electrochemical Detection of Serotonin Using t-ZrO2 Nanoparticles Modified Carbon Paste Electrode. J. Electrochem. Soc. 2020, 167, 155512. [Google Scholar] [CrossRef]
- Mok, Z.H.; Proctor, G.; Thanou, M. Emerging nanomaterials for dental treatments. Emerg. Top. Life Sci. 2020, 4, 613–625. [Google Scholar]
- Khan, M.; Shaik, M.R.; Khan, S.T.; Adil, S.F.; Kuniyil, M.; Khan, M.; Al-Warthan, A.A.; Siddiqui, M.R.H.; Tahir, M.N. Enhanced Antimicrobial Activity of Biofunctionalized Zirconia Nanoparticles. ACS Omega 2020, 5, 1987–1996. [Google Scholar] [CrossRef]
- Ashiq, M.N.; Aman, A.; Alshahrani, T.; Iqbal, M.F.; Razzaq, A.; Najam-Ul-Haq, M.; Shah, A.; Nisar, J.; Tyagi, D.; Ehsan, M.F. Enhanced electrochemical properties of silvercoated zirconia nanoparticles for supercapacitor application. J. Taibah Univ. Sci. 2021, 15, 10–16. [Google Scholar] [CrossRef]
- Shelke, G.B.; Patil, D.R. Gas Sensing Performance of Pure and Modified Nanostructured Screen Printed Zirconia Thick Films. Int. J. Eng. Res. Technol. 2019, 8, 198–202. [Google Scholar]
- Chikere, C.O.; Faisal, N.H.; Kong-Thoo-Lin, P.; Fernandez, C. Interaction between Amorphous Zirconia Nanoparticles and Graphite: Electrochemical Applications for Gallic Acid Sensing Using Carbon Paste Electrodes in Wine. Nanomater 2020, 10, 537. [Google Scholar] [CrossRef]
- Annu, A.; Sivasankari, C.; Krupasankar, U. Synthesis and characterization of ZrO2 nanoparticle by leaf extract bioreduction process for its biological studies. Mater. Today Proc. 2020, 33, 5317–5323. [Google Scholar] [CrossRef]
- Demirbas, E. Adsorption of Cobalt(II) Ions from Aqueous Solution onto Activated Carbon Prepared from Hazelnut Shells. Adsor. Sci. Technol. 2003, 21, 951–963. [Google Scholar] [CrossRef]
- Patel, H. Charcoal as an adsorbent for textile wastewater Treatment. Sep. Sci. Technol. 2018, 53, 2797–2812. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, A.K.; Kumar, P.S.; Hoang, T.K.A.; Sekar, K.; Chong, K.Y.; Khoo, K.S.; Ng, H.S.; Show, P.L. A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review. Chemosphere 2022, 303, 135146. [Google Scholar] [CrossRef]
- Zou, W.; Bai, H.; Zhao, L.; Li, K.; Han, R. Characterization and properties of zeolite as adsorbent for removal of uranium (VI) from solution in fixed bed column. J. Nucl. Chem. 2011, 288, 779–788. [Google Scholar] [CrossRef]
- Gua, M.; Haoa, L.; Wang, Y.; Li, X.; Chen, Y.; Li, W.; Jiang, L. The selective heavy metal ions adsorption of zinc oxide nanoparticles from dental wastewater. Chem. Phys. 2020, 534, 110750. [Google Scholar] [CrossRef]
- Roto, R. Surface Modification of Fe3O4 as Magnetic Adsorbents for Recovery of Precious Metals. In Advanced Surface Engineering Research; Chowdhury, M.A., Ed.; Intechopen: London, UK, 2018; pp. 127–145. [Google Scholar] [CrossRef]
- Viana da Silva, A.F.; Fagundes, A.P.; Macuvele, D.L.P.; Urano de Carvalho, E.F.; Durazzo, M.; Padoin, N.; Soares, C.; Riella, H.G. Green synthesis of zirconia nanoparticles based on Eucleanatalensis plant extract: Optimization of reaction conditions and evaluation of adsorptive properties. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123915. [Google Scholar] [CrossRef]
- Navı´o, J.A.; Hidalgo, M.C.; Colo´n, G.; Botta, S.G.; Litter, M.I. Preparation and Physicochemical Properties of ZrO2 and Fe/ZrO2 Prepared by a Sol-Gel Technique. Langmuir 2001, 17, 202–210. [Google Scholar] [CrossRef]
- Gurushantha, K.; Anantharaju, K.S.; Nagabhushana, H.; Sharma, S.C.; Vidya, Y.S.; Shivakumara, C.; Nagaswarupa, H.P.; Prashantha, S.C.; Anilkumar, M.R. Facile green fabrication of iron-doped cubic ZrO2 nanoparticles by Phyllanthusacidus: Structural, photocatalytic and photoluminescent properties. J. Mol. Catal. A Chem. 2015, 397, 36–47. [Google Scholar] [CrossRef]
- Behbahani, A.; Rowshanzamir, S.; Esmaeilifar, A. Hydrothermal synthesis of zirconia nanoparticles from commercial zirconia. Procedia Eng. 2012, 42, 908–917. [Google Scholar] [CrossRef]
- Singh, A.K.; Nakate, U.T. Microwave Synthesis, Characterization and Photoluminiscence Properties of Nanocrystalline Zirconia. Sci. World J. 2014, 2014, 349457. [Google Scholar] [CrossRef]
- Dang, Y.Y.; Bhuiyan, M.S.; Paranthaman, M.P. Zirconium oxide nanostructures prepared by anodic oxidation. U.S. Dep. Energy J. Undergrad. Res. 2008, 8, 48–53. [Google Scholar]
- Zhu, H.Y.; Liu, B.; Shen, M.M.; Kong, Y.; Hong, X.; Hu, Y.H.; Ding, W.P.; Dong, L.; Chen, Y. Effect of maltose for the crystallization of tetragonal zirconia. Mater. Lett. 2004, 58, 3107–3110. [Google Scholar] [CrossRef]
- Nimare, P.; Koser, A.A. Biological Synthesis of ZrO2 Nanoparticle Using Azadirachta Indica Leaf Extract. Int. Res. J. Eng. Technol. 2016, 3, 1910–1912. [Google Scholar]
- Al-Zaqri, N.; Muthuvel, A.; Jothibas, M.; Alsalme, A.; Alharthi, F.A.; Mohana, V. Biosynthesis of zirconium oxide nanoparticles using Wrightiatinctoria leaf extract: Characterization, photocatalytic degradation and antibacterial activities. Inorg. Chem. Commun. 2021, 127, 108507. [Google Scholar] [CrossRef]
- Shinde, H.M.; Bhosale1, T.T.; Gavade, N.L.; Babar, S.B.; Kamble, R.J.; Shirke, B.S.; Garadkar, K.M. Biosynthesis of ZrO2 nanoparticles from Ficusbenghalensis leaf extract for photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 14055–14064. [Google Scholar]
- Majedi, A.; Abbasi, A.; Davar, F. Green synthesis of zirconia nanoparticles using the modified Pechini method and characterization of its optical and electrical Properties. J. Sol-Gel Sci. Technol. 2016, 77, 542–552. [Google Scholar] [CrossRef]
- Shanthi, S.; Tharani, S.S.N. Green Synthesis of Zirconium Dioxide (ZrO2) Nano Particles Using Acalypha Indica Leaf Extract. Int. J. Eng. Appl. Sci. 2016, 3, 23–25. [Google Scholar]
- Hasan, I.M.A.; Tawfik, A.R.; Assaf, F.H. GC/MS screening of buckthorn phytochemicals and their use to synthesize ZnO nanoparticles for photocatalytic degradation of malachite Green dye in water. Water Sci. Technol. 2021, 85, 666. [Google Scholar] [CrossRef]
- Huang, Y.; Haw, C.; Zheng, Z.; Kang, J.; Zheng, J.; Wang, H. Biosynthesis of zinc oxide nanomaterials from plant extracts and future green prospects: A topical review. Adv. Sustain. Syst. 2021, 5, 2000266. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomedi. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Marwa, C.; Fikri-Benbrahim, K.; Ou-Yahia, D.; Farah, A. African peppermint (Menthapiperita) from Morocco: Chemical composition and antimicrobial properties of essential oil. J. Adv. Pharm. Technol. Res. 2017, 8, 86–90. [Google Scholar]
- González-Montaña, J.R.; Escalera, F.; Alonso, A.J.; Lumillos, J.M.; Robles, R.; Alonso, M. Relationship between Vitamin B12 and Cobalt Metabolism in Domestic Ruminant: An Update. Animal 2020, 10, 1855. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Kumar, R.; Thakur, A.; Pori, P.; Kumara, R.; Kanojia, R. Cobalt Toxicity/Poisoning with analytical aspects and its Manaagment. Int. J. Med. Lab. Res. 2019, 4, 29–36. [Google Scholar] [CrossRef]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A.K. Selected aspects of the action of cobalt ions in the human body. Cent. Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Arnaiz, S.; Tate, R.J.; Grant, M.H. Cobalt neurotoxicity: Transcriptional Effect of elevated cobalt blood levels in the rodent brain. Toxics 2022, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J.; Ayres, D.C. Chemical Principles of Environmental Pollution; Chapman and Hall: Oxford, UK, 1993. [Google Scholar]
- Caramalau, C.; Bulgariu, L.; Macobeanu, M. Kinetic study of cobalt adsorption on peat activated by simple chemical treatment. Envron. Eng. Manag. J. 2009, 8, 1351–1358. [Google Scholar]
- Caramalau, C.; Bulgariu, L.; Macobeanu, M. Adsorption Characteristics of Cobalt(II) ions from aqueous solution on Romanian peat moss. Environ. Eng. Manag. J. 2009, 8, 1089–1095. [Google Scholar]
- Basahel, S.N.; Ali1, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Manivasakan, P.; Rajendran, V.; Rauta, P.R.; Sahu, B.B.; Panda, B.K. Synthesis of Monoclinic and Cubic ZrO2 Nanoparticles from Zircon. J. Am. Ceram. Soc. 2011, 94, 1410–1420. [Google Scholar] [CrossRef]
- Andrade, A.B.; Ferreira, N.S.; Valerio, M.E.G. Particle size effects on structural and optical properties of BaF2 nanoparticles. RSC Adv. 2017, 7, 26839. [Google Scholar] [CrossRef]
- Bokuniaeva, A.O.; Vorokh, A.S. Estimation of particle size using the Debye equation and the Scherrer formula for polyphasic TiO2 powder. J. Phys. Conf. Ser. 2019, 1410, 012057. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure. Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.P.; Macena, M.; Esteves, B.; Guiné, R.P.F. Ideal pH for the adsorption of metal ions Cr6+, Ni2+, Pb2+ in aqueous solution with different adsorbent materials. Open Agric. 2021, 6, 115–123. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge VIII. Update. Adv. Colloid Interface Sci. 2020, 275, 102064. [Google Scholar] [CrossRef] [PubMed]
- Al-Maliky, E.A.; Gzar, H.A.; Al-Azawy, M.G. Determination of Point of Zero Charge (PZC) of Concrete Particles Adsorbents. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1184, 012004. [Google Scholar] [CrossRef]
- Abate, G.Y.; Alene, A.N.; Habte, A.T.; Addis, Y.A. Adsorptive removal of basic green dye from aqueous solution using humic acid modified magnetite nanoparticles: Kinetics, equilibrium and thermodynamic studies. J. Polym. Environ. 2021, 29, 967–984. [Google Scholar] [CrossRef]
- Azman, A.; Ngadi, N.; Awg Zaini, D.K.; Jusoh, M.; Mohamad, Z.; Arsad, A. Effect of adsorption parameter on the removal of aspirin using tyre waste adsorbent. Chem. Eng. Trans. 2019, 72, 157–162. [Google Scholar]
- Gulipalli, C.H.S.; Prasad, B.; Wasewar, K.L. Bach study, Equilibrium and kinetics of adsorption of selenium using rich husk ash (RHA). J. Eng. Sci. Technol. 2011, 6, 586–605. [Google Scholar]
- Altintig, E.; Yenigun, M.; Sari, A.; Altundag, H.; Tuzen, M.; Saleh, T.A. Facile synthesis of zinc oxide nanoparticles loaded activated carbon as an eco-friendly adsorbent for ultra-removal of malachite green from water. Environ. Technol. Innov. 2021, 21, 101305. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Arghavan, S.M.A.; Allahyari, E.; Arghavan, F.S.; Othmani, A.; Nasseh, N. Adsorption of malachite green dye onto almond peel waste: A study focusing on application of the ANN approach for optimization of the effect of environmental parameters. Biomass Convers. Biorefin. 2022, 1–13. [Google Scholar] [CrossRef]
- Kim, Y.-H. Adsorption Characteristics of Cobalt on ZrO2 and Al2O3 Adsorbents in High-Temperature Water. Sep. Sci. Technol. 2000, 35, 2327–2341. [Google Scholar] [CrossRef]
- Barrow, G.M. Physical Chemistry, 5th ed.; McGrow-Hill: Singapore, 1988. [Google Scholar]
- Tang, R.; Hong, W.; Srinivasakannan, C.; Liu, X.; Wang, X.; Duan, X. A novel mesoporous Fe-silica aerogel composite with phenomenal adsorption capacity for malachite green. Sep. Purif. Technol. 2022, 281, 119950. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Essaadaoui, Y.; Lebkiri, A.; Rifi, E.; Kadiri, L.; Ouass, A. Adsorption of cobalt from aqueous solutions onto Bark of Eucalyptus. Mediterranean. J. Chem. 2018, 7, 145–155. [Google Scholar]
- Ramos, S.N.C.; Xavier, A.L.P.; Teodoro, F.S.; Gil, L.F.; Gurgel, L.V.A. Removal of cobalt(II), copper(II), and nickel(II) ions from aqueous solutions using phthalate-functionalized sugarcane bagasse: Mono-and multicomponent adsorption in batch mode. Ind. Crops Prod. 2016, 79, 116–130. [Google Scholar] [CrossRef]
- Ketsela, G.; Animen, Z.; Talema, A. Adsorption of Lead (II), Cobalt (II) and Iron (II) From Aqueous Solution by Activated Carbon Prepared from White Lupine (GIBITO) HSUK. J. Thermodyn. Catal. 2020, 11, 203. [Google Scholar]
- Rengaraj, S.; Moon, S. Kinetics of adsorption of Co(II) removal from water and wastewater by ion exchange resins. Water Res. 2002, 36, 1783–1793. [Google Scholar] [CrossRef]
- Wanga, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Wang, L.; Pan, L.; Zhang, X.; Zou, J. Rational design, synthesis, adsorption principles and applications of metal oxide adsorbents: A review. Nanoscale 2020, 12, 4790–4815. [Google Scholar] [CrossRef]
- Mahadevan, H.; Nimina, P.V.M.; Krishnan, K.A. An environmental green approach for the effective removal of malachite green from estuarine waters using Pistaciavera L. shell-based active carbon. Sustain. Water Resour. Manag. 2022, 8, 38. [Google Scholar]
- Hasan, I.M.A.; Tawfik, A.R.; Assaf, F.H. Biosynthesis of α-MoO3 nanoparticles and its adsorption performance of cadmium from aqueous solutions. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 035007. [Google Scholar] [CrossRef]
- Khan, M.I.; Kazmi, S.A. Spectrophotometric determination of cobalt(II). J. Chem. Soc. Pak. 1979, 1, 73–74. [Google Scholar]










| ZrO2570 | ZrO2700 | |
|---|---|---|
| Average crystallite size, nm (XRD) | 6.27 | 7.26 |
| Average particle size, nm (TEM) | 7.5 | 8.1 |
| Surface area, m2/g (BET) | 94.805 | 62.384 |
| Surface area, m2/g (BJH) | 80.329 | 61.222 |
| Mean pore diameter, nm (BET) | 3.922 | 7.588 |
| Mean pore diameter, nm (BJH) | 3.92 | 7.32 |
| Total pore volume, cm3/g (BET) | 0.09296 | 0.1183 |
| Pore volume, cm3/g (NLDFT) | 0.0698 | 0.06098 |
| Pore volume, cm3/g (BJH) | 0.08513 | 0.1164 |
| T, K | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | n | KF | R2 | |
| 298 | 8.995 | 0.039 | 0.973 | 3.810 | 2.420 | 0.840 |
| C0 | Pseudo 1st Order | Pseudo 2nd Order | Elovich | qeexp | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K1 | qecalc | R2 | K2 | qecalc | R2 | β | α | R2 | ||
| 50 | 0.078 | 6.207 | 0.955 | 0.0125 | 9.225 | 0.976 | 0.9613 | 1.749 | 0.993 | 5.469 |
| 100 | 0.052 | 8.669 | 0.901 | 0.009 | 12.500 | 0.977 | 0.617 | 2.316 | 0.965 | 7.258 |
| 150 | 0.052 | 5.744 | 0.676 | 0.010 | 13.643 | 0.989 | 0.668 | 2.048 | 0.974 | 8.523 |
| 200 | 0.084 | 6.187 | 0.818 | 0.0141 | 14.045 | 0.980 | 1.348 | 0.832 | 0.999 | 8.995 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, I.M.A.; Salah El-Din, H.; AbdElRaady, A.A. Peppermint-Mediated Green Synthesis of Nano ZrO2 and Its Adsorptive Removal of Cobalt from Water. Inorganics 2022, 10, 257. https://doi.org/10.3390/inorganics10120257
Hasan IMA, Salah El-Din H, AbdElRaady AA. Peppermint-Mediated Green Synthesis of Nano ZrO2 and Its Adsorptive Removal of Cobalt from Water. Inorganics. 2022; 10(12):257. https://doi.org/10.3390/inorganics10120257
Chicago/Turabian StyleHasan, Ibrahem Mohamed Abouzeid, Hanan Salah El-Din, and Ahmed A. AbdElRaady. 2022. "Peppermint-Mediated Green Synthesis of Nano ZrO2 and Its Adsorptive Removal of Cobalt from Water" Inorganics 10, no. 12: 257. https://doi.org/10.3390/inorganics10120257
APA StyleHasan, I. M. A., Salah El-Din, H., & AbdElRaady, A. A. (2022). Peppermint-Mediated Green Synthesis of Nano ZrO2 and Its Adsorptive Removal of Cobalt from Water. Inorganics, 10(12), 257. https://doi.org/10.3390/inorganics10120257