Nonstoichiometric Strontium Ferromolybdate as an Electrode Material for Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Sr2Fe1+xMo1−xO6−δ Properties
2.1. Crystal Structure
2.2. Thermodynamic Stability
2.3. Iron and Molybdenum Valence States
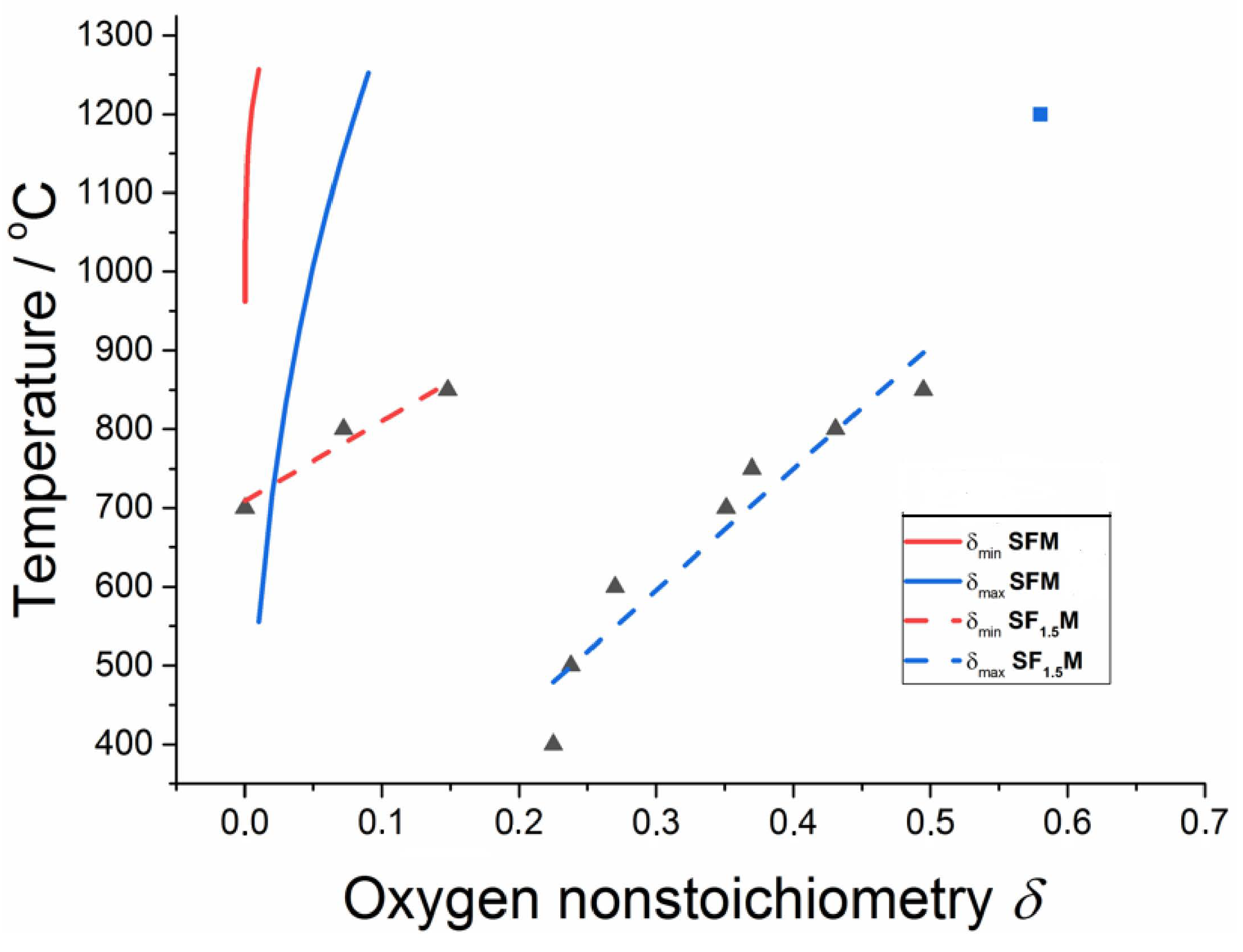
2.4. Oxygen Vacancy Formation Energy and Oxygen Non-Stoichiometry
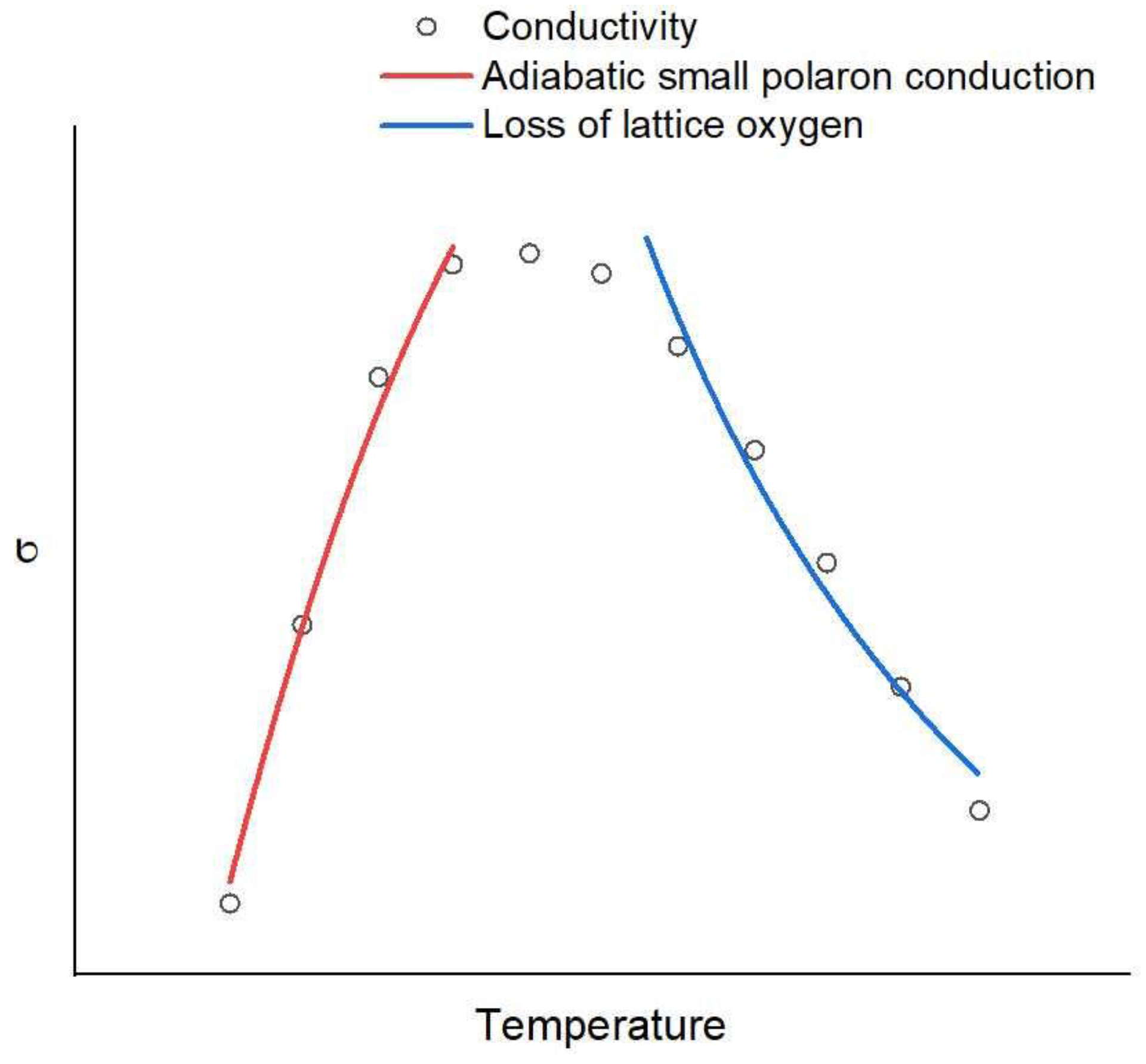
2.5. Electrical Conductivity
3. A- and B-Site Substitution
3.1. A-Site Substitution
3.2. B-Site Substitution
4. Performance of Sr2Fe1+xMo1−xO6−δ-Based SOFCs
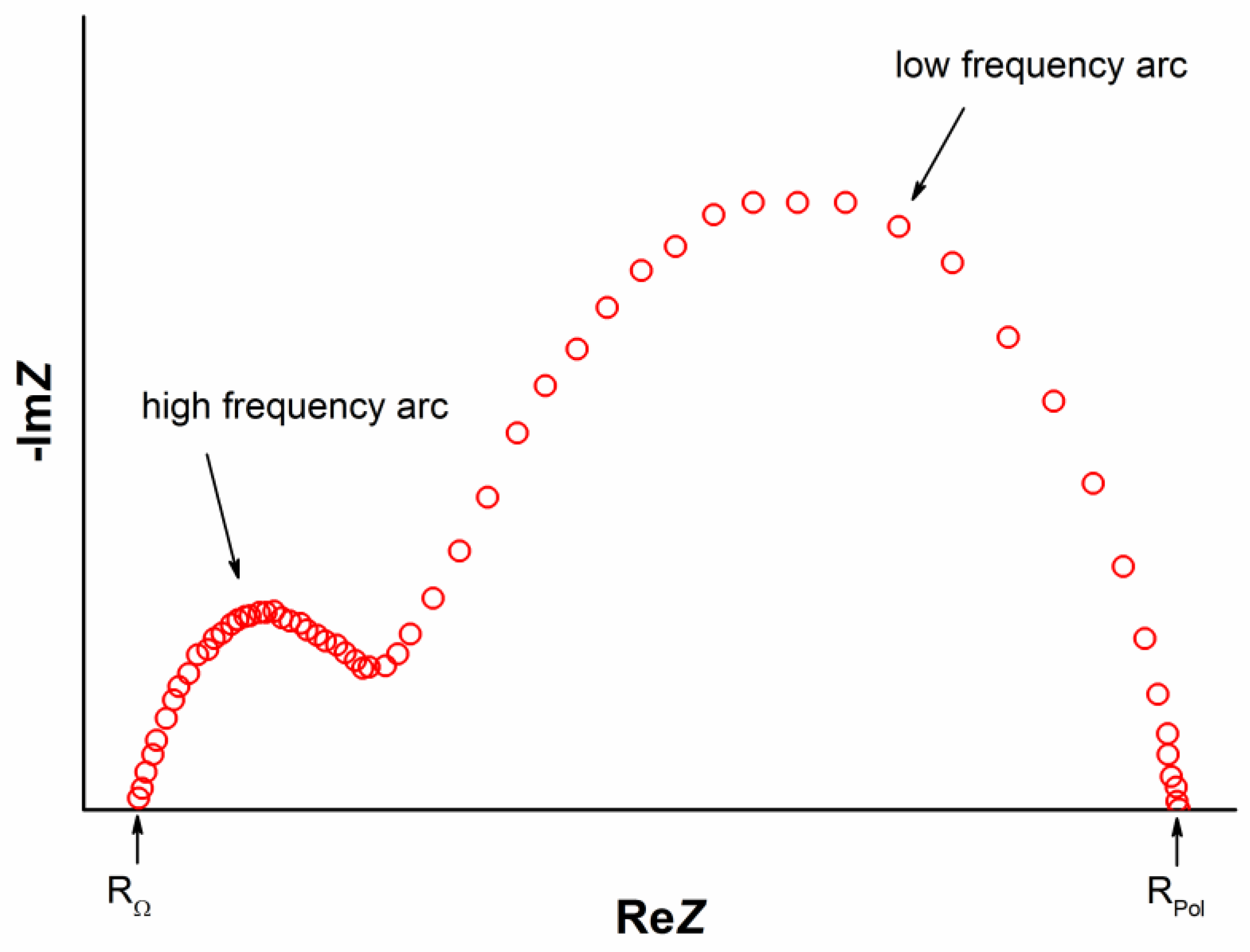
4.1. Polarization Resistance
4.2. Sr2Fe1+xMo1−xO6−δ-Based SOFCs Operated with Hydrogen Fuel
4.3. Sr2Fe1+xMo1−xO6−δ-Based SOFCs Operated with Hydrocarbon Fuel
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steele, B.C.H.; Heinzel, A. Materials for Fuel-Cell Technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.Q. Solid Oxide Fuel Cell Technology—Features and Applications. Solid State Ion. 2004, 174, 271–277. [Google Scholar] [CrossRef]
- Cai, W.; Zhou, Q.; Xie, Y.; Liu, J.; Long, G.; Cheng, S.; Liu, M. A Direct Carbon Solid Oxide Fuel Cell Operated on a Plant Derived Biofuel with Natural Catalyst. Appl. Energy 2016, 179, 1232–1241. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of Solid Oxide Fuel Cell Materials: Cathode, Anode, and Electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Ndubuisi, A.; Abouali, S.; Singh, K.; Thangadurai, V. Recent Advances, Practical Challenges, and Perspectives of Intermediate Temperature Solid Oxide Fuel Cell Cathodes. J. Mater. Chem. A 2022, 10, 2196–2227. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode Materials for Solid Oxide Fuel Cells: A Review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Pavone, M.; Ritzmann, A.M.; Carter, E.A. Oxide Ion Transport in Sr2Fe1.5Mo0.5O6−δ, a Mixed Ion-Electron Conductor: New Insights from First Principles Modeling. Phys. Chem. Chem. Phys. 2013, 15, 6250–6259. [Google Scholar] [CrossRef]
- Afroze, S.; Karim, A.H.; Cheok, Q.; Eriksson, S.; Azad, A.K. Latest Development of Double Perovskite Electrode Materials for Solid Oxide Fuel Cells: A Review. Front. Energy 2019, 13, 770–797. [Google Scholar] [CrossRef]
- Skutina, L.; Filonova, E.; Medvedev, D.; Maignan, A. Undoped Sr2MMoO6 Double Perovskite Molybdates (M = Ni, Mg, Fe) as Promising Anode Materials for Solid Oxide Fuel Cells. Materials 2021, 14, 1715. [Google Scholar] [CrossRef] [PubMed]
- Falcón, H.; Barbero, J.A.; Araujo, G.; Casais, M.T.; Martínez-Lope, M.J.; Alonso, J.A.; Fierro, J.L.G. Double Perovskite Oxides A2FeMoO6−δ (A=Ca, Sr and Ba) as Catalysts for Methane Combustion. Appl. Catal. B Environ. 2004, 53, 37–45. [Google Scholar] [CrossRef]
- Bastidas, D.M.; Tao, S.; Irvine, J.T.S. A Symmetrical Solid Oxide Fuel Cell Demonstrating Redox Stable Perovskite Electrodes. J. Mater. Chem. 2006, 16, 1603–1605. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Xiao, G.; Zhao, F.; Chen, F. A Novel Electrode Material for Symmetrical SOFCs. Adv. Mater. 2010, 22, 5478–5482. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, C.; Jin, C.; Han, M.; Chen, F. Ba0.9Co0.7Fe0.2Nb0.1O3−δ as Cathode Material for Intermediate Temperature Solid Oxide Fuel Cells. Electrochem. Commun. 2011, 13, 882–885. [Google Scholar] [CrossRef]
- Choi, H.J.; Kwak, M.; Kim, T.W.; Seo, D.W.; Woo, S.K.; Kim, S.D. Redox Stability of La0.6Sr0.4Fe1−xScxO3−δ for Tubular Solid Oxide Cells Interconnector. Ceram. Int. 2017, 43, 7929–7934. [Google Scholar] [CrossRef]
- Marinha, D.; Dessemond, L.; Djurado, E. Comprehensive Review of Current Developments in IT-SOFCs. Curr. Inorg. Chem. 2013, 3, 2–22. [Google Scholar] [CrossRef]
- Morales, M.; Roa, J.J.; Tartaj, J.; Segarra, M. A Review of Doped Lanthanum Gallates as Electrolytes for Intermediate Temperature Solid Oxides Fuel Cells: From Materials Processing to Electrical and Thermo-Mechanical Properties. J. Eur. Ceram. Soc. 2016, 36, 1–16. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; León-Reina, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Chemical Stability and Compatibility of Double Perovskite Anode Materials for SOFCs. Solid State Ion. 2013, 239, 1–7. [Google Scholar] [CrossRef]
- da Silva, F.S.; de Souza, T.M. Novel Materials for Solid Oxide Fuel Cell Technologies: A Literature Review. Int. J. Hydrog. Energy 2017, 42, 26020–26036. [Google Scholar] [CrossRef]
- Huang, K.; Tichy, R.; Goodenough, J.B.; Milliken, C. Superior Perovskite Oxide-Ion Conductor; Strontium- and Magnesium-Doped LaGaO3: III, Performance Tests of Single Ceramic Fuel Cells. J. Am. Ceram. Soc. 1998, 81, 2581–2585. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze Der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Wang, Y.; Li, Y. Sr2Fe2-XMoxO6-δ Perovskite as an Anode in a Solid Oxide Fuel Cell: Effect of the Substitution Ratio. Catal. Today 2016, 259, 417–422. [Google Scholar] [CrossRef]
- Markov, A.A.; Savinskaya, O.A.; Patrakeev, M.V.; Nemudry, A.P.; Leonidov, I.A.; Pavlyukhin, Y.T.; Ishchenko, A.V.; Kozhevnikov, V.L. Structural Features, Nonstoichiometry and High-Temperature Transport in SrFe1−xMoxO3−δ. J. Solid State Chem. 2009, 182, 799–806. [Google Scholar] [CrossRef]
- Markov, A.A.; Leonidov, I.A.; Patrakeev, M.V.; Kozhevnikov, V.L.; Savinskaya, O.A.; Ancharova, U.V.; Nemudry, A.P. Structural Stability and Electrical Transport in SrFe1−xMoxO3−δ. Solid State Ion. 2008, 179, 1050–1053. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Q.; Wang, S.; Komvokis, V.G.; Amiridis, M.D.; Heyden, A.; Ma, S.; Chen, F. Synthesis and Characterization of Mo-Doped SrFeO3−δ as Cathode Materials for Solid Oxide Fuel Cells. J. Power Sources 2012, 202, 63–69. [Google Scholar] [CrossRef]
- Fernández-Ropero, A.J.; Porras-Vázquez, J.M.; Cabeza, A.; Slater, P.R.; Marrero-López, D.; Losilla, E.R. High Valence Transition Metal Doped Strontium Ferrites for Electrode Materials in Symmetrical SOFCs. J. Power Sources 2014, 249, 405–413. [Google Scholar] [CrossRef]
- Liang, F.; Partovi, K.; Jiang, H.; Luo, H.; Caro, J. B-Site La-Doped BaFe0.95-xLaxZr0.05O3−δ Perovskite-Type Membranes for Oxygen Separation. J. Mater. Chem. A 2013, 1, 746–751. [Google Scholar] [CrossRef]
- Liu, G.Y.; Rao, G.H.; Feng, X.M.; Yang, H.F.; Ouyang, Z.W.; Liu, W.F.; Liang, J.K. Structural Transition and Atomic Ordering in the Non-Stoichiometric Double Perovskite Sr2FexMo2−xO6. J. Alloys Compd. 2003, 353, 42–47. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Q.; Nuansaeng, S.; Chen, F. Sr2Fe1+xMo1−xO6−δ as Anode Materials for Solid Oxide Fuel Cells. ECS Trans. 2012, 45, 355–362. [Google Scholar] [CrossRef]
- Gou, M.; Ren, R.; Sun, W.; Xu, C.; Meng, X.; Wang, Z.; Qiao, J.; Sun, K. Nb-Doped Sr2Fe1.5Mo0.5O6−δ Electrode with Enhanced Stability and Electrochemical Performance for Symmetrical Solid Oxide Fuel Cells. Ceram. Int. 2019, 45, 15696–15704. [Google Scholar] [CrossRef]
- Liu, Q.; Bugaris, D.E.; Xiao, G.; Chmara, M.; Ma, S.; Zur Loye, H.C.; Amiridis, M.D.; Chen, F. Sr2Fe1.5Mo0.5O6−δ as a Regenerative Anode for Solid Oxide Fuel Cells. J. Power Sources 2011, 196, 9148–9153. [Google Scholar] [CrossRef]
- Bugaris, D.E.; Hodges, J.P.; Huq, A.; Chance, W.M.; Heyden, A.; Chen, F.; Loye, H.C. Zur Investigation of the High-Temperature Redox Chemistry of Sr2Fe1.5Mo0.5O6−δ via in Situ Neutron Diffraction. J. Mater. Chem. A 2014, 2, 4045–4054. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Bugaris, D.E.; Pavone, M.; Hodges, J.P.; Huq, A.; Chen, F.; Zur Loye, H.C.; Carter, E.A. Unveiling Structure-Property Relationships in Sr2Fe1.5Mo0.5O6−δ, an Electrode Material for Symmetric Solid Oxide Fuel Cells. J. Am. Chem. Soc. 2012, 134, 6826–6833. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Q.; Dong, X.; Huang, K.; Chen, F. Sr2Fe4/3Mo2/3O6 as Anodes for Solid Oxide Fuel Cells. J. Power Sources 2010, 195, 8071–8074. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, S.; Lin, Y.; Chen, F. Ni-Doped Sr2Fe1.5Mo0.5O6 as Anode Materials for Solid Oxide Fuel Cells. ECS Trans. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Liu, G.Y.; Rao, G.H.; Feng, X.M.; Yang, H.F.; Ouyang, Z.W.; Liu, W.F.; Liang, J.K. Atomic Ordering and Magnetic Properties of Non-Stoichiometric Double-Perovskite Sr2FexMo2−xO6. J. Phys. Condens. Matter 2003, 15, 2053–2060. [Google Scholar] [CrossRef]
- Dai, N.; Wang, Z.; Jiang, T.; Feng, J.; Sun, W.; Qiao, J.; Rooney, D.; Sun, K. A New Family of Barium-Doped Sr2Fe1.5Mo0.5O6−δ Perovskites for Application in Intermediate Temperature Solid Oxide Fuel Cells. J. Power Sources 2014, 268, 176–182. [Google Scholar] [CrossRef]
- Xia, T.; Meng, X.; Luo, T.; Zhan, Z.L. Synthesis and Evaluation of Ca-Doped Sr2Fe1.5Mo0.5O6−δ as Symmetrical Electrodes for High Performance Solid Oxide Fuel Cells. Wuji Cailiao Xuebao/J. Inorg. Mater. 2019, 34, 1109–1114. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Z.; He, B.; Wang, S.; Wu, X.; Xia, C. Effect of Co Doping on the Electrochemical Properties of Sr2Fe1.5Mo0.5O6 Electrode for Solid Oxide Fuel Cell. Int. J. Hydrog. Energy 2013, 38, 4108–4115. [Google Scholar] [CrossRef]
- Dai, N.; Feng, J.; Wang, Z.; Jiang, T.; Sun, W.; Qiao, J.; Sun, K. Synthesis and Characterization of B-Site Ni-Doped Perovskites Sr2Fe1.5−xNixMo0.5O6−δ (x = 0, 0.05, 0.1, 0.2, 0.4) as Cathodes for SOFCs. J. Mater. Chem. A 2013, 1, 14147–14153. [Google Scholar] [CrossRef]
- Feng, J.; Yang, G.; Dai, N.; Wang, Z.; Sun, W.; Rooney, D.; Qiao, J.; Sun, K. Investigation into the Effect of Fe-Site Substitution on the Performance of Sr2Fe1.5Mo0.5O6−δ Anodes for SOFCs. J. Mater. Chem. A 2014, 2, 17628–17634. [Google Scholar] [CrossRef]
- Sun, W.; Li, P.; Xu, C.; Dong, L.; Qiao, J.; Wang, Z.; Rooney, D.; Sun, K. Investigation of Sc Doped Sr2Fe1.5Mo0.5O6 as a Cathode Material for Intermediate Temperature Solid Oxide Fuel Cells. J. Power Sources 2017, 343, 237–245. [Google Scholar] [CrossRef]
- He, B.; Gong, C.; Wang, Z.; Jia, L.; Zhao, L. Novel, Cobalt-Free, and Highly Active Sr2Fe1.5Mo0.5−xSnxO6−δ Cathode Materials for Intermediate Temperature Solid Oxide Fuel Cells. Int. J. Hydrog. Energy 2017, 42, 10308–10316. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, W.; Wang, W.; Wang, Z.; Sun, W.; Zhang, J.; Sun, K. The Ca Element Effect on the Enhancement Performance of Sr2Fe1.5Mo0.5O6−δ Perovskite as Cathode for Intermediate-Temperature Solid Oxide Fuel Cells. J. Power Sources 2016, 331, 400–407. [Google Scholar] [CrossRef]
- Hou, M.; Sun, W.; Li, P.; Feng, J.; Yang, G.; Qiao, J.; Wang, Z.; Rooney, D.; Feng, J.; Sun, K. Investigation into the Effect of Molybdenum-Site Substitution on the Performance of Sr2Fe1.5Mo0.5O6−δ for Intermediate Temperature Solid Oxide Fuel Cells. J. Power Sources 2014, 272, 759–765. [Google Scholar] [CrossRef]
- Hodges, J.P.; Short, S.; Jorgensen, J.D.; Xiong, X.; Dabrowski, B.; Mini, S.M.; Kimball, C.W. Evolution of Oxygen-Vacancy Ordered Crystal Structures in the Perovskite Series SrnFenO3n-1 (n = 2, 4, 8, and ∞), and the Relationship to Electronic and Magnetic Properties. J. Solid State Chem. 2000, 151, 190–209. [Google Scholar] [CrossRef]
- Schmidt, M.; Campbell, S.J. Crystal and Magnetic Structures of Sr2Fe2O5 at Elevated Temperature. J. Solid State Chem. 2001, 156, 292–304. [Google Scholar] [CrossRef]
- Mizusaki, J.; Okayasu, M.; Yamauchi, S.; Fueki, K. Nonstoichiometry and Phase Relationship of the SrFeO2.5−SrFeO3 System at High Temperature. J. Solid State Chem. 1992, 99, 166–172. [Google Scholar] [CrossRef]
- Majewski, P.; Rager, J.; Schurr, C.; Aldinger, F. Phase Relations and Homogeneity Region of Sr(Fe,Mo)O3 at 1200 °C in Air. Int. J. Inorg. Mater. 2001, 3, 733–736. [Google Scholar] [CrossRef]
- Fang, T.T.; Ko, T.F. Factors Affecting the Preparation of Sr2Fe2−xMoxO6. J. Am. Ceram. Soc. 2003, 86, 1453–1455. [Google Scholar] [CrossRef]
- Rager, J.; Zipperle, M.; Sharma, A.; MacManus-Driscoll, J.L. Oxygen Stoichiometry in Sr2FeMoO6, the Determination of Fe and Mo Valence States, and the Chemical Phase Diagram of SrO-Fe3O4-MoO3. J. Am. Ceram. Soc. 2004, 87, 1330–1335. [Google Scholar] [CrossRef]
- Miao, G.; Yuan, C.; Chen, T.; Zhou, Y.; Zhan, W.; Wang, S. Sr2Fe1+xMo1−xO6−δ as Anode Material of Cathode-Supported Solid Oxide Fuel Cells. Int. J. Hydrog. Energy 2016, 41, 1104–1111. [Google Scholar] [CrossRef]
- Merkulov, O.V.; Markov, A.A.; Naumovich, E.N.; Shalaeva, E.V.; Leonidov, I.A.; Patrakeev, M.V. Non-Uniform Electron Conduction in Weakly Ordered SrFe1−xMoxO3−δ. Dalt. Trans. 2019, 48, 4530–4537. [Google Scholar] [CrossRef] [PubMed]
- Zallen, R. The Physics of Amorphous Solids. In The Physics of Amorphous Solids; Wiley: New York, NY, USA, 2007; pp. 1–304. ISBN 9783527617968. [Google Scholar]
- Nakamura, T.; Kunihara, K.; Hirose, Y. Stable Po2-Region of Ordered Perovskites Ca2FeMoO6 and Sr2FeMoO6 at 1200 °C. Mater. Res. Bull. 1981, 16, 321–326. [Google Scholar] [CrossRef]
- Wright, J.H.; Virkar, A.V.; Liu, Q.; Chen, F. Electrical Characterization and Water Sensitivity of Sr2Fe1.5Mo0.5O6−δ as a Possible Solid Oxide Fuel Cell Electrode. J. Power Sources 2013, 237, 13–18. [Google Scholar] [CrossRef]
- Hayes, J.R.; Grosvenor, A.P. An Investigation of the Fe and Mo Oxidation States in Sr2Fe2−xMoxO6 (0.25 ≤ x ≤ 1.0) Double Perovskites by X-Ray Absorption Spectroscopy. J. Alloys Compd. 2012, 537, 323–331. [Google Scholar] [CrossRef]
- Besse, M.; Cros, V.; Barthélémy, A.; Jaffrès, H.; Vogel, J.; Petroff, F.; Mirone, A.; Tagliaferri, A.; Bencok, P.; Decorse, P.; et al. Experimental Evidence of the Ferrimagnetic Ground State of Sr2FeMoO6 Probed by X-Ray Magnetic Circular Dichroism. Europhys. Lett. 2002, 60, 608–614. [Google Scholar] [CrossRef]
- Kuepper, K.; Balasz, I.; Hesse, H.; Winiarski, A.; Prince, K.C.; Matteucci, M.; Wett, D.; Szargan, R.; Burzo, E.; Neumann, M. Electronic and Magnetic Properties of Highly Ordered Sr2FeMoO6. Phys. Status Solidi Appl. Res. 2004, 201, 3252–3256. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; He, Q.; He, T. Double-Perovskites A2FeMoO6−δ (A = Ca, Sr, Ba) as Anodes for Solid Oxide Fuel Cells. J. Power Sources 2010, 195, 6356–6366. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Y.; Li, Y. Direct CH4 Fuel Cell Using Sr2FeMoO6 as an Anode Material. J. Power Sources 2011, 196, 6104–6109. [Google Scholar] [CrossRef]
- Yasukawa, Y.; Lindén, J.; Chan, T.S.; Liu, R.S.; Yamauchi, H.; Karppinen, M. Iron Valence in Double-Perovskite (Ba,Sr,Ca)2FeMoO6: Isovalent Substitution Effect. J. Solid State Chem. 2004, 177, 2655–2662. [Google Scholar] [CrossRef][Green Version]
- Woodward, P.; Hoffmann, R.D.; Sleieht, A.W. Order-Disorder in A2M3+M5+O6 Perovskites. J. Mater. Res. 1994, 9, 2118–2127. [Google Scholar] [CrossRef]
- Das, T.; Nicholas, J.D.; Qi, Y. Long-Range Charge Transfer and Oxygen Vacancy Interactions in Strontium Ferrite. J. Mater. Chem. A 2017, 5, 4493–4506. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Pavone, M.; Carter, E.A. Effect of Antisite Defects on the Formation of Oxygen Vacancies in Sr2FeMoO6: Implications for Ion and Electron Transport. Chem. Mater. 2011, 23, 4525–4536. [Google Scholar] [CrossRef]
- Reuter, K.; Scheffler, M. Composition, Structure, and Stability of RuO2(110) as a Function of Oxygen Pressure. Phys. Rev. B 2002, 65, 035406. [Google Scholar] [CrossRef]
- Adeagbo, W.A.; Hoffmann, M.; Ernst, A.; Hergert, W.; Saloaro, M.; Paturi, P.; Kokko, K. Tuning the Probability of Defect Formation via Substrate Strains in Sr2FeMoO6 Films. Phys. Rev. Mater. 2018, 2, 083604. [Google Scholar] [CrossRef]
- Onuma, S.; Yashiro, K.; Miyoshi, S.; Kaimai, A.; Matsumoto, H.; Nigara, Y.; Kawada, T.; Mizusaki, J.; Kawamura, K.; Sakai, N.; et al. Oxygen Nonstoichiometry of the Perovskite-Type Oxide La1−xCaxCrO3−δ (x = 0.1, 0.2, 0.3). Solid State Ion. 2004, 174, 287–293. [Google Scholar] [CrossRef]
- Suchaneck, G.; Kalanda, N.; Artsiukh, E.; Gerlach, G. Challenges in Sr2FeMoO6−δ Thin Film Deposition. Phys. Status Solidi Basic Res. 2020, 257, 1900312. [Google Scholar] [CrossRef]
- MacChesney, J.B.; Sherwood, R.C.; Potter, J.F. Electric and Magnetic Properties of the Strontium Ferrates. J. Chem. Phys. 1965, 43, 1907–1913. [Google Scholar] [CrossRef]
- Emin, D.; Holstein, T. Studies of Small-Polaron Motion IV. Adiabatic Theory of the Hall Effect. Ann. Phys. 1969, 53, 439–520. [Google Scholar] [CrossRef]
- Stevenson, J.W.; Armstrong, T.R.; Carneim, R.D.; Pederson, L.R.; Weber, W.J. Electrochemical Properties of Mixed Conducting Perovskites La1−xMxCo1−yFeyO3−δ (M = Sr, Ba, Ca). J. Electrochem. Soc. 1996, 143, 2722–2729. [Google Scholar] [CrossRef]
- Zhen, S.; Sun, W.; Tang, G.; Rooney, D.; Sun, K.; Ma, X. Evaluation of Strontium-Site-Deficient Sr2Fe1.4Co0.1Mo0.5O6−δ-Based Perovskite Oxides as Intermediate Temperature Solid Oxide Fuel Cell Cathodes. Int. J. Hydrog. Energy 2016, 41, 9538–9546. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yang, Z.; Lei, Z.; Jin, C.; Liu, Y.; Wang, Y.; Peng, S. Co-Substituted Sr2Fe1.5Mo0.5O6−δ as Anode Materials for Solid Oxide Fuel Cells: Achieving High Performance via Nanoparticle Exsolution. J. Power Sources 2019, 438, 226989. [Google Scholar] [CrossRef]
- Tomioka, Y.; Okuda, T.; Okimoto, Y.; Kumai, R.; Kobayashi, K.; Tokura, Y. Magnetic and Electronic Properties of a Single Crystal of Ordered Double Perovskite. Phys. Rev. B 2000, 61, 422–427. [Google Scholar] [CrossRef]
- Yanagihara, H.; Salamon, M.B.; Lyanda-Geller, Y.; Xu, S.; Moritomo, Y. Magnetotransport in Double Perovskite Sr2FeMoO6: Role of Magnetic and Nonmagnetic Disorder. Phys. Rev. B 2001, 64, 214407. [Google Scholar] [CrossRef]
- Snyder, G.J.; Hiskes, R.; DiCarolis, S. Intrinsic Electrical Transport and Magnetic Properties of La0.67Ca0.33MnO3 and La0.67Sr0.33MnO3 MOCVD and Bulk Material. Phys. Rev. B 1996, 53, 14434–14444. [Google Scholar] [CrossRef]
- De Teresa, J.; Ibarra, M.; Blasco, J.; García, J.; Marquina, C.; Algarabel, P.; Arnold, Z.; Kamenev, K. Spontaneous Behavior and Magnetic Field and Pressure Effects Perovskite. Phys. Rev. B 1996, 54, 1187–1193. [Google Scholar] [CrossRef]
- Pi, L.; Zheng, L.; Zhang, Y. Transport Mechanism in Polycrystalline. Phys. Rev. B 2000, 61, 8917–8921. [Google Scholar] [CrossRef]
- Akimoto, T.; Moritomo, Y.; Nakamura, A.; Furukawa, N. Observation of Anomalous Single-Magnon Scattering in Half-Metallic Ferromagnets by Chemical Pressure Control. Phys. Rev. Lett. 2000, 85, 3914–3917. [Google Scholar] [CrossRef]
- Dyson, F.J. Thermodynamic Behavior of an Ideal Ferromagnet. Phys. Rev. 1956, 102, 1230–1244. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.J.; Zhang, C.L.; Almasan, C.C.; Habermeier, H.U. Spin-Wave Scattering at Low Temperatures in Manganite Films. Phys. Rev. B 2003, 67, 134405. [Google Scholar] [CrossRef]
- Kubo, K.; Ohatata, N. A Quantum Theory of Double Exchange. I. J. Phys. Soc. Jpn. 1972, 33, 21–32. [Google Scholar] [CrossRef]
- Urushibara, A.; Moritomo, Y.; Arima, T.; Asamitsu, A.; Kido, G.; Tokura, Y. Insulator-Metal Transition and Giant Magnetoresistance in La1−xSrxMnO3. Phys. Rev. B 1995, 51, 14103–14109. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, H.D.; Feng, S.J.; Fan, X.J.; Li, X.G.; Wang, Z.D. Competition between Ferromagnetic Metallic and Paramagnetic Insulating Phases in Manganites. J. Appl. Phys. 2002, 92, 1406–1410. [Google Scholar] [CrossRef]
- Zhao, G.M.; Smolyaninova, V.; Prellier, W.; Keller, H. Electrical Transport in the Ferromagnetic State of Manganites: Small-Polaron Metallic Conduction at Low Temperatures. Phys. Rev. Lett. 2000, 84, 6086–6089. [Google Scholar] [CrossRef] [PubMed]
- Saloaro, M.; Majumdar, S.; Huhtinen, H.; Paturi, P. Absence of Traditional Magnetoresistivity Mechanisms in Sr2FeMoO6 Thin Films Grown on SrTiO3, MgO and NdGaO3 Substrates. J. Phys. Condens. Matter 2012, 24, 366003. [Google Scholar] [CrossRef]
- Niebieskikwiat, D.; Sánchez, R.; Caneiro, A.; Morales, L.; Vásquez-Mansilla, M.; Rivadulla, F.; Hueso, L. High-Temperature Properties of the Double Perovskite: Electrical Resistivity, Magnetic Susceptibility, and ESR. Phys. Rev. B 2000, 62, 3340–3345. [Google Scholar] [CrossRef]
- Maignan, A.; Raveau, B.; Martin, C.; Hervieu, M. Large Intragrain Magnetoresistance above Room Temperature in the Double Perovskite Ba2FeMoO6. J. Solid State Chem. 1999, 144, 224–227. [Google Scholar] [CrossRef]
- Bastow, T.J. T3 2 Dependence of the High Temperature Electrical Resistivity of Metals. Phys. Lett. A 1977, 60, 487–489. [Google Scholar] [CrossRef]
- Tai, L.W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and Electrical Properties of La1−xSrxCo1−yFeyO3. Part 2. The System La1-xSrxCo0.2Fe0.8O3. Solid State Ion. 1995, 76, 273–283. [Google Scholar] [CrossRef]
- Li, S.; Lü, Z.; Huang, X.; Su, W. Thermal, Electrical, and Electrochemical Properties of Nd-Doped Ba0.5Sr0.5Co0.8Fe0.2O3−δ as a Cathode Material for SOFC. Solid State Ion. 2008, 178, 1853–1858. [Google Scholar] [CrossRef]
- Graves, C.; Sudireddy, B.R.; Mogensen, M. Molybdate Based Ceramic Negative-Electrode Materials for Solid Oxide Cells. ECS Trans. 2010, 28, 173–192. [Google Scholar] [CrossRef]
- Rath, M.K.; Lee, K.T. Superior Electrochemical Performance of Non-Precious Co-Ni-Mo Alloy Catalyst-Impregnated Sr2FeMoO6−δ as an Electrode Material for Symmetric Solid Oxide Fuel Cells. Electrochim. Acta 2016, 212, 678–685. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Xiao, G.; Yang, Z.; Han, M.; Chen, F. Sulfur-Tolerant Hierarchically Porous Ceramic Anode-Supported Solid-Oxide Fuel Cells with Self-Precipitated Nanocatalyst. ChemElectroChem 2015, 2, 672–678. [Google Scholar] [CrossRef]
- Ding, H.; Tao, Z.; Liu, S.; Yang, Y. A Redox-Stable Direct-Methane Solid Oxide Fuel Cell (SOFC) with Sr2FeNb0.2Mo0.8O6−δ Double Perovskite as Anode Material. J. Power Sources 2016, 327, 573–579. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, H.; Yi, S.; Xia, Q.; Gong, Y.; Zhang, Y.; Cheng, X.; Li, Y.; Gu, L.; Świerczek, K. High-Performance Anode Material Sr2FeMo0.65Ni0.35O6−δ with in Situ Exsolved Nanoparticle Catalyst. ACS Nano 2016, 10, 8660–8669. [Google Scholar] [CrossRef]
- Liu, Q.; Xiao, G.; Howell, T.; Reitz, T.L.; Chen, F. A Novel Redox Stable Catalytically Active Electrode for Solid Oxide Fuel Cells. ECS Trans. 2011, 35, 1357–1366. [Google Scholar] [CrossRef]
- Xiao, G.; Chen, F. Ni Modified Ceramic Anodes for Direct-Methane Solid Oxide Fuel Cells. Electrochem. Commun. 2011, 13, 57–59. [Google Scholar] [CrossRef]
- Yang, Z.; Pang, Z.; Zhu, T.; Zheng, Z.; Han, M. Fabrication and Performance of Ceramic Anode-Supported Solid Oxide Fuel Cells. ECS Trans. 2013, 57, 549–554. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Q.; Zhao, F.; Zhang, L.; Xia, C.; Chen, F. Sr2Fe1.5Mo0.5O6 as Cathodes for Intermediate-Temper ature Solid Oxide Fuel Cells with La0.8Sr0.2Ga0.87Mg0.13O3 Electrolyte. J. Electrochem. Soc. 2011, 158, B455. [Google Scholar] [CrossRef]
- Huang, K.; Lee, H.Y.; Goodenough, J.B. Sr-and Ni-doped LaCoO3 and LaFeO3 perovskites: New cathode materials for solid-oxide fuel cells. J. Electrochem. Soc. 1998, 145, 3220. [Google Scholar] [CrossRef]
- Yang, G.; Feng, J.; Sun, W.; Dai, N.; Hou, M.; Hao, X.; Qiao, J.; Sun, K. The characteristic of strontium-site deficient perovskites SrxFe1.5Mo0.5O6−δ (x = 1.9–2.0) as intermediate-temperature solid oxide fuel cell cathodes. J. Power. Sources 2014, 268, 771–777. [Google Scholar] [CrossRef]
- Wang, X.; Sui, Y.; Cheng, J.; Qian, Z.; Miao, J.; Liu, Z.; Zhu, R.; Su, W.; Tang, J.; Ong, C.K. Magnetic and Magneto-Transport Properties of Double Perovskite Sr2FeMoO6 with Co Doping. J. Phys. Condens. Matter 2007, 19, 026215. [Google Scholar] [CrossRef]
- Suthirakun, S.; Ammal, S.C.; Muñoz-García, A.B.; Xiao, G.; Chen, F.; Zur Loye, H.C.; Carter, E.A.; Heyden, A. Theoretical Investigation of H2 Oxidation on the Sr2Fe1.5Mo0.5O6 (001) Perovskite Surface under Anodic Solid Oxide Fuel Cell Conditions. J. Am. Chem. Soc. 2014, 136, 8374–8386. [Google Scholar] [CrossRef] [PubMed]
- Kobsiriphat, W.; Madsen, B.D.; Wang, Y.; Shah, M.; Marks, L.D.; Barnett, S.A. Nickel- and Ruthenium-Doped Lanthanum Chromite Anodes: Effects of Nanoscale Metal Precipitation on Solid Oxide Fuel Cell Performance. J. Electrochem. Soc. 2010, 157, B279. [Google Scholar] [CrossRef]
- Chroneos, A.; Yildiz, B.; Tarancón, A.; Parfitt, D.; Kilner, J.A. Oxygen Diffusion in Solid Oxide Fuel Cell Cathode and Electrolyte Materials: Mechanistic Insights from Atomistic Simulations. Energy Environ. Sci. 2011, 4, 2774–2789. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Ran, R.; Cai, R.; Shao, Z.; Farrusseng, D. A New Symmetric Solid-Oxide Fuel Cell with La0.8Sr0.2Sc0.2Mn0.8O3−δ Perovskite Oxide as Both the Anode and Cathode. Acta Mater. 2009, 57, 1165–1175. [Google Scholar] [CrossRef]
- Zhou, W.; Ran, R.; Shao, Z.; Zhuang, W.; Jia, J.; Gu, H.; Jin, W.; Xu, N. Barium- and Strontium-Enriched (Ba0.5Sr0.5)1+xCo0.8Fe0.2O3−δ Oxides as High-Performance Cathodes for Intermediate-Temperature Solid-Oxide Fuel Cells. Acta Mater. 2008, 56, 2687–2698. [Google Scholar] [CrossRef]
- McIntosh, S.; Adler, S.B.; Vohs, J.M.; Gorte, R.J. Effect of Polarization on and Implications for Characterization of LSM-YSZ Composite Cathodes. Electrochem. Solid-State Lett. 2004, 7, A111–A114. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zhang, Y.; Xiao, G.; Chen, F.; Xia, C. Enhancement in Surface Exchange Coefficient and Electrochemical Performance of Sr2Fe1.5Mo0.5O6 Electrodes by Ce0.8Sm0.2O1.9 Nanoparticles. Electrochem. Commun. 2011, 13, 711–713. [Google Scholar] [CrossRef]
- Ding, H.; Tao, Z.; Liu, S.; Zhang, J. A High-Performing Sulfur-Tolerant and Redox-Stable Layered Perovskite Anode for Direct Hydrocarbon Solid Oxide Fuel Cells. Sci. Rep. 2015, 5, 18129. [Google Scholar] [CrossRef]
- Flipsen, S.F.J. Power Sources Compared: The Ultimate Truth? J. Power Sources 2006, 162, 927–934. [Google Scholar] [CrossRef]
- Walker, E.; Ammal, S.C.; Suthirakun, S.; Chen, F.; Terejanu, G.A.; Heyden, A. Mechanism of Sulfur Poisoning of Sr2Fe1.5Mo0.5O6−δ Perovskite Anode under Solid Oxide Fuel Cell Conditions. J. Phys. Chem. C 2014, 118, 23545–23552. [Google Scholar] [CrossRef]
- Shi, N.; Xie, Y.; Yang, Y.; Xue, S.; Li, X.; Zhu, K.; Huan, D.; Peng, R.; Xia, C.; Lu, Y. Review of Anodic Reactions in Hydrocarbon Fueled Solid Oxide Fuel Cells and Strategies to Improve Anode Performance and Stability. Mater. Renew. Sustain. Energy 2020, 9, 6. [Google Scholar] [CrossRef]
- Hua, B.; Li, M.; Sun, Y.F.; Zhang, Y.Q.; Yan, N.; Chen, J.; Li, J.; Etsell, T.; Sarkar, P.; Luo, J.L. Biogas to Syngas: Flexible on-Cell Micro-Reformer and NiSn Bimetallic Nanoparticle Implanted Solid Oxide Fuel Cells for Efficient Energy Conversion. J. Mater. Chem. A 2016, 4, 4603–4609. [Google Scholar] [CrossRef]
| Bond Configuration | Ef(VO), eV | |||
|---|---|---|---|---|
| x = 0 | x = 0.5 | x = 1.0 | x = 1.5 | |
| Fe-O-Mo | 2.95 | 2.89 | 2.58 | |
| Fe-O-Fe | 2.30 | 2.19 | ||
| Co-O-Mo | 2.78 | 2.41 | 2.2 | |
| Co-O-Co | 1.80 | 1.37 | ||
| Fe-O-Co | 2.08 | 1.74 | ||
| Compound | Temperature Range, °C | Ea, eV | Ref. |
|---|---|---|---|
| LSCF | 100–500 | 0.10 | [91] |
| BSCF | 45–470 | 0.365 | [92] |
| SMgM | 450–850 | 0.279 | [93] |
| S1.8Sm0.2MgM | 0.195 | ||
| S1.6Sm0.4MgM | 0.113 | ||
| S1.4Sm0.6MgM | 0.080 | ||
| S1.2Sm0.8MgM | 0.087 | ||
| SFO | 400–500 | 0.204 | [25] |
| SF1.9M | 400–600 | 0.221 | |
| SF1.8M | 400–600 | 0.164 | |
| SF1.6M | 410–700 | 0.162 | |
| SF1.5M | 400–750 | 0.156 | |
| SF1.5M | 40–440 | 0.236 | [39] |
| SF1.0C0.5M | 40–530 | 0.193 | |
| SF0.5C1.5M | 40–530 | 0.137 | |
| SF1.5M | 300–450 | 0.067 | [45] |
| Compound | ρ(0), mΩ cm | ν | Rν, µΩ cm T-ν | Ref. |
|---|---|---|---|---|
| SF1.33M | 18.8 | 1.54 | 1.482 | [34] |
| SFM | 1.85 | 2.07 | 0.0013 | [93] |
| SFM | 3.01 | 1.75 | 0.014 | [94] |
| Compound | Temperature Range, °C | Ea, eV | Ref. |
|---|---|---|---|
| SF1.5M | 475–800 | 0.167 | [29] |
| SF1.5M | 475–800 | 0.292 | [35] |
| S1.9F1.4Ni0.1M | 400–800 | 0.240 | |
| SF1.5M | 200–800 | 0.225 | [56] |
| SF1.5M | 400–750 | 0.15 | [32] |
| SF1.5M (SF1.4N0.1M) | 300–800 | 0.115 (0.1) | [30] |
| SFM0.8N0.2 | 600–800 | 0.281 | [96] |
| SFM0.65Ni0.35 | 250–700 | 0.05 | [97] |
| Configuration Anode-Electrolyte-Cathode | del, µm | T, °C | Pmax, W/cm2 | Ref. |
|---|---|---|---|---|
| SFM/LSGM/SDC/SmBC2 | 300 | 850 | 0.831 | [60] |
| 800 | 0.584 | |||
| 750 | 0,412 | |||
| SFM/LSGM/SDC/SmBC2 | 300 | 850 | 0.735 1 | [60] |
| 800 | 0.476 1 | |||
| 750 | 0.183 1 | |||
| BFM/LSGM/SDC/SmBC2 | 300 | 850 | 0.561 | [60] |
| 800 | 0.338 | |||
| 750 | 0.206 | |||
| CaFM/LSGM/SDC/SmBC2 | 300 | 850 | 0.186 | [60] |
| SFM/LSGM/BSCF | 300 | 850 | 0.864 | [61] |
| 800 | 0.603 | |||
| 750 | 0.436 | |||
| SFN0.8M0.2/LSGM/PBCO | 200 | 800 | 0.520 | [96] |
| 700 | 0.375 | |||
| 600 | 0.130 | |||
| 550 | 0.061 | |||
| SF1.33M/LGSM/LSCF | 300 | 800 | 0.547 | [34] |
| 750 | 0.392 | |||
| 700 | 0.268 | |||
| SF1.33M/LGSM/LSCF | 300 | 800 | 0.472 1 | [34] |
| SFM/LSGM/BSCF | 300 | 850 | 0.864 | [61] |
| 800 | 0.603 | |||
| 750 | 0.436 | |||
| SF1.33M/LGSM/LSCF | 300 | 800 | 0.472 1 | [34] |
| SF1.33M/LGSM/LSCF | 265 | 850 | 0.532 | [98] |
| 800 | 0.340 | |||
| 750 | 0.200 | |||
| SF1.33M/LGSM/LSCF SF1.5M/LGSM/LSCF | NA | 800 | 0.588 0.474 | [29] |
| SF1.33Mo0.66/LGSM/LSCF SF1.5M/LGSM/LSCF | NA | 800 | 0.473 1 0.432 1 | [29] |
| Ni-SF1.5M/LGSM/LSCF | 300 | 800 | 1134 | [99] |
| SF1.5M-GDC/CeGdO/BCFN | 50 | 700 | 0.188 | [100] |
| 650 | 0.100 | |||
| 600 | 0.039 | |||
| S1.9F1.4Ni0.1M/LSGM/CLO/LSCF | NA | 850 | 1.160 | [35] |
| 800 | 0.968 | |||
| 750 | 0.730 | |||
| SF1.5M-GDC/GDC/GDC-LSCF | 35 | 700 | 0.22 | [95] |
| 600 | 0.14 | |||
| LSMn/YSZ-S2FM/YSZ/YSZ-LSFSc | 24 | 800 | 0.462 | [52] |
| 750 | 0.324 | |||
| SF1.4M/LSGM/BSCF SF1.5M/LSGM/BSCF SF1.6M/LSGM/BSCF | 300 | 800 | 0.514 0.508 0.482 | [22] |
| SF1.4M/LSGM/BSCF SF1.5M/LSGM/BSCF SF1.6M/LSGM/BSCF | 300 | 750 | 0.387 0.385 0.380 | [22] |
| SF1.3Co0.3M0.4/LSGM/LSCF | 170 | 850 | 1.09 | [74] |
| 800 | 0.81 | |||
| 750 | 0.50 | |||
| 700 | 0.30 | |||
| SF1.3Co0.3M0.4/LSGM/LSCF | 170 | 850 | 0.981 2 | [74] |
| 800 | 0.808 2 | |||
| 750 | 0.604 2 | |||
| Ni-LDC/LDC/LSGM/SF1.5M | 300 | 850 | 0.613 | [101] |
| 800 | 0.468 | |||
| 750 | 0.349 | |||
| Ni-LDC/LDC/LSGM/SF1.5M | 265 | 850 | 0.613 | [98] |
| 800 | 0.468 | |||
| 750 | 0.349 | |||
| Ni-YSZ/YSZ/SDC/S1.8B0.2F1.5M | 700 | 800 | 1.63 | [37] |
| 750 | 1.3 | |||
| 700 | 0.87 | |||
| 650 | 0.41 | |||
| Ni-YSZ/YSZ/SDC/SF1.5Mo0.4N0.1 | 400 | 800 | 1.102 | [45] |
| 750 | 0.920 | |||
| 700 | 0.671 | |||
| 650 | 0.421 | |||
| Ni-YSZ/YSZ/SDC/S1.8B0.2F1.5M Ni-YSZ/YSZ/SDC/S1.6B0.4F1.5M Ni-YSZ/YSZ/SDC/S1.4B0.6F1.5M | 400 | 800 | 1.06 1.26 0.94 | [44] |
| Ni-YSZ/YSZ/SDC/SF1.4Ni0.1M | 10 | 800 | 1.77 | [40] |
| 750 | 1.21 | |||
| 700 | 0.79 | |||
| 650 | 0.33 | |||
| Ni-YSZ/LSGM/SDC/SF1.5M Ni-YSZ/LSGM/SDC/SF1.45Sc0.05M | 400 | 800 | 0.91 1.23 | [42] |
| Ni-ScSZ/ScSZ/SDC/SF1.4Co0.1M Ni-ScSZ/ScSZ/SDC/S1.95F1.4Co0.1M Ni-ScSZ/ScSZ/SDC/S1.9F1.4Co0.1M | 11 | 800 | 0.88 1.16 0.96 | [73] |
| SF1.5M/LSGM/SF1.5M | 265 | 900 | 0.835 | [12] |
| SF1.5M/LSGM/SF1.5M | 265 | 900 | 0.835 | [98] |
| SF1.5M/LSGM/SF1.5M | 243 | 800 | 0.531 | [30] |
| 750 | 0.365 | |||
| 700 | 0.244 | |||
| 650 | 0.124 | |||
| SF1.4N0.1M/LSGM/SF1.5M | 236 | 800 | 0.374 | [30] |
| 750 | 0.228 | |||
| 700 | 0.156 | |||
| 650 | 0.092 | |||
| S1.4Ca0.6F1.5M/LSGM/S1.4Ca0.6F1.5M | 35 | 800 | 1.050 | [38] |
| 750 | 0.880 | |||
| 700 | 0.660 | |||
| 600 | 0.410 | |||
| SF1.4Ni0.1M/LSGM/SF1.4Ni0.1Mo0.5 | 310 | 800 | 0.530 3 | [41] |
| 750 | 0.380 | |||
| 700 | 0.258 | |||
| 650 | 0.164 | |||
| SF1.5M/LSGM/SF1.5M0.2Sn0.3 | 400 | 800 | 0.618 | [43] |
| 750 | 0.431 | |||
| 700 | 0.262 |
| Configuration Anode-Electrolyte-Cathode | Fuel | del, µm | T, °C | Pmax, W/cm2 | Ref. |
|---|---|---|---|---|---|
| SFM/LSGM/BSCF | CH4 | 300 | 850 | 0.605 | [61] |
| 800 | 0.429 | ||||
| SF1.33M/LGSM/LSCF | CH4 | 300 | 800 | 0.13 | [34] |
| SF1.33M/LGSM/LSCF SF1.5M/LGSM/LSCF | CH4 | NA | 800 | 0.079 0.041 | [29] |
| SF1.5M/LSGM/SF1.5M | CH4 | 265 | 900 | 0.23 | [99] |
| SF1.5M/LSGM/SF1.5M | CH4 | 400 | 900 | 0.250 | [31] |
| 850 | 0.125 | ||||
| Ni-SF1.5M/LGSM/LSCF | CH4 | 300 | 800 | 0.663 | [99] |
| SF1.3Co0.3M/LSGM/LSCF | CH4 | 170 | 850 | 0.290 | [74] |
| 750 | 0.151 | ||||
| 700 | 0.057 | ||||
| SFM-YSZ/YSZ/YSZ-LSFSc | C3H8 | 24 | 800 | 0.331 | [52] |
| 750 | 0.173 | ||||
| SF1.4M/LSGM/BSCF SF1.5M/LSGM/BSCF SF1.6M/LSGM/BSCF | CH3OH | 300 | 800 | 0.415 0.395 0.382 | [22] |
| SF1.4M/LSGM/BSCF SF1.5M/LSGM/BSCF SF1.6M/LSGM/BSCF | CH3OH | 300 | 750 | 0.341 0.297 0.205 | [22] |
| Configuration Anode-Electrolyte-Cathode | T, °C | Rp, Ω cm2 | Ref. |
|---|---|---|---|
| SFM/LSGM/SDC/SmBC2 | 850 | 0.284 | [60] |
| 800 | 0.327 | ||
| 750 | 0.583 | ||
| SFM-YSZ/YSZ/YSZ-LSFSc0.1O | 750 | 0.52 2.11 1 | [52] |
| SFM-YSZ/YSZ/YSZ-LSFSc0.1 SF1.2M-YSZ/YSZ/YSZ-LSFSc0.1 SF1.35M-YSZ/YSZ/YSZ-LSFSc0.1 SF1.5M-YSZ/YSZ/YSZ-LSFSc0.1 | 800 | 0.152 0.176 0.193 0.235 | [52] |
| SF1.33M/LGSM/LSCF | 800 | ~0.2 | [34] |
| 750 | 0.26 | ||
| 700 | 0.45 | ||
| SF1.5M/LGSM/LSCF | 850 | 0.40 | [98] |
| 800 | 0.76 | ||
| 750 | 1.73 | ||
| SF1.3Co0.3M/LSGM/LSCF | 850 | 0.152 | [74] |
| 800 | 0.223 | ||
| 750 | 0.369 | ||
| 700 | 0.575 | ||
| SF1.3Co0.3M/LSGM/LSCF | 850 | 0.442 2 | [74] |
| 800 | 0.842 2 | ||
| 750 | 1.979 2 | ||
| Ni-LDC/LDC/LGSM/SF1.33M | 800 | 0.32 | [101] |
| Ni-YSZ/YSZ/SDC/SF1.5M | 750 | 0.42 | [37] |
| 700 | 0.92 | ||
| Ni-YSZ/YSZ/SDC/SF1.5M0.4N0.1 | 800 | 0.168 | [45] |
| SDC-SF1.65M/LGSM/SF1.5M | 750 | 0.11 0.27 | [111] |
| S1.4C0.6F1.5M/LSGM/S1.4C0.6F1.5M | 800 | 0.155 | [38] |
| 750 | 0.179 | ||
| 700 | 0.220 | ||
| 650 | 0.359 | ||
| SF1.2Co0.3M/LSGM/SF1.2Co0.3M | 850 | 0.152 | [74] |
| 800 | 0.223 | ||
| 750 | 0.369 | ||
| 700 | 0.575 | ||
| SF1.5M/LSGM/SF1.5M0.2Sn0.3 | 800 | 0.22 | [43] |
| 750 | 0.35 | ||
| 700 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchaneck, G.; Artiukh, E. Nonstoichiometric Strontium Ferromolybdate as an Electrode Material for Solid Oxide Fuel Cells. Inorganics 2022, 10, 230. https://doi.org/10.3390/inorganics10120230
Suchaneck G, Artiukh E. Nonstoichiometric Strontium Ferromolybdate as an Electrode Material for Solid Oxide Fuel Cells. Inorganics. 2022; 10(12):230. https://doi.org/10.3390/inorganics10120230
Chicago/Turabian StyleSuchaneck, Gunnar, and Evgenii Artiukh. 2022. "Nonstoichiometric Strontium Ferromolybdate as an Electrode Material for Solid Oxide Fuel Cells" Inorganics 10, no. 12: 230. https://doi.org/10.3390/inorganics10120230
APA StyleSuchaneck, G., & Artiukh, E. (2022). Nonstoichiometric Strontium Ferromolybdate as an Electrode Material for Solid Oxide Fuel Cells. Inorganics, 10(12), 230. https://doi.org/10.3390/inorganics10120230






