The Use of Plasmonic Spectroscopy for Detecting Ultra-Low Concentrations of Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurements of Hydrogen Peroxide Solutions
2.2. Measurements of Mixtures of Sodium Chloride Solutions
3. Results
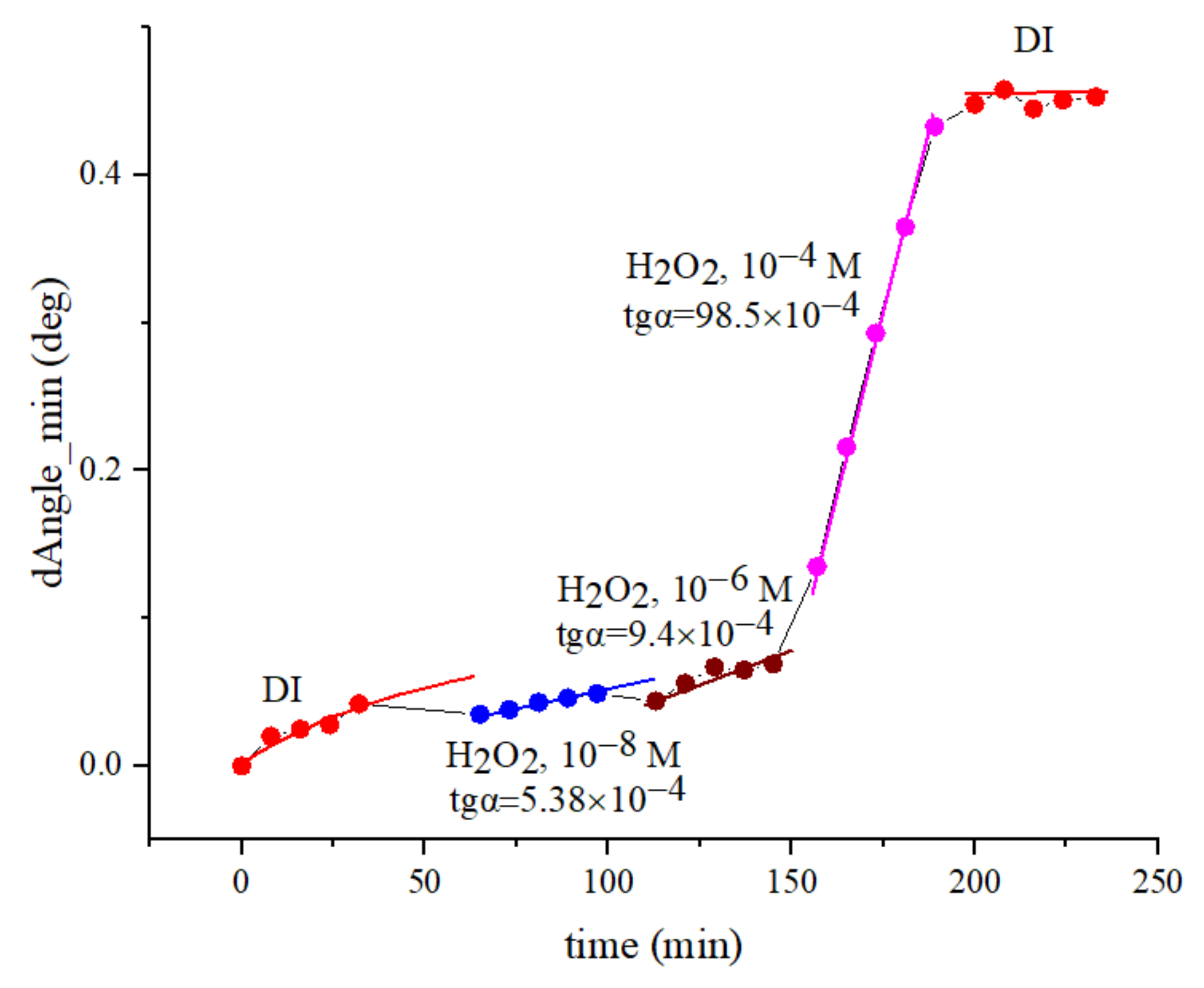
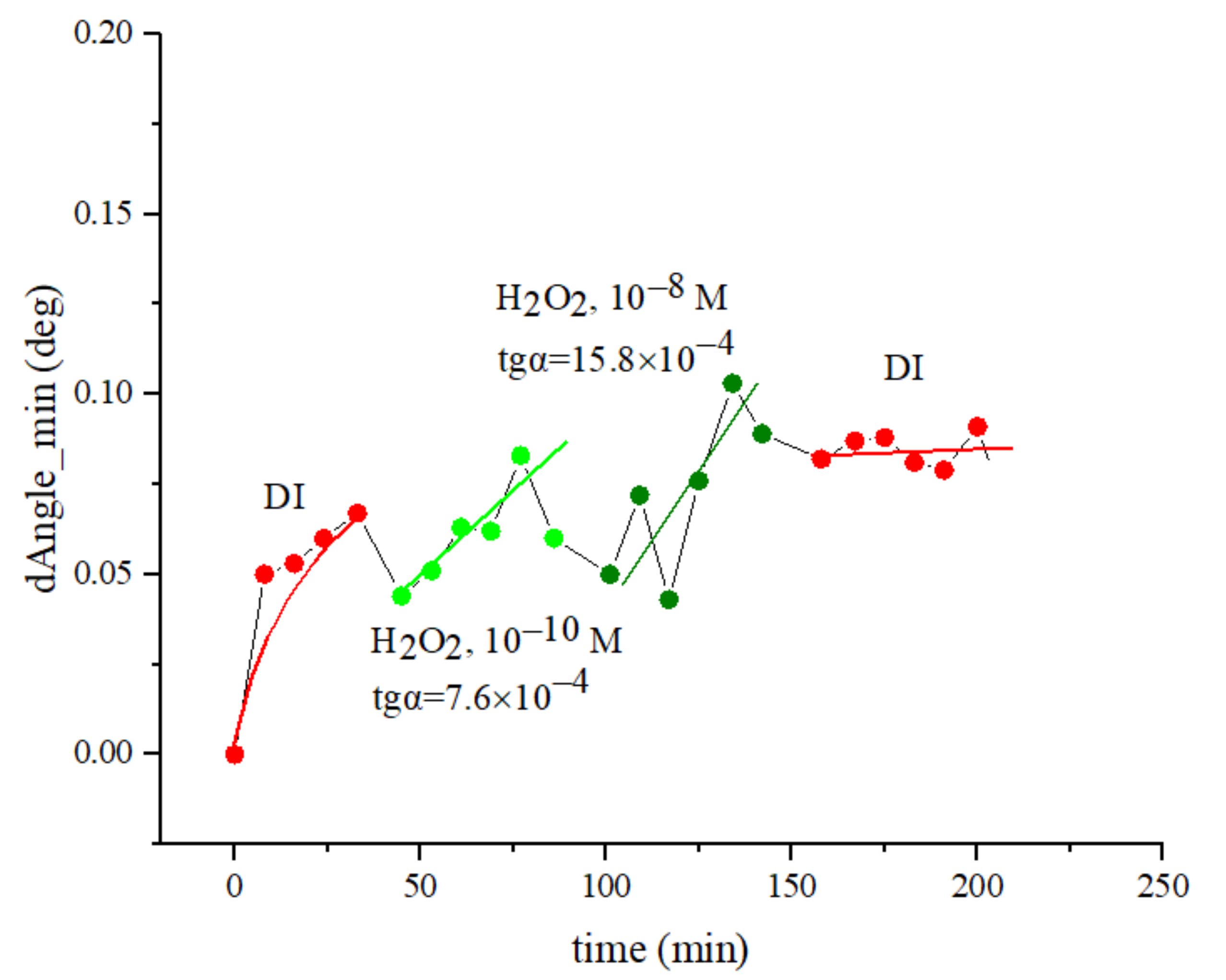
3.1. Studying the Hydrogen Peroxide Solutions
3.1.1. Measuring the SEW Excitation Angle on a Two-Layer Au/AgI substrate
3.1.2. Measuring the SEW Excitation Angle on a Two-Layer Au/Ag substrate
3.2. Studying High Dilutions of a Sodium Chloride Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobyshev, V.I.; Tomkevich, M.S.; Petrushanko, I.Y. Experimental study of potentiated aqueous solutions. Biophysics 2005, 50, 416–420. [Google Scholar]
- Rey, L. Thermoluminescence of ultra-high dilutions of lithium chloride and sodium chloride. Chem. Phys. A-Stat. Mech. Its Appl. 2003, 323, 67–74. [Google Scholar] [CrossRef]
- Elia, V.; Niccoli, M. New physico-chemical properties of extremely diluted aqueous solutions. J. Therm. Anal. Calorim. 2004, 75, 815–836. [Google Scholar] [CrossRef]
- Lobyshev, V.I. Dielectric characteristics of highly deluted aqueous diclofenac solutions in the frequency range of 20 Hz to 10 MHz. Phys. Wave Phenom. 2019, 27, 119–127. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Masalimov, Z.K.; Chernikov, A.V. Heat-induced generation of reactive oxygen species during reduction of dissolved air oxygen. Dokl. Biol. Sci. 2001, 381, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Lyakhov, G.A.; Pustovoy, V.I.; Shcherbakov, I.A. Vibration-Vortex Mechanism of Radical-Reaction Activation in an Aqueous Solution. Physical Analogies. Phys. Wave Phenom. 2021, 29, 108–113. [Google Scholar] [CrossRef]
- Kononov, M.A.; Pustovoy, V.I.; Svetikov, V.V. Specific features of the excitation of surface plasmons at the interface between metal and aqueous solution of extremely low concentration. Phys. Wave Phenom. 2020, 28, 4–7. [Google Scholar] [CrossRef]
- Agranovich, V.M.; Mills, D.L. (Eds.) Surface Polaritons: Electromagnetic Waves on Surfaces and Interfaces of Media; Nauka: Moscow, Russia, 1985; p. 528. [Google Scholar]
- Abronin, I.A.; Burshtein, K.Y.; Zhidomirov, G.M. Quantum-chemical calculations of the influence of a solvent on the electronic structure and reactivity of molecules. J. Struct. Chem. 1980, 21, 145–164. [Google Scholar] [CrossRef]
- Kononov, M.A.; Pustovoy, V.I.; Svetikov, V.V.; Usievich, B.A. Excitation of Surface Electromagnetic Waves at the Silver/NaCl Aqueous Solution Interface. Front. Phys. 2021, 9, 636979. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Kononov, M.A.; Savranskii, V.V.; Valyanskii, S.I. A simple optical method for determination of buffer layer quality. Bull. Lebedev Phys. Inst. 2003, 2, 3–9. [Google Scholar]
- Valyansky, S.I.; Vinogradov, S.V.; Kononov, M.A.; Kononov, V.M.; Savransky, V.V.; Tishkov, V.V. A system for monitoring and controlling metal film growth on glass prisms for the use in surface plasmon excitation devices. Appl. Phys. 2017, 4, 5–9. [Google Scholar]
- Petrov, S.I.; Epstein, O.I. Effect of potentiated solutions on mercury (II) signal in inversion voltammetry. Bull. Exp. Biol. Med. 2003, 135, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Penkov, N.V. Peculiarities of the perturbation of water structure by ions with various hydration in concentrated solutions of CaCl2, CsCl, KBr, and KI. Phys. Wave Phenom. 2019, 27, 128–134. [Google Scholar] [CrossRef]
- Ryzhkina, I.; Murtazina, L.; Gainutdinov, K.; Konovalov, A. Diluted Aqueous Dispersed Systems of 4-Aminopyridine: The Relationship of Self-Organization, Physicochemical Properties, and Influence on the Electrical Characteristics of Neurons. Front. Chem. 2021, 9, 623860. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, A.I.; Ryzhkina, I.S. Formation of nanoassociates as a key to understanding of physicochemical and biological properties of highly dilute aqueous solutions. Rus. Chem. Bull. 2014, 63, 1–14. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kononov, M.; Molkova, E.; Pustovoy, V.; Svetikov, V. The Use of Plasmonic Spectroscopy for Detecting Ultra-Low Concentrations of Substances. Photonics 2022, 9, 281. https://doi.org/10.3390/photonics9050281
Kononov M, Molkova E, Pustovoy V, Svetikov V. The Use of Plasmonic Spectroscopy for Detecting Ultra-Low Concentrations of Substances. Photonics. 2022; 9(5):281. https://doi.org/10.3390/photonics9050281
Chicago/Turabian StyleKononov, Michael, Elena Molkova, Vladimir Pustovoy, and Vladimir Svetikov. 2022. "The Use of Plasmonic Spectroscopy for Detecting Ultra-Low Concentrations of Substances" Photonics 9, no. 5: 281. https://doi.org/10.3390/photonics9050281
APA StyleKononov, M., Molkova, E., Pustovoy, V., & Svetikov, V. (2022). The Use of Plasmonic Spectroscopy for Detecting Ultra-Low Concentrations of Substances. Photonics, 9(5), 281. https://doi.org/10.3390/photonics9050281



