Performance Improvement of Graded Bandgap Solar Cell via Optimization of Energy Levels Alignment in Si Quantum Dot, TiO2 Nanoparticles, and Porous Si
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Colloidal ZnSiQDs
2.2. Synthesis of TiO2NPs
2.3. Synthesis of PSi Layers
2.4. Synthesis of GBQDSCs
2.5. Charactrization
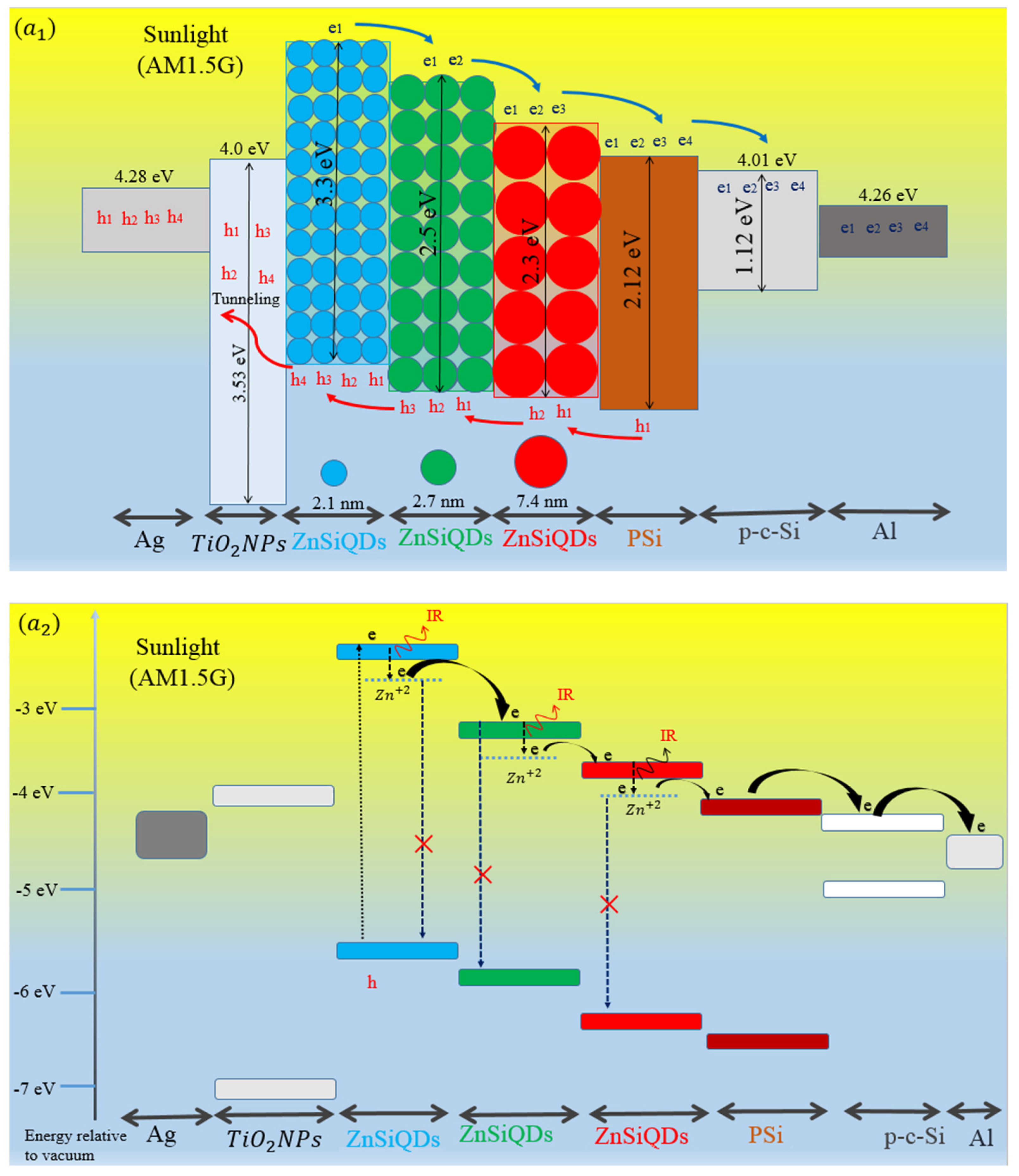
3. Results and Discussion
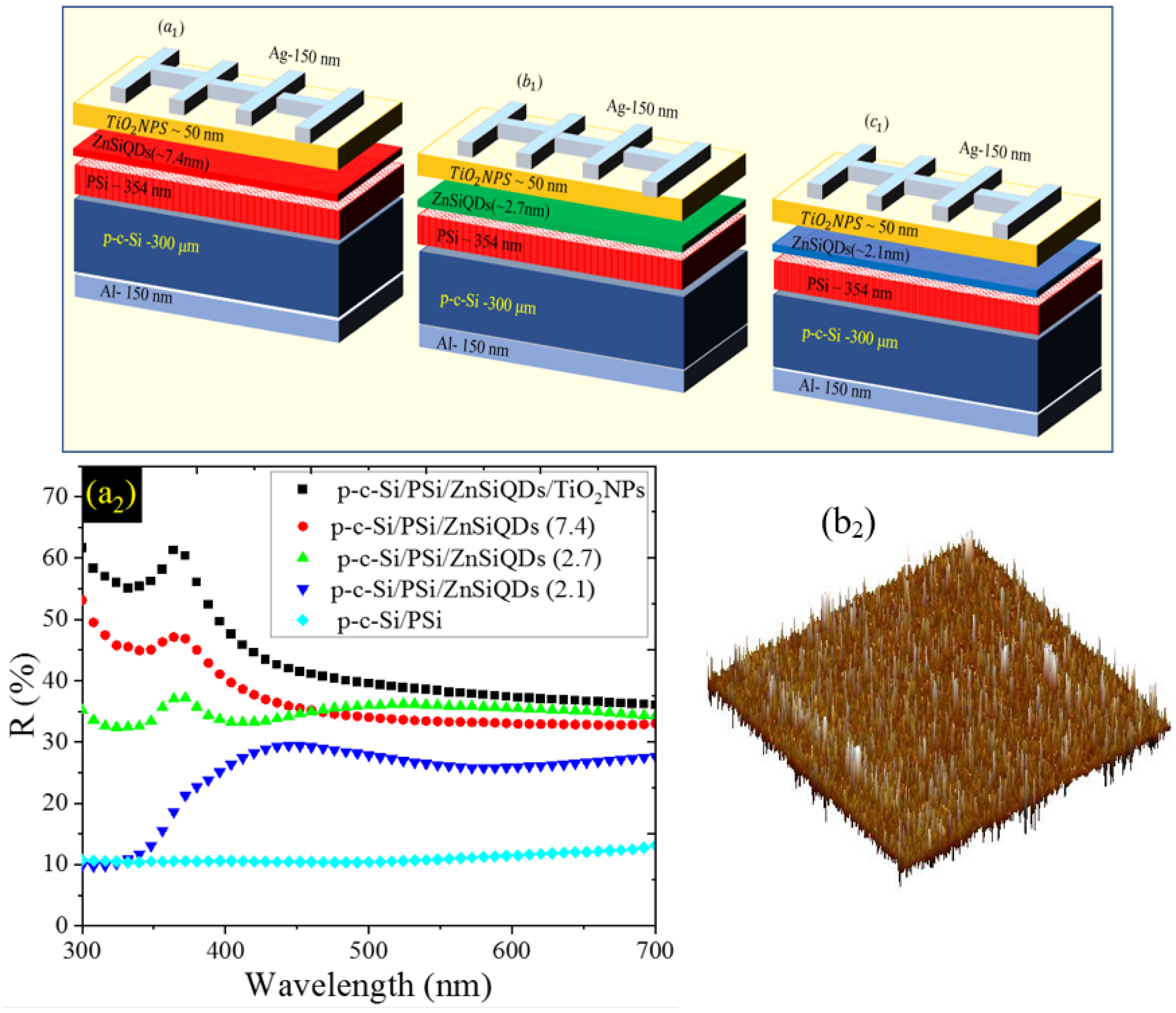
3.1. Structure and Morphology of ZnPSi and ZnSiQDs
3.2. Effect of PSi on Cell’s Performance
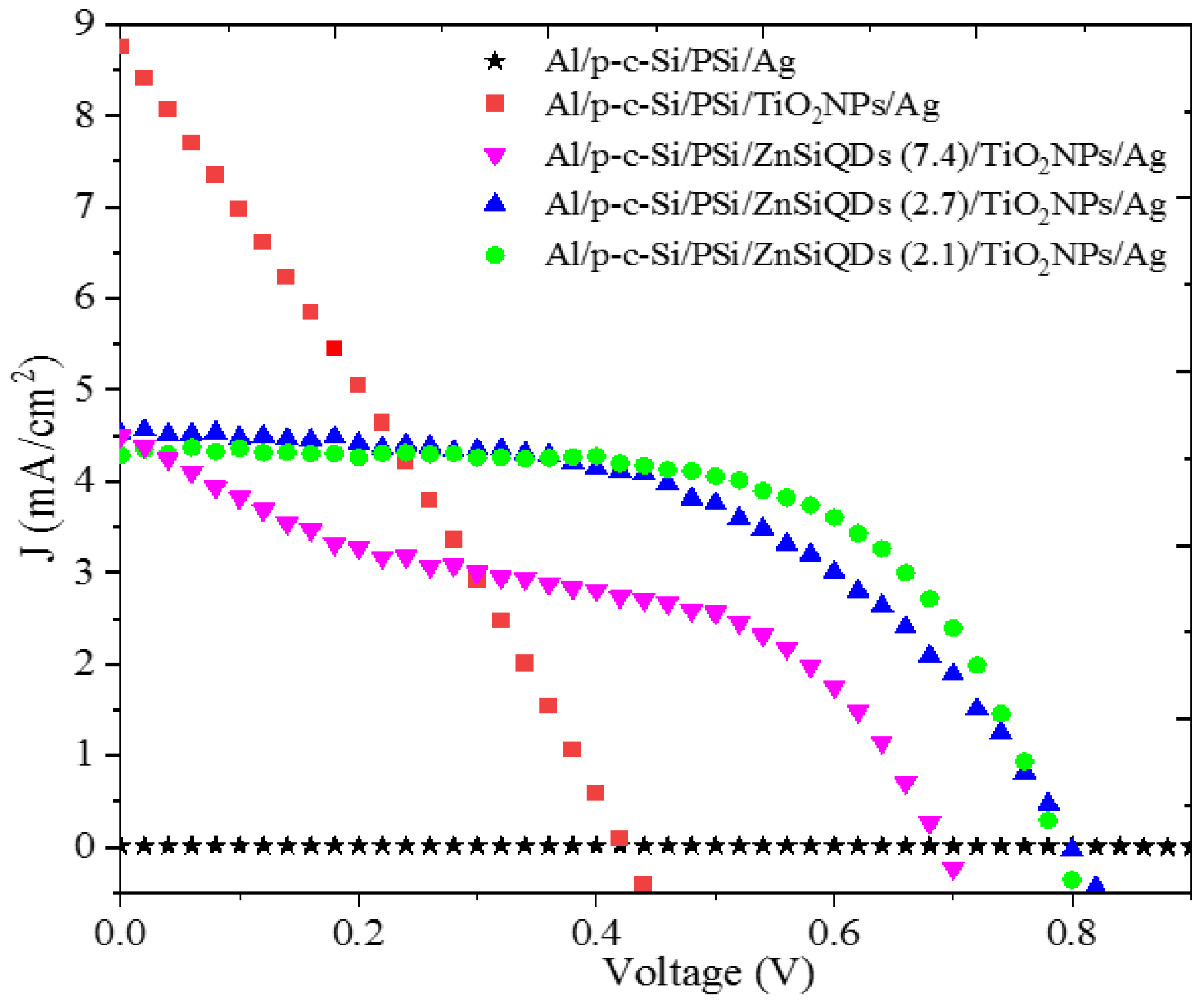
3.3. Effect of TiO2 NPs on Cell’s Performance
3.4. Effect of Deposited Single Layer of ZnSiQDs (SL-ZnSiQDs) on Cell’s Performance
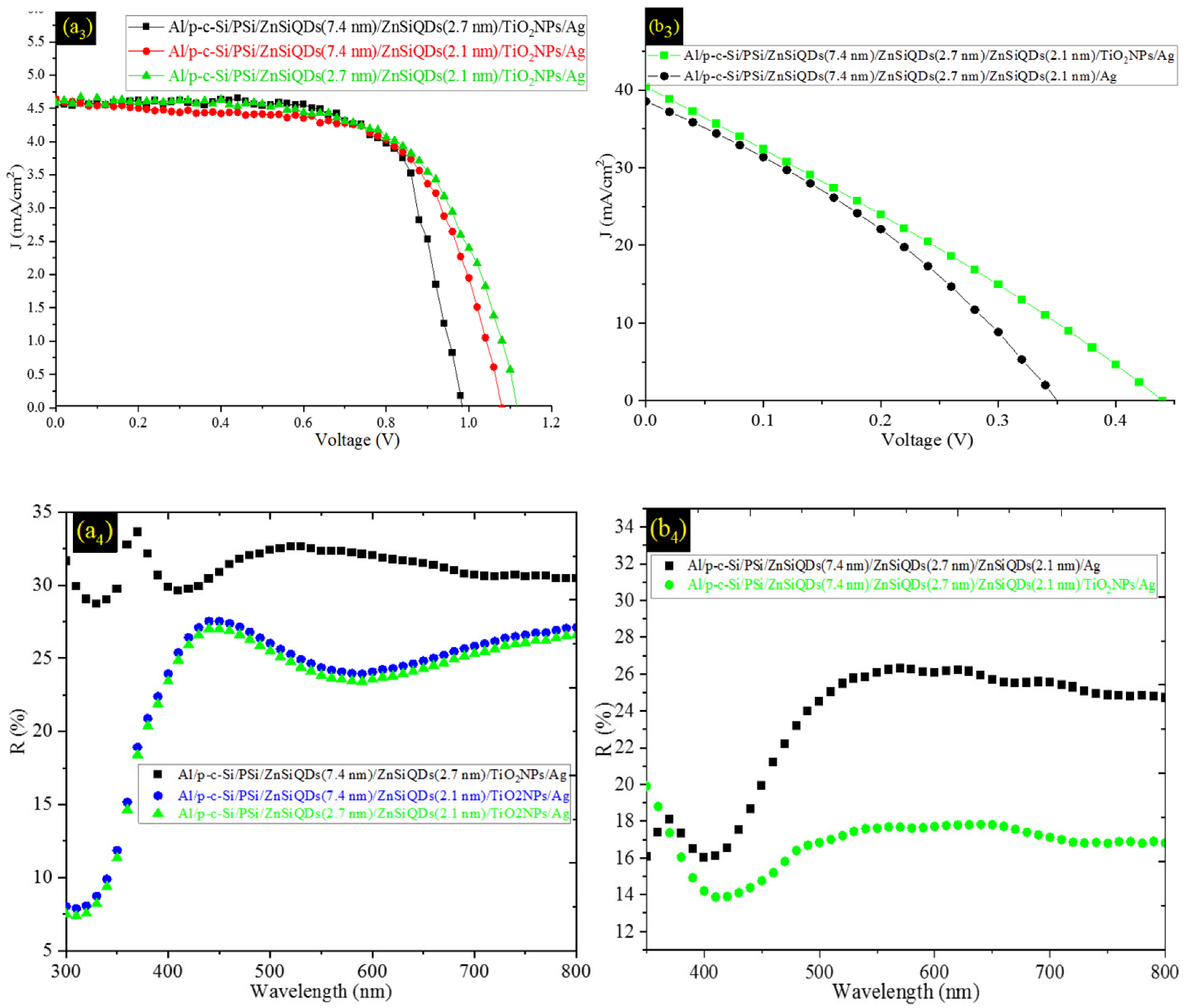
3.5. Effect of Deposited ZnSiQDs Double and Triple Layers (DL-ZnSiQDs and TL-ZnSiQDs) on Cell’s Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaurasiya, N.; Kumar, U.; Sikarwar, S.; Yadav, B.C.; Yadawa, P.K. Synthesis of TiO2 nanorods using wet chemical method and their photovoltaic and humidity sensing applications. Sens. Int. 2021, 2, 100095. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, D.H.; Lee, H.S.; Jang, C.W.; Kim, J.M.; Seo, S.W.; Kim, S.; Choi, S.H. Enhancement of efficiency in graphene/porous silicon solar cells by co-doping graphene with gold nanoparticles and bis(trifluoromethanesulfonyl)-amide. J. Mater. Chem. C 2017, 5, 9005–9011. [Google Scholar] [CrossRef]
- Kralik, M.; Hola, M.; Jurecka, S. Optical properties of porous silicon solar cells for use in transport. Commun.-Sci. Lett. Univ. Zilina 2019, 21, 53–58. [Google Scholar] [CrossRef]
- Raghunathan, D. Black silicon for higher efficiency in solar cells. Appl. Mech. Mater. 2015, 787, 92–96. [Google Scholar] [CrossRef]
- Chuang, C.-H.M.; Brown, P.R.; Bulović, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, R.; Manso-silva, M. Composites Hybrid porous silicon/silver nanostructures for the development of enhanced photovoltaic devices. J. Mater. Sci. 2020, 55, 5458–5470. [Google Scholar] [CrossRef]
- Garnett, E.; Yang, P. Light trapping in silicon nanowire solar cells. Nano Lett. 2010, 10, 1082–1087. [Google Scholar] [CrossRef]
- Selopal, G.S.; Zhao, H.; Wang, Z.M.; Rosei, F. Core/Shell Quantum Dots Solar Cells. Adv. Funct. Mater. 2020, 30, 1908762. [Google Scholar] [CrossRef]
- Oh, J.; Yuan, H.C.; Branz, H.M. An 18.2%-efficient black-silicon solar cell achieved through control of carrier recombination in nanostructures. Nat. Nanotechnol. 2012, 7, 743–748. [Google Scholar] [CrossRef]
- Morozova, S.; Alikina, M.; Vinogradov, A.; Pagliaro, M. Silicon Quantum Dots: Synthesis, Encapsulation, and Application in Light-Emitting Diodes. Front. Chem. 2020, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Gelloz, B.; Juangsa, F.B.; Nozaki, T.; Asaka, K.; Koshida, N.; Jin, L. Si/SiO2 Core/Shell Luminescent Silicon Nanocrystals and Porous Silicon Powders with High Quantum Yield, Long Lifetime, and Good Stability. Front. Phys. 2019, 7, 47. [Google Scholar] [CrossRef]
- Cheng, X.; Lowe, S.B.; Reece, J.; Gooding, J.J.; Lowe, S.B. Chem Soc Rev Colloidal silicon quantum dots: From preparation to the modification of self-assembled monolayers. Chem. Soc. Rev. 2014, 43, 2680–2700. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, J.L.; Curtis, C.L.; Credo, G.M.; Sailor, M.J.; Kavanagh, K.L. Luminescent colloidal silicon suspensions from porous silicon. Science 1992, 255, 66–68. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.; Liao, F.; Dang, Q.; Shao, M. Fluorescent-stable and water-soluble two-component-modified silicon quantum dots and their application for bioimaging. J. Lumin. 2019, 215, 116644. [Google Scholar] [CrossRef]
- Gallach-Pérez, D.; Muñoz-Noval, A.; García-Pelayo, L.; Manso-Silván, M.; Torres-Costa, V. Tunnel conduction regimes, white-light emission and band diagram of porous silicon–zinc oxide nanocomposites. J. Lumin. 2017, 191, 107–111. [Google Scholar] [CrossRef]
- Harizi, A.; Laatar, F.; Ezzaouia, H. Physical properties enhancement of porous silicon treated with In2O3 as a antireflective coating. Results Phys. 2019, 12, 1716–1724. [Google Scholar] [CrossRef]
- Herbert, F.W.; Krishnamoorthy, A.; Van Vliet, K.J.; Yildiz, B. Quantification of electronic band gap and surface states on FeS2 (100). Surf. Sci. 2013, 618, 53–61. [Google Scholar] [CrossRef]
- Shen, G.; Du, Z.; Pan, Z.; Du, J.; Zhong, X. Solar paint from TiO2 particles supported quantum dots for photoanodes in quantum dot–sensitized solar cells. ACS Omega 2018, 3, 1102–1109. [Google Scholar] [CrossRef]
- Green, M.A.; Bremner, S.P. Energy conversion approaches and materials for high-efficiency photovoltaics. Nat. Mater. 2017, 16, 23–34. [Google Scholar] [CrossRef]
- Tian, J.; Shen, T.; Liu, X.; Fei, C.; Lv, L.; Cao, G. Enhanced performance of PbS-quantum-dot-sensitized solar cells via optimizing precursor solution and electrolytes. Sci. Rep. 2016, 6, 23094. [Google Scholar] [CrossRef]
- Latif, H.; Ashraf, S.; Ra, M.S.; Imtiaz, A.; Sattar, A.; Zaheer, S.; Ammara, S.; Usman, A. A novel, PbS quantum dot-Sensitized solar cell structure with TiO2—fMWCNTS nano-composite fi lled meso-porous anatase TiO2 photoanode. Sol. Energy 2020, 204, 617–623. [Google Scholar] [CrossRef]
- Conibeer, G.; Green, M.; Cho, E.-C.; König, D.; Cho, Y.-H.; Fangsuwannarak, T.; Scardera, G.; Pink, E.; Huang, Y.; Puzzer, T. Silicon quantum dot nanostructures for tandem photovoltaic cells. Thin Solid Films 2008, 516, 6748–6756. [Google Scholar] [CrossRef]
- Nelson, J. The Physics of Solar Cells; World Scientific Publishing Company: Singapore, 2003; ISBN 1848168233. [Google Scholar]
- Rosiles-Perez, C.; Sidhik, S.; Ixtilico-Cortés, L.; Robles-Montes, F.; López-Luke, T.; Jiménez-González, A.E. High short-circuit current density in a non-toxic Bi2S3 Quantum dot sensitized solar cell. Mater. Today Energy 2021, 21, 100783. [Google Scholar] [CrossRef]
- Rao, H.; Zhou, M.; Pan, Z.; Zhong, X. Quantum dot materials engineering boosting the quantum dot sensitized solar cell efficiency over 13%. J. Mater. Chem. A 2020, 8, 10233–10241. [Google Scholar] [CrossRef]
- Reiss, P.; Carriere, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of semiconductor nanocrystals, focusing on nontoxic and earth-abundant materials. Chem. Rev. 2016, 116, 10731–10819. [Google Scholar] [CrossRef]
- Almomani, M.S.; Ahmed, N.M.; Rashid, M.; Almessiere, M.A.; Altowyan, A.S. Broadband visible emission from photoelectrochemical etched porous silicon quantum dots containing zinc. Mater. Chem. Phys. 2021, 258, 123935. [Google Scholar] [CrossRef]
- Almomani, M.S.; Ahmed, N.M.; Rashid, M.; Almessiere, M.A.; Altowyan, A.S. White, blue and green emission from Si QDs derived from zinc incorporated porous silicon. J. Lumin. 2021, 232, 117845. [Google Scholar] [CrossRef]
- Brachmann, E.; Seifert, M.; Oswald, S.; Menzel, S.; Gemming, T. Evaluation of Surface Cleaning Procedures for CTGS Substrates for SAW Technology with XPS. Materials (Basel) 2017, 10, 1373. [Google Scholar] [CrossRef]
- Lee, D.-S.; Liu, T.-K. Preparation of TiO2 sol using TiCl4 as a precursor. J. Sol-Gel Sci. Technol. 2002, 25, 121–136. [Google Scholar] [CrossRef]
- Sasirekha, N.; Rajesh, B.; Chen, Y.W. Synthesis of TiO2 sol in a neutral solution using TiCl4 as a precursor and H2O2 as an oxidizing agent. Thin Solid Films 2009, 518, 43–48. [Google Scholar] [CrossRef]
- Ischenko, A.A.; Fetisov, G.V.; Aslalnov, L.A. Nanosilicon: Properties, Synthesis, Applications, Methods of Analysis and Control; CRC Press: Boca Raton, FL, USA, 2014; ISBN 0429073755. [Google Scholar]
- Hussein, M.J.; Yunus, W.M.M.; Kamari, H.M.; Zakaria, A.; Oleiw, H.F. Effect of current density and etching time on photoluminescence and energy band gap of p-type porous silicon. Opt. Quantum Electron. 2016, 48, 194. [Google Scholar] [CrossRef]
- Ramesh, M.; Nagaraja, H.S. Effect of current density on morphological, structural and optical properties of porous silicon. Mater. Today Chem. 2017, 3, 10–14. [Google Scholar] [CrossRef]
- Abdelhameed, M.; Jevasuwan, W.; Subramani, T.; Chen, J.; Fukata, N. Efficiency enhancement of Si nanostructure hybrid solar cells by optimizing non-radiative energy transfer from Si quantum dots. Nano Energy 2021, 82, 105728. [Google Scholar] [CrossRef]
- Ingham, B.; Toney, M.F. X-ray diffraction for characterizing metallic films. In Metallic Films for Electronic, Optical and Magnetic Applications; Elsevier: Burlington, MA, USA, 2014; pp. 3–38. [Google Scholar]
- Almomani, M.S.; Ahmed, N.M.; Rashid, M.; Ali, M.K.M.; Akhdar, H.; Aldaghri, O.; Ibnaouf, K.H. Enhancement of Temperature Fluorescence Brightness of Zn@Si Core-Shell Quantum Dots Produced via a Unified Strategy. Nanomaterials 2021, 11, 3158. [Google Scholar] [CrossRef]
- Norazmi, F.S.; Chaudhary, K.T.; Mazalan, E.; Hader, Z.; Ali, J. Effect of various amount of ammonium hydroxide on morphology of silica nanoparticles grown by sol-gel. Malays. J. Fundam. Appl. Sci. 2018, 14, 482–484. [Google Scholar] [CrossRef]
- Singh, M.; Goyal, M.; Devlal, K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef]
- Kadhum, H.A.; Mahdi, W.; Ahmed, S.; Rheima, M. Improved PSi/c–Si and Ga/PSi/c–Si nanostructures dependent solar cell efficiency. Appl. Phys. A 2020, 126, 802. [Google Scholar] [CrossRef]
- Gong, X.; Bustillo, J.; Blanc, L.; Gautier, G. FEM simulation on elastic parameters of porous silicon with different pore shapes. Int. J. Solids Struct. 2020, 190, 238–243. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X. Pore-size dependence of the heat conduction in porous silicon and phonon spectral energy density analysis. Phys. Lett. A 2020, 384, 126503. [Google Scholar] [CrossRef]
- Sohn, H. Refractive index of porous silicon. Handb. Porous Silicon 2014, 1, 12. [Google Scholar]
- Naderi, N.; Moghaddam, M. Ultra-sensitive UV sensors based on porous silicon carbide thin films on silicon substrate. Ceram. Int. 2020, 46, 13821–13826. [Google Scholar] [CrossRef]
- Solanki, C.S.; Bilyalov, R.R.; Poortmans, J.; Celis, J.; Nijs, J. Self-Standing Porous Silicon Films by One-Step Anodizing. J. Electrochem. Soc. 2004, 151, C307–C314. [Google Scholar] [CrossRef]
- Herino, R.; Bomchil, G.; Barla, K.; Bertrand, C.; Ginoux, J.L. Porosity and pore size distributions of porous silicon layers. J. Electrochem. Soc. 1987, 134, 1994. [Google Scholar] [CrossRef]
- Virgili, U.R.I.; Of, C.; Silicon, P.; Optical, M. Fundamentals of porous silicon and applications. In Design, Fabrication and Characterization of Porous Silicon Multilayer Optical Devices; Universitat Rovira I Virgili: Tarragona, Spain, 2007. [Google Scholar]
- Nayef, U.M.; Muayad, M.W. Typical of morphological properties of porous silicon. Int. J. Basic Appl. Sci. IJBAS-IJENS 2013, 13, 15–17. [Google Scholar]
- Wijayanti, S.; Suryana, R. Variation of etching time on formation of porous silicon on p-type Si (111) using electrochemical anodization method. J. Phys. Conf. Ser. 2021, 1825, 12067. [Google Scholar] [CrossRef]
- Kulathuraan, K.; Mohanraj, K.; Natarajan, B. Structural, optical and electrical characterization of nanostructured porous silicon: Effect of current density. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kopani, M.; Mikula, M.; Kosnac, D.; Vojtek, P.; Gregus, J.; Vavrinsky, E.; Jergel, M.; Pincik, E. Effect of etching time on structure of p-type porous silicon. Appl. Surf. Sci. 2018, 461, 44–47. [Google Scholar] [CrossRef]
- Ramizy, A.; Hassan, Z.; Omar, K. Porous silicon nanowires fabricated by electrochemical and laser-induced etching. J. Mater. Sci. Mater. Electron. 2011, 22, 717–723. [Google Scholar] [CrossRef]
- Ogawa, S.; Uehara, N.; Ohmukai, M.; Tsutsumi, Y. The Effect of Surface Roughness on Photoluminescence of Porous Silicon. MRS Online Proc. Libr. 2000, 638, 5281. [Google Scholar] [CrossRef]
- Sahin, G. Effect of wavelength on the electrical parameters of a vertical parallel junction silicon solar cell illuminated by its rear side in frequency domain Results in Physics Effect of wavelength on the electrical parameters of a vertical parallel junction sili. Results Phys. 2017, 6, 107–111. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, J.H.; Kim, J.H.; Jang, C.W.; Seo, S.W.; Lee, H.S.; Kim, S.; Choi, S.-H. Graphene/porous silicon Schottky-junction solar cells. J. Alloys Compd. 2017, 715, 291–296. [Google Scholar] [CrossRef]
- Saslow, W.M. Chapter 7-Ohm’s Law: Electric Current Is Driven by emf, and Limited byElectrical Resistance. Electr. Magn. Light 2002, 281–335. [Google Scholar] [CrossRef]
- Almomani, M.S.; Ahmed, N.M.; Rashid, M.; Suardi, N.; Almessiere, M.A.; Madkhali, N.; Aldaghri, O.A.; Ibnaouf, K.H. Photovoltaic Performance of Spherical TiO2 Nanoparticles Derived from Titanium Hydroxide Ti(OH)4: Role of Annealing Varying Temperature. Energies 2022, 15, 1648. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, Y.; Mi, Y.; Wang, W.; Lei, Z. Highly ordered micro-meso-macroporous Co-N-doped carbon polyhedrons from bimetal-organic frameworks for rechargeable Zn-air batteries. J. Colloid Interface Sci. 2021, 598, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ostadebrahim, M.; Dehghani, H. The study of the treatment of cerium-zinc sulfide passivation layer on photovoltaic performance of CdSe0. 2S0. 8 ternary quantum dot sensitized solar cells. J. Power Sources 2021, 507, 230266. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, P.; Li, D.; Zeng, X.; Shan, D. Size-Dependent and Enhanced Photovoltaic Performance of Solar Cells Based on Si Quantum Dots. Energies 2020, 13, 4845. [Google Scholar] [CrossRef]
- Song, D.; Cho, E.-C.; Conibeer, G.; Flynn, C.; Huang, Y.; Green, M.A. Structural, electrical and photovoltaic characterization of Si nanocrystals embedded SiC matrix and Si nanocrystals/c-Si heterojunction devices. Sol. Energy Mater. Sol. Cells 2008, 92, 474–481. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, J.; Chen, W.; Yuan, L.; Teh, Z.L.; Yang, J.; Cui, X.; Conibeer, G.; Patterson, R.; Huang, S. Enhancing PbS Colloidal Quantum Dot Tandem Solar Cell Performance by Graded Band Alignment. J. Phys. Chem. Lett. 2019, 10, 5729–5734. [Google Scholar] [CrossRef]
- Bi, Y.; Pradhan, S.; Akgul, M.Z.; Gupta, S.; Stavrinadis, A.; Wang, J.; Konstantatos, G. Colloidal quantum dot tandem solar cells using chemical vapor deposited graphene as an atomically thin intermediate recombination layer. ACS Energy Lett. 2018, 3, 1753–1759. [Google Scholar] [CrossRef]
- Shi, G.; Wang, Y.; Liu, Z.; Han, L.; Liu, J.; Wang, Y.; Lu, K.; Chen, S.; Ling, X.; Li, Y.; et al. Stable and Highly Efficient PbS Quantum Dot Tandem Solar Cells Employing a Rationally Designed Recombination Layer. Adv. Energy Mater. 2017, 7, 1602667. [Google Scholar] [CrossRef]
- Lv, Y.; Tong, H.; Cai, W.; Zhang, Z.; Chen, H.; Zhou, X. Boosting the efficiency of commercial available carbon-based perovskite solar cells using Zinc-doped TiO2 nanorod arrays as electron transport layer. J. Alloys Compd. 2021, 851, 156785. [Google Scholar] [CrossRef]
- Deb, P.; Dhar, J.C. Graphene oxide charge blocking layer with high K TiO2 nanowire for improved capacitive memory. J. Alloys Compd. 2021, 868, 159095. [Google Scholar] [CrossRef]
- Hou, B.; Cho, Y.; Kim, B.S.; Hong, J.; Park, J.B.; Ahn, S.J.; Sohn, J.I.; Cha, S.; Kim, J.M. Highly monodispersed PbS quantum dots for outstanding cascaded-junction solar cells. ACS Energy Lett. 2016, 1, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Muthalif, M.P.A.; Choe, Y. Surface modification of CuS counter electrodes by hydrohalic acid treatment for improving interfacial charge transfer in quantum-dot-sensitized solar cells. J. Colloid Interface Sci. 2021, 595, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Akhil, S.; Kusuma, J.; Akash, S.; Balakrishna, R.G. Perovskite-like ceramic hole transport material for quantum dot sensitized solar cells. Sol. Energy 2021, 224, 355–360. [Google Scholar] [CrossRef]
- Bin Jin, B.; Kong, S.Y.; Zhang, G.Q.; Chen, X.Q.; Ni, H.S.; Zhang, F.; Wang, D.J.; Zeng, J.H. Voltage-assisted SILAR deposition of CdSe quantum dots to construct a high performance of ZnS/CdSe/ZnS quantum dot-sensitized solar cells. J. Colloid Interface Sci. 2021, 586, 640–646. [Google Scholar] [CrossRef]
- Chen, M.X.; Bai, Y.Q.; Guan, X.N.; Chen, J.W.; Zeng, J.H. Phosphating passivation layer for quantum dot sensitized solar cells. Thin Solid Films 2021, 727, 138678. [Google Scholar] [CrossRef]
- Prasad, A.K.; Jo, I.-R.; Kang, S.-H.; Ahn, K.-S. Novel method for synthesis of reduced graphene oxide–Cu2S and its application as a counter electrode in quantum-dot-sensitized solar cells. Appl. Surf. Sci. 2021, 564, 150393. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, X.; Zhang, S.; Liu, L.; Wan, L.; Guo, H.; Yang, X.; Cheng, Z.; Hu, L.; Niu, H. Spray-coated copper antimony sulfide (CuSbS2) thin film: A novel counter electrode for quantum dot-sensitized solar cells. Mater. Sci. Semicond. Process. 2021, 124, 105613. [Google Scholar] [CrossRef]
- Bin Jin, B.; Huang, H.S.; Kong, S.Y.; Zhang, G.Q.; Yang, B.; Jiang, C.X.; Zhou, Y.; Wang, D.J.; Zeng, J.H. Antimony tin oxide/lead selenide composite as efficient counter electrode material for quantum dot-sensitized solar cells. J. Colloid Interface Sci. 2021, 598, 492–499. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, S.; Fang, Y.; Xie, D.; Zhou, X.; Lin, Y. Effect of linkers with different chemical structures on photovoltaic performance of CdSe quantum dot-sensitized solar cells. Electrochim. Acta 2021, 367, 137452. [Google Scholar] [CrossRef]
- Simi, N.J.; Bernadsha, S.B.; Thomas, A.; Ison, V.V. Quantum dot sensitized solar cells using type-II CdSe-Cu2Se core-shell QDs. Results Opt. 2021, 4, 100088. [Google Scholar] [CrossRef]
- Jo, I.-R.; Lee, Y.-H.; Kim, H.; Ahn, K.-S. Multifunctional nitrogen-doped graphene quantum dots incorporated into mesoporous TiO2 films for quantum dot-sensitized solar cells. J. Alloys Compd. 2021, 870, 159527. [Google Scholar] [CrossRef]
- Pandi, D.V.; Saraswathi, V.; Muthukumarasamy, N.; Agilan, S.; Balraju, P.; Velauthapillai, D. C-axis oriented ZnO nanorods based quantum dot solar cells. Opt. Mater. (Amst.) 2021, 112, 110774. [Google Scholar] [CrossRef]
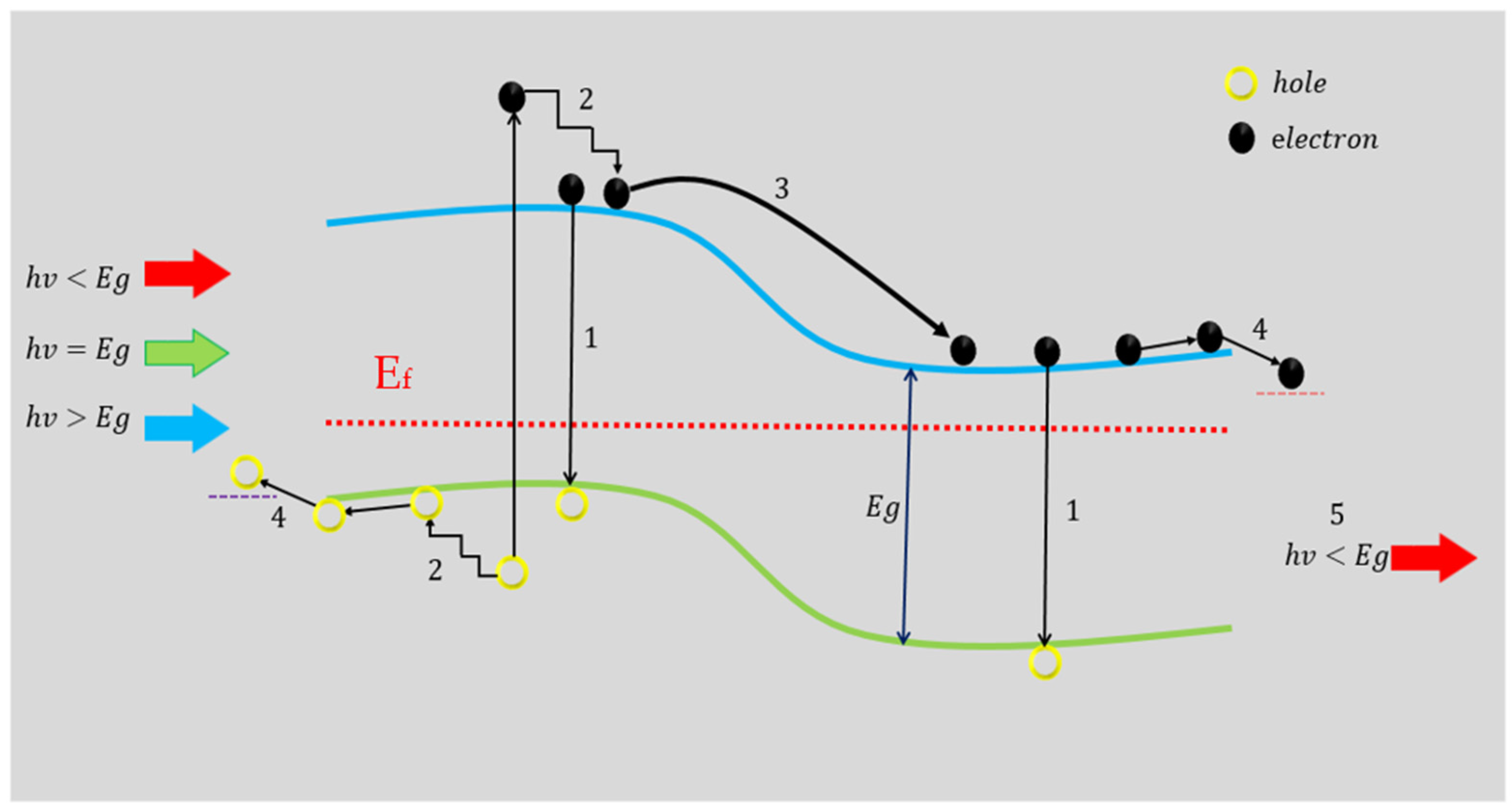


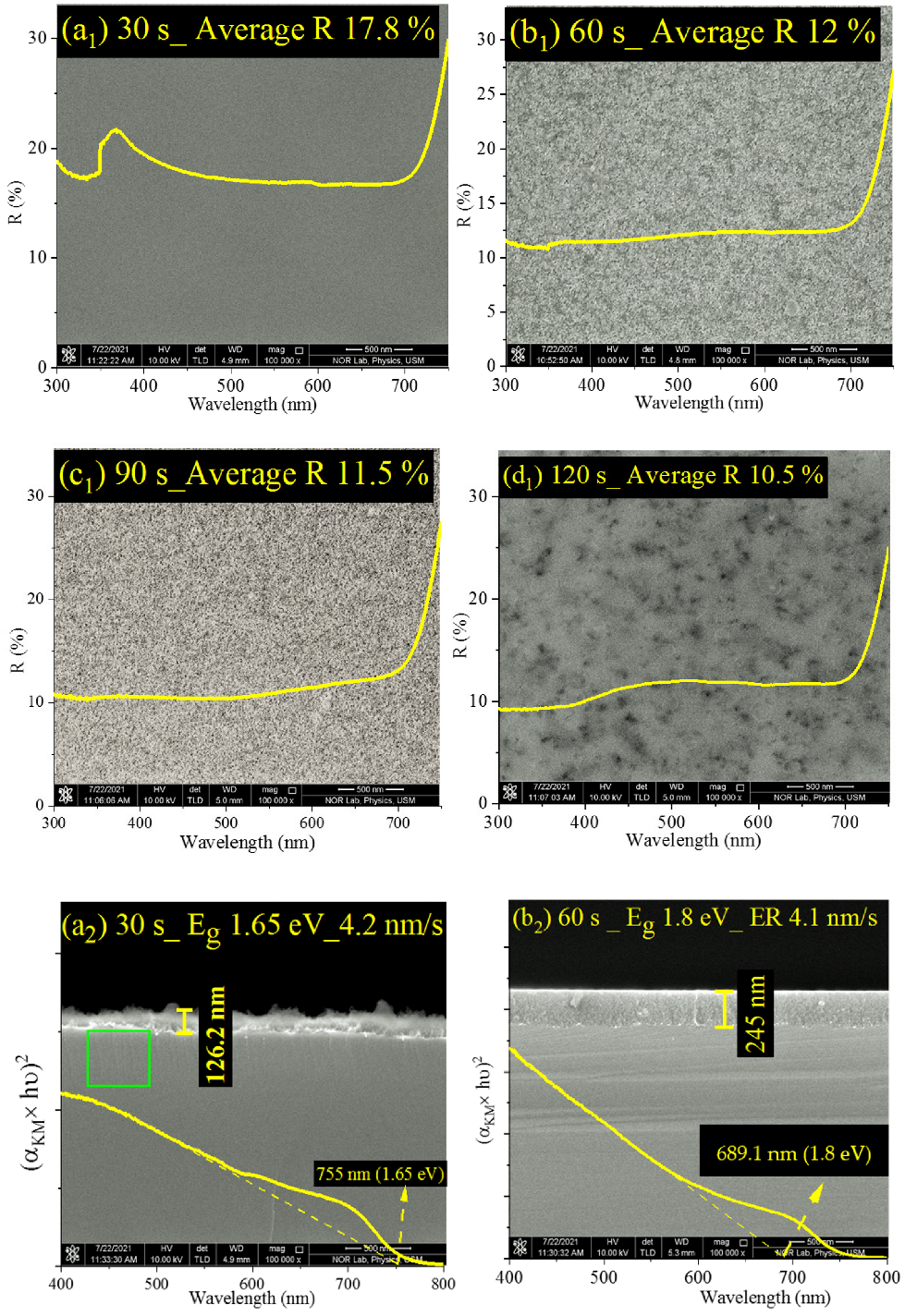
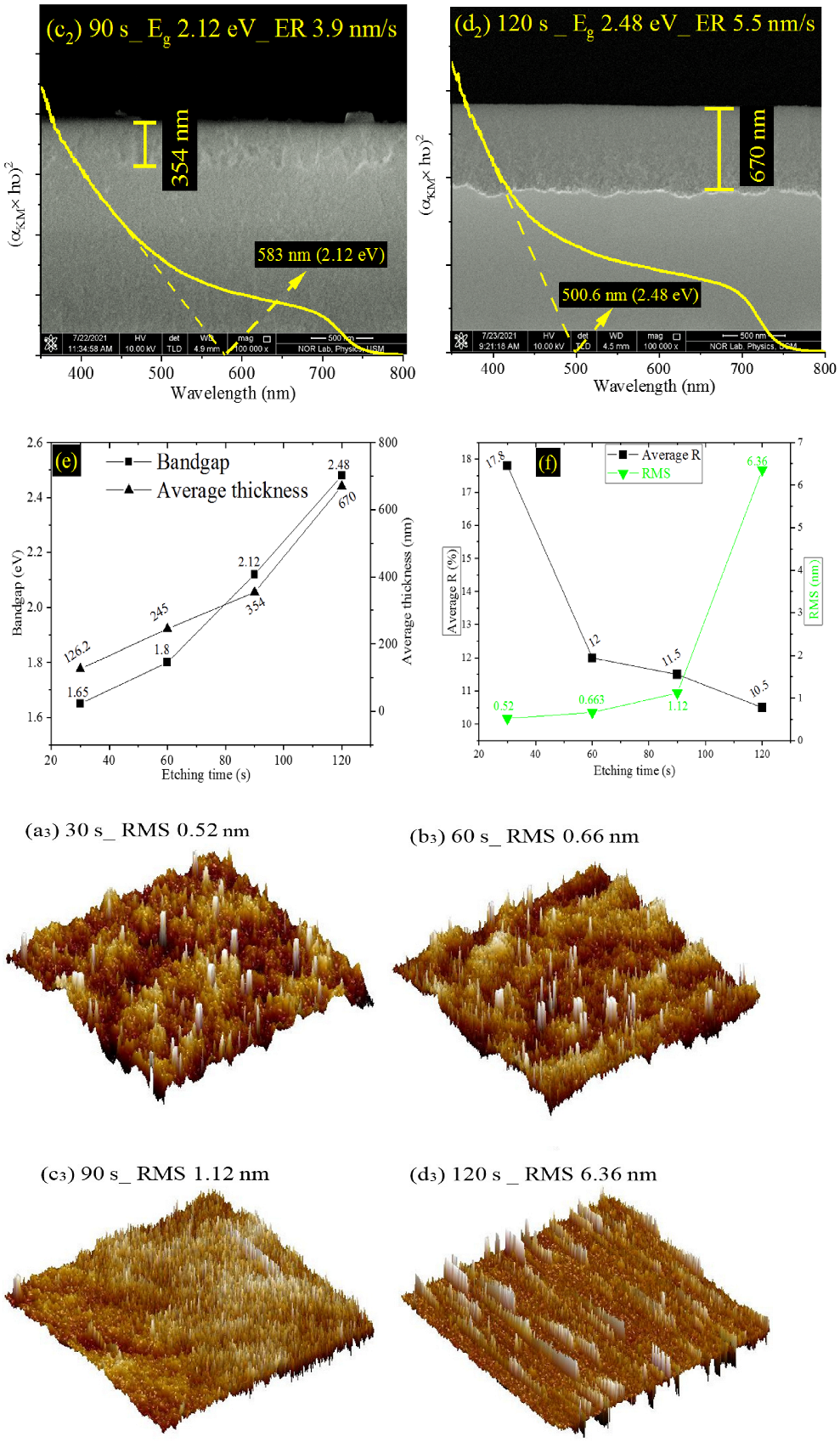
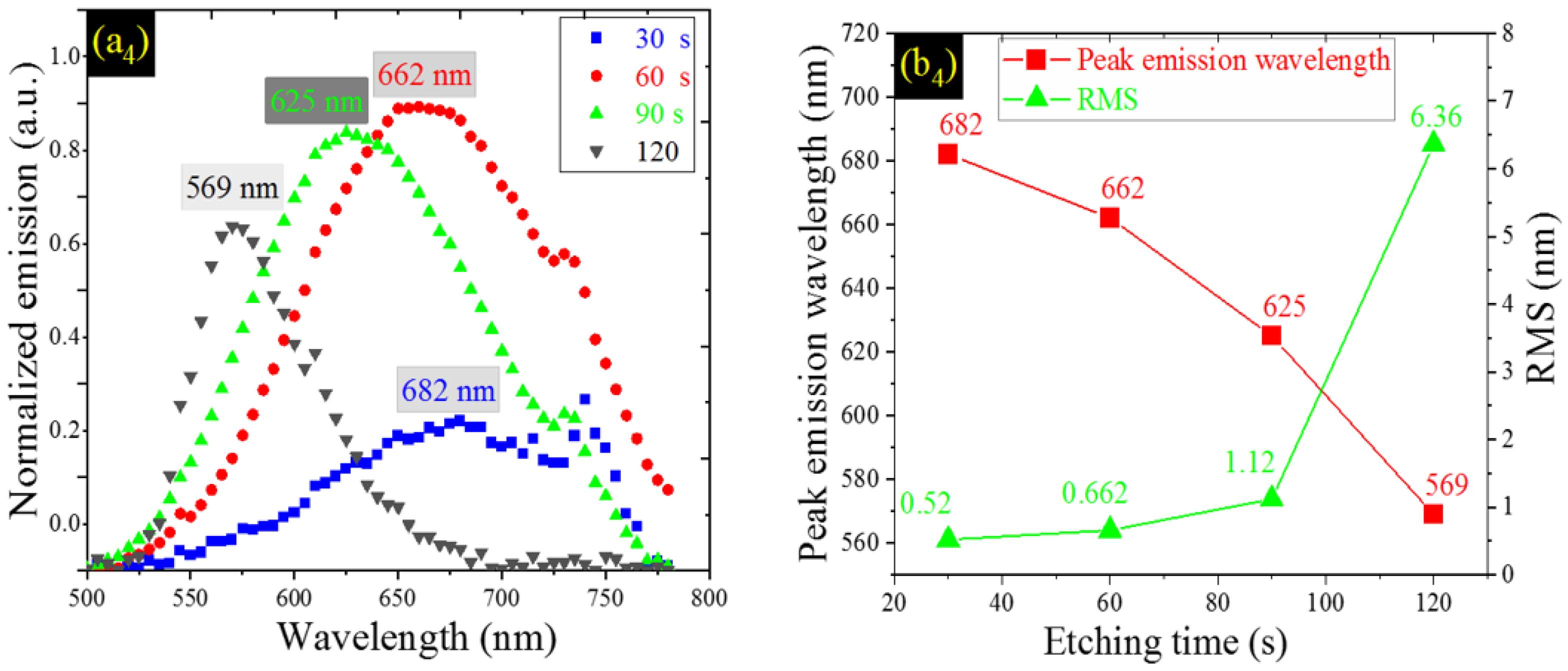

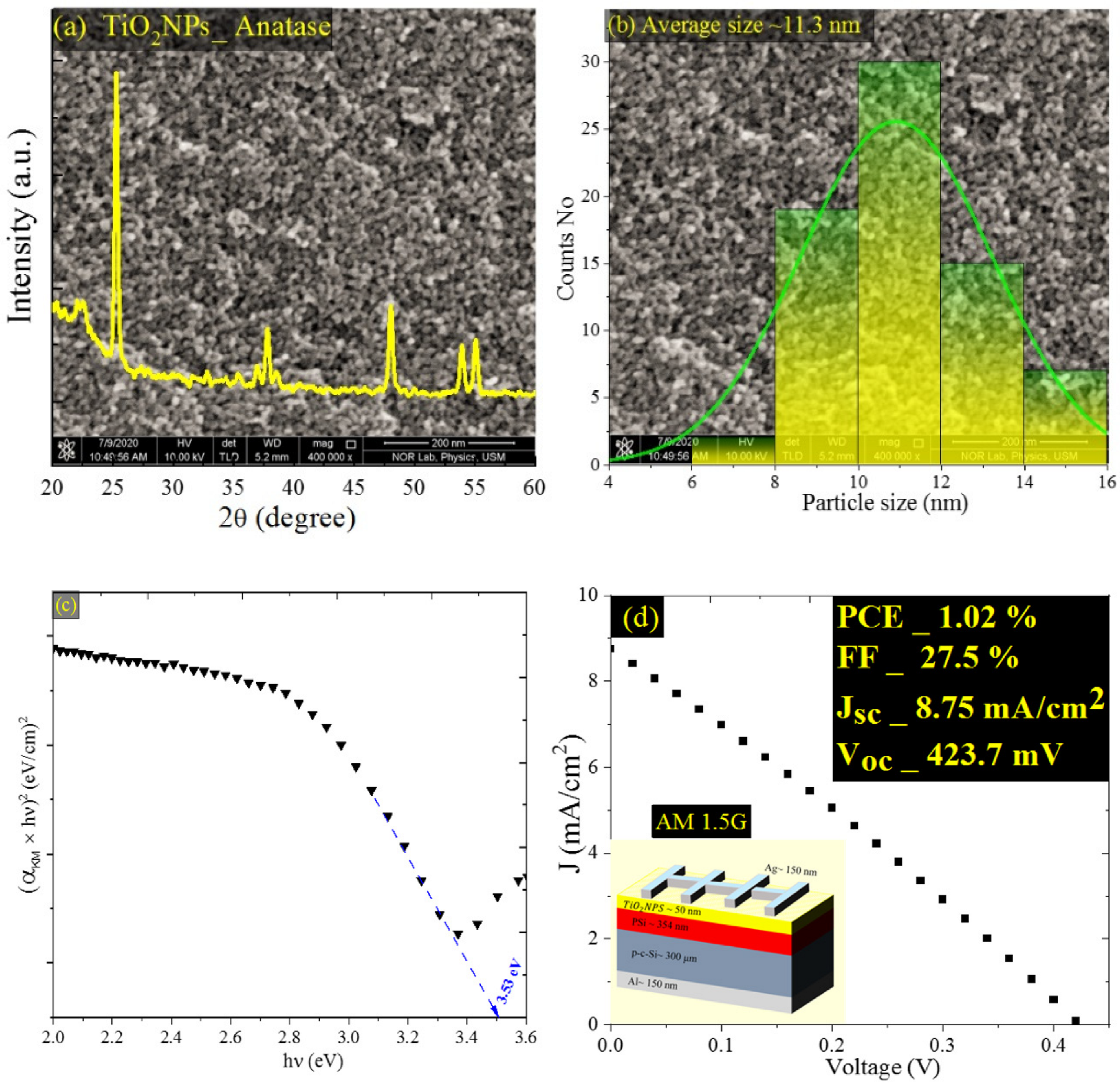


| Etching Time (s) | JSC (mA/cm2) | VOC (V) | FF (%) | PCE or η (%) |
|---|---|---|---|---|
| 0 | 3.6 × 10−3 | 0.54 | 61 | 1.19 × 10−3 |
| 30 | 6.41 × 10−3 | 0.85 | 50 | 2.74 × 10−3 |
| 60 | 6.37 × 10−3 | 0.98 | 64 | 4.05 × 10−3 |
| 90 | 1.54 × 10−2 | 0.88 | 54 | 7.39 × 10−3 |
| 120 | 2.59 × 10−3 | 0.48 | 47 | 5.92 × 10−4 |
| Solar Cell Structure | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Al/p-c-Si/PSi/TiO2NPs/Ag | 8.75 | 423.7 | 27.5 | 1.02 |
| Al/p-c-Si/PSi/ZnSiQDs (7.4 nm)/TiO2NPs/Ag | 4.49 | 695 | 41.03 | 1.28 |
| Al/p-c-Si/PSi/ZnSiQDs (2.7 nm)/TiO2NPs/Ag | 4.52 | 801.1 | 51.88 | 1.88 |
| Al/p-c-Si/PSi/ZnSiQDs (2.1 nm)/TiO2NPs/Ag | 4.28 | 789.6 | 64.11 | 2.17 |
| Solar Cell Structure | PCE (%) | FF (%) | JSC (mA/cm2) | VOC (mV) |
|---|---|---|---|---|
| Aluminum/p-c-Si/PSi/Silver | 0.0074 | 54.52 | 0.0154 | 879.6 |
| Al/p-c-Si/PSi/TiO2NP/Ag | 1.02 | 27.5 | 8.75 | 423.7 |
| Al/p-c-Si/PSi/ZnSiQDs (7.4 nm)/TiO2NP/Ag | 1.28 | 41.03 | 4.49 | 695 |
| Al/p-c-Si/PSi/ZnSiQDs (2.7 nm)/TiO2NP/Ag | 1.88 | 51.88 | 4.52 | 801.1 |
| Al/p-c-Si/PSi/ZnSiQDs (2.1 nm)/TiO2NP/Ag | 2.17 | 64.11 | 4.28 | 789.6 |
| Al/p-c-Si/PSi/ZnSiQDs(7.4 nm)/ZnSiQDs(2.7 nm)/TiO2NPs/Ag | 3.19 | 71.35 | 4.55 | 983.32 |
| Al/p-c-Si/PSi/ZnSiQDs(7.4 nm)/ZnSiQDs(2.1 nm)/TiO2NPs/Ag | 3.23 | 64.34 | 4.64 | 1079.7 |
| Al/p-c-Si/PSi/ZnSiQDs(2.7 nm)/ZnSiQDs(2.1 nm)/TiO2NPs/Ag | 3.3 | 64.48 | 4.58 | 1116.93 |
| Al/p-c-Si/PSi/ZnSiQDs(7.4 nm)/ZnSiQDs(2.7 nm)/ZnSiQDs(2.1 nm)/Ag | 4.4 | 32.6 | 38.6 | 351.9 |
| Al/p-c-Si/PSi/ZnSiQDs(7.4 nm)/ZnSiQDs(2.7 nm)/ZnSiQDs(2.1 nm)/TiO2NPs/Ag | 4.9 | 27.6 | 40.4 | 440.8 |
| QD | VOC (V) | JSC (mA/cm2) | FF (%) | η (%) | Ref. |
|---|---|---|---|---|---|
| M-ZnS/CdSeS | 0.6 | 17.57 | 49 | 5.15 | [59] |
| CdS/CdSe/ZnS | 0.614 | 15.37 | 45.1 | 4.25 | [68] |
| CdS | 0.69 | 3.93 | 62.5 | 1.73 | [69] |
| ZnS/CdSe/ZnS | 0.559 | 14.8 | 49.45 | 4.34 | [70] |
| ZnPh | 0.54 | 18.03 | 46.22 | 4.5 | [71] |
| CdS/CdSe/ZnS | 0.56 | 17.2 | 44 | 4.26 | [72] |
| CdS/CdSe | 0.53 | 11.86 | 34 | 2.15 | [73] |
| CdS/CdSe | 0.59 | 17.18 | 55 | 5.59 | [74] |
| CdSe | 0.636 | 16 | 54 | 5.5 | [75] |
| CuSe/CdSe | 0.55 | 17.29 | 51 | 4.83 | [76] |
| N-G/CdSe | 0.48 | 17.78 | 51.88 | 4.88 | [77] |
| Bi2S3 | 0.21 | 21.37 | 37 | 1.63 | [24] |
| CdSe/ZnO | 0.48 | 15.7 | 37.4 | 2.82 | [78] |
| TL-ZnSiQDs | 0.44 | 40.4 | 27.6 | 4.9 | Present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almomani, M.S.; Ahmed, N.M.; Rashid, M.; Ibnaouf, K.H.; Aldaghri, O.A.; Madkhali, N.; Cabrera, H. Performance Improvement of Graded Bandgap Solar Cell via Optimization of Energy Levels Alignment in Si Quantum Dot, TiO2 Nanoparticles, and Porous Si. Photonics 2022, 9, 843. https://doi.org/10.3390/photonics9110843
Almomani MS, Ahmed NM, Rashid M, Ibnaouf KH, Aldaghri OA, Madkhali N, Cabrera H. Performance Improvement of Graded Bandgap Solar Cell via Optimization of Energy Levels Alignment in Si Quantum Dot, TiO2 Nanoparticles, and Porous Si. Photonics. 2022; 9(11):843. https://doi.org/10.3390/photonics9110843
Chicago/Turabian StyleAlmomani, Mohammad S., Naser M. Ahmed, Marzaini Rashid, Khalid Hassan Ibnaouf, Osamah A. Aldaghri, Nawal Madkhali, and Humberto Cabrera. 2022. "Performance Improvement of Graded Bandgap Solar Cell via Optimization of Energy Levels Alignment in Si Quantum Dot, TiO2 Nanoparticles, and Porous Si" Photonics 9, no. 11: 843. https://doi.org/10.3390/photonics9110843
APA StyleAlmomani, M. S., Ahmed, N. M., Rashid, M., Ibnaouf, K. H., Aldaghri, O. A., Madkhali, N., & Cabrera, H. (2022). Performance Improvement of Graded Bandgap Solar Cell via Optimization of Energy Levels Alignment in Si Quantum Dot, TiO2 Nanoparticles, and Porous Si. Photonics, 9(11), 843. https://doi.org/10.3390/photonics9110843









