Ethylammonium Bromide- and Potassium-Added CH3NH3PbI3 Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2019, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hutter, E.M.; Rieder, P.; Grill, I.; Hanisch, J.; Aygüler, M.F.; Hufnagel, A.G.; Handloser, M.; Bein, T.; Hartschuh, A.; et al. Understanding the role of cesium and rubidium additives in perovskite solar cells: Trap states, charge transport, and recombination. Adv. Energy Mater. 2018, 8, 1703057. [Google Scholar] [CrossRef]
- Tyrren-Cruz, S.H.; Saliba, M.; Mayer, T.M.; Juarez-Santiesteban, H.; Mathew, X.; Nienhaus, L.; Tress, W.; Erodici, P.M.; Sher, M.-J.; Bawendi, G.M.; et al. Enhanced charge carrier mobility and lifetime suppress hysteresis and improve efficiency in planar perovskite solar cells. Energy Environ. Sci. 2018, 11, 78–86. [Google Scholar] [CrossRef]
- Tailor, N.K.; Abdi-Jalebi, M.; Gupta, V.; Hu, H.; Dar, M.I.; Li, G.; Satapathi, S. Recent progress in morphology optimization in perovskite solar cell. J. Mater. Chem. A 2020, 8, 21356–21386. [Google Scholar] [CrossRef]
- Oku, T. Crystal structures of perovskite halide compounds used for solar cells. Rev. Adv. Mater. Sci. 2020, 59, 264–305. [Google Scholar] [CrossRef]
- Rao, M.K.; Sangeetha, D.N.; Selvakumar, M.; Sudhakar, Y.N.; Mahesha, M.G. Review on persistent challenges of perovskite solar cells’ stability. Sol. Energy 2021, 218, 469–491. [Google Scholar] [CrossRef]
- Oku, T.; Kandori, S.; Taguchi, M.; Suzuki, A.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Polysilane-inserted methylammonium lead iodide perovskite solar cells doped with formamidinium and potassium. Energies 2020, 13, 4776. [Google Scholar] [CrossRef]
- Zaccaria, F.; Zhang, B.; Goldoni, L.; Imran, M.; Zito, J.; van Beek, B.; Lauciello, S.; De Trizio, L.; Manna, L.; Infante, I. The reactivity of CsPbBr3 nanocrystals toward acid/base ligands. ACS Nano 2022, 16, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kong, W.; Li, W.; Chen, H.; Li, D.; Wang, W.; Xu, B.; Cheng, C.; Jen, A.K.Y. Enhanced stability and photovoltage for inverted perovskite solar cells via precursor engineering. J. Mater. Chem. A 2019, 26, 15880–15886. [Google Scholar] [CrossRef]
- Zhang, M.; Yun, J.S.; Ma, Q.; Zheng, J.; Lau, C.F.J.; Deng, X.; Kim, J.; Kim, D.; Seidel, J.; Green, M.A.; et al. High-efficiency rubidium-incorporated perovskite solar cells by gas quenching. ACS Energy Lett. 2017, 2, 438–444. [Google Scholar] [CrossRef]
- Takada, K.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Fabrication and characterization of ethylammonium- and rubidium-added perovskite solar cells. Chem. Proc. 2022, 9, 14. [Google Scholar] [CrossRef]
- Tang, Z.; Bessho, T.; Awai, F.; Kinoshita, T.; Maitani, M.M.; Jono, R.; Murakami, T.N.; Wang, H.; Kubo, T.; Uchida, S.; et al. Hysteresis-free perovskite solar cells made of potassium-doped organometal halide perovskite. Sci. Rep. 2017, 7, 12183. [Google Scholar] [CrossRef]
- Zhao, W.; Yao, Z.; Yu, F.; Yang, D.; Liu, S. Alkali metal doping for improved CH3NH3PbI3 perovskite solar cells. Adv. Sci. 2018, 5, 1700131. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef]
- Machiba, H.; Oku, T.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Fabrication and evaluation of K-doped MA0.8FA0.1K0.1PbI3(Cl) perovskite solar cells. Chem. Phys. Lett. 2019, 730, 117–123. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, L.; Hao, X.; Tang, Z.; Lai, H.; Zhang, J.; Wanga, W.; Feng, L. Beneficial effects of potassium iodide incorporation on grain boundaries and interfaces of perovskite solar cells. RSC Adv. 2019, 9, 28561–28568. [Google Scholar] [CrossRef]
- Kandori, S.; Oku, T.; Nishi, K.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Fabrication and characterization of potassium- and formamidinium-added perovskite solar cells. J. Ceram. Soc. Jpn. 2020, 128, 805–811. [Google Scholar] [CrossRef]
- Hsu, H.-L.; Chang, C.-C.; Chen, C.-P.; Jiang, B.-H.; Jeng, R.-J.; Cheng, C.-H. High-performance and high-durability perovskite photovoltaic devices prepared using ethylammonium iodide as an additive. J. Mater. Chem. A 2015, 3, 9271–9277. [Google Scholar] [CrossRef]
- Nishi, K.; Oku, T.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Photovoltaic Characteristics of CH3NH3PbI3 Perovskite Solar Cells Added with Ethylammonium Bromide and Formamidinium Iodide. Coatings 2020, 10, 410. [Google Scholar] [CrossRef]
- Oku, T.; Zushi, M.; Imanishi, Y.; Suzuki, A.; Suzuki, K. Microstructures and photovoltaic properties of perovskite-type CH3NH3PbI3 compounds. Appl. Phys. Express 2014, 7, 121601. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y. Effects of annealing on CH3NH3PbI3 (Cl) perovskite photovoltaic devices. J. Ceram. Soc. Jpn. 2018, 126, 56–60. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T. Stability Characterization of PbI2-added CH3NH3PbI3−xClx photovoltaic devices. ACS Appl. Mater. Interfaces 2018, 10, 44443–44451. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T. Effects of co-addition of sodium chloride and copper (ii) bromide to mixed-cation mixed-halide perovskite photovoltaic devices. ACS Appl. Energy Mater. 2020, 9, 7272–7283. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y.; Ueoka, N. Highly (100) -oriented CH3NH3PbI3 (Cl) perovskite solar cells prepared with NH4Cl using an air blow method. RSC Adv. 2018, 8, 10389–10395. [Google Scholar] [CrossRef]
- Taguchi, M.; Suzuki, A.; Oku, T.; Ueoka, N.; Minami, S.; Okita, M. Effects of annealing temperature on decaphenylcyclopentasilane-inserted CH3NH3PbI3 perovskite solar cells. Chem. Phys. Lett. 2019, 737, 136822. [Google Scholar] [CrossRef]
- Oku, T.; Taguchi, M.; Suzuki, A.; Kitagawa, K.; Asakawa, Y.; Yoshida, S.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Effects of polysilane addition to chlorobenzene and high temperature annealing on CH3NH3PbI3 perovskite photovoltaic devices. Coatings 2021, 11, 665. [Google Scholar] [CrossRef]
- Suzuki, A.; Taguchi, M.; Oku, T.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Additive effects of methyl ammonium bromide or formamidinium bromide in methylammonium lead iodide perovskite solar cells using decaphenylcyclopentasilane. J. Mater. Sci. Mater. Electron. 2021, 32, 26449–26464. [Google Scholar] [CrossRef]
- Mizuno, S.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Photovoltaic properties and microstructures of polysilane-added perovskite solar cells. Chem. Proc. 2022, 9, 20. [Google Scholar] [CrossRef]
- Oku, T.; Ueoka, N.; Suzuki, K.; Suzuki, A.; Yamada, M.; Sakamoto, H.; Minami, S.; Fukunishi, S.; Kohno, K.; Miyauchi, S. Fabrication and characterization of perovskite photovoltaic devices with TiO2 nanoparticle layers. AIP Conf. Proc. 2017, 1807, 020014. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Tanaka, H.; Suzuki, A.; Sakamoto, H.; Yamada, M.; Minami, S.; Miyauchi, S.; Tsukada, S. Effects of PbI2 addition and TiO2 electron transport layers for perovskite solar cells. Jpn. J. Appl. Phys. 2018, 57, 08RE05. [Google Scholar] [CrossRef]
- Chen, H.-W.; Sakai, N.; Ikegami, M.; Miyasaka, T. Emergence of hysteresis and transient ferroelectric response in organo-lead halide perovskite solar cells. J. Phys. Chem. Lett. 2014, 6, 164–169. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Perovskite structures by tuning tolerance factor: Formation of formamidinium and cesium lead iodide solid-state alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Tanaka, H.; Oku, T.; Ueoka, N. Structural stabilities of organic-inorganic perovskite crystals. Jpn. J. Appl. Phys. 2018, 57, 08RE12. [Google Scholar] [CrossRef]
- Ferdowsi, P.; Ochoa-Martinez, E.; Alonso, S.S.; Steiner, U.; Saliba, M. Ultrathin polymeric films for interfacial passivation in wide band-gap perovskite solar cells. Sci. Rep. 2020, 10, 22260. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A. Effects of doping with Na, K, Rb, and formamidinium cations on (CH3NH3)0.99Rb0.01Pb0.99Cu0.01I3−x(Cl, Br)x perovskite photovoltaic cells. AIP Adv. 2020, 10, 125023. [Google Scholar] [CrossRef]
- Suzuki, A.; Kitagawa, K.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T. Additive effects of copper and alkali metal halides into methylammonium lead iodide perovskite solar cells. Electron. Mater. Lett. 2022, 18, 176–186. [Google Scholar] [CrossRef]
- Ono, I.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of guanidinium addition to CH3NH3PbI3 perovskite solar cells inserted with decaphenylpentasilane. Chem. Proc. 2022, 9, 13. [Google Scholar] [CrossRef]
- Suzuki, A.; Kishimoto, K.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T. Additive effect of lanthanide compounds into perovskite layer on photovoltaic properties and electronic structures. Synth. Met. 2022, 287, 117092. [Google Scholar] [CrossRef]
- Enomoto, A.; Suzuki, A.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of Cu, K and guanidinium addition to CH3NH3PbI3 perovskite solar cells. J. Electron. Mater. 2022, 51, 4317–4328. [Google Scholar] [CrossRef]
- Suzuki, A.; Oku, T. Effects of mixed-valence states of Eu-doped FAPbI3 perovskite crystals studied by first-principles calculation. Mater. Adv. 2021, 2, 2609–2616. [Google Scholar] [CrossRef]
- Kishimoto, T.; Oku, T.; Suzuki, A.; Ueoka, N. Additive effects of guanidinium iodide on CH3NH3PbI3 perovskite solar cells. Phys. Status Solidi A 2021, 218, 2100396. [Google Scholar] [CrossRef]
- Okumura, R.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of adding alkali metals and organic cations to Cu-based perovskite solar cells. Appl. Sci. 2022, 12, 1710. [Google Scholar] [CrossRef]
- Ono, I.; Oku, T.; Suzuki, A.; Asakawa, Y.; Terada, S.; Okita, M.; Fukunishi, S.; Tachikawa, T. Fabrication and characterization of CH3NH3PbI3 solar cells with added guanidinium and inserted with decaphenylpentasilane. Jpn. J. Appl. Phys. 2022, 61, SB1024. [Google Scholar] [CrossRef]
- Hayase, S. Research following pb perovskite solar cells. Electrochemistry 2017, 85, 222–225. [Google Scholar] [CrossRef]
- Jeyakumar, R.; Bag, A.; Nekovei, R.; Radhakrishnan, R. Interface studies by simulation on methylammonium lead iodide based planar perovskite solar cells for high efficiency. Sol. Energy 2019, 190, 104–111. [Google Scholar] [CrossRef]
- Ku, W.; Eguiluz, A.G. Band-gap problem in semiconductors revisited: Effects of core states and many-body self-consistency. Phys. Rev. Lett. 2002, 89, 126401. [Google Scholar] [CrossRef]
- Tan, H.; Liu, H.; Li, Y.; Duan, W.; Zhang, S. Understanding the origin of bandgap problem in transition and post-transition metal oxides. J. Chem. Phys. 2019, 151, 124703. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Kohn, W. Nobel Lecture: Electronic structure of matter—Wave functions and density functionals. Rev. Mod. Phys. 1999, 71, 1253–1266. [Google Scholar] [CrossRef]
- Ong, K.P.; Wu, S.; Nguyen, T.H.; Singh, D.J.; Fan, Z.; Sullivan, M.B.; Dang, C. Multi band gap electronic structure in CH3NH3PbI3. Sci. Rep. 2019, 9, 2144. [Google Scholar] [CrossRef]
- Oku, T.; Nakagawa, J.; Iwase, M.; Kawashima, A.; Yoshida, K.; Suzuki, A.; Akiyama, T.; Tokumitsu, K.; Yamada, M.; Nakamura, M. Microstructures and photovoltaic properties of polysilane-based solar cells. Jpn. J. Appl. Phys. 2013, 52, 04CR07. [Google Scholar] [CrossRef]
- Oku, T.; Nomura, J.; Suzuki, A.; Tanaka, H.; Fukunishi, S.; Minami, S.; Tsukada, S. Fabrication and characterization of CH3NH3PbI3 perovskite solar cells added with polysilanes. Int. J. Photoenergy 2018, 2018, 8654963. [Google Scholar] [CrossRef]


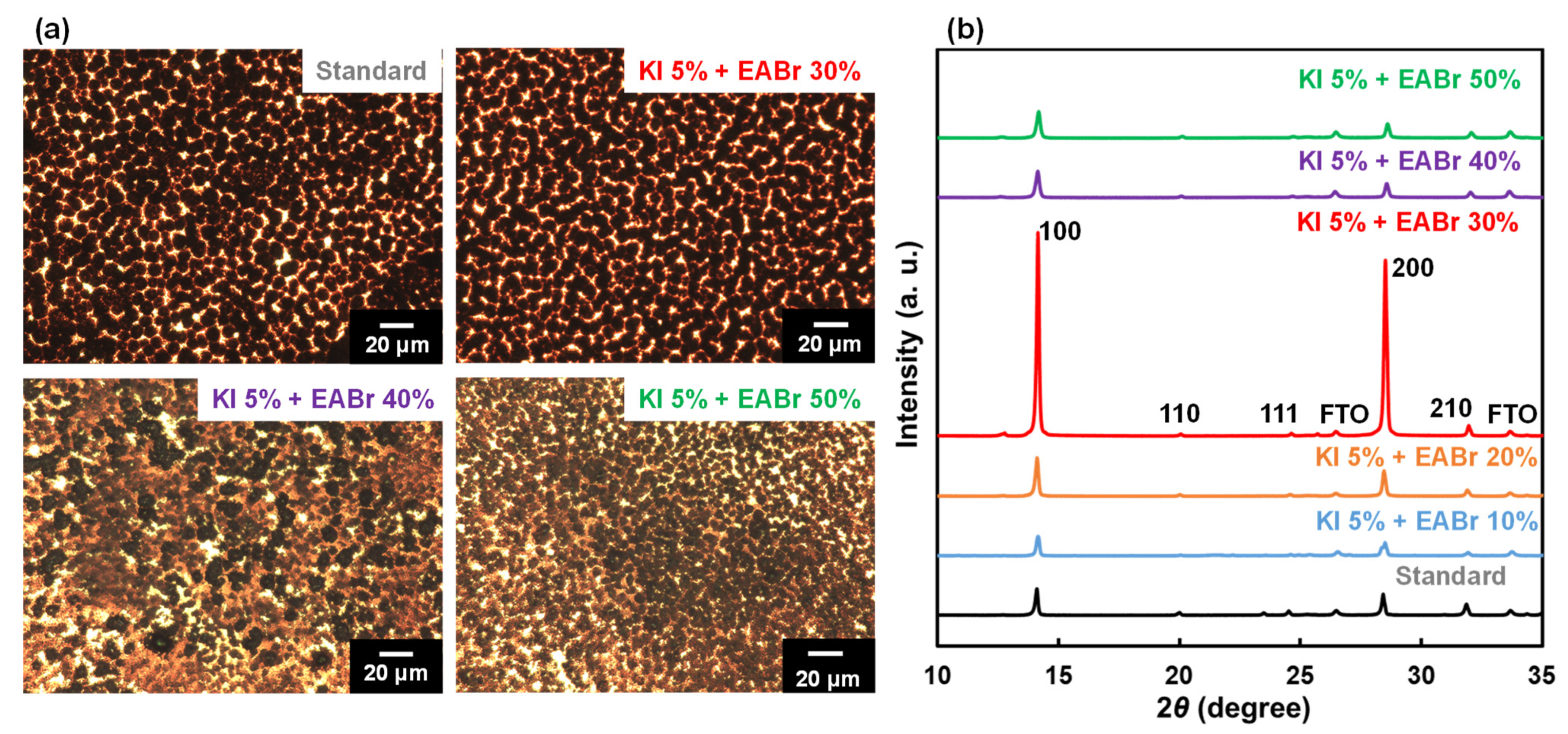
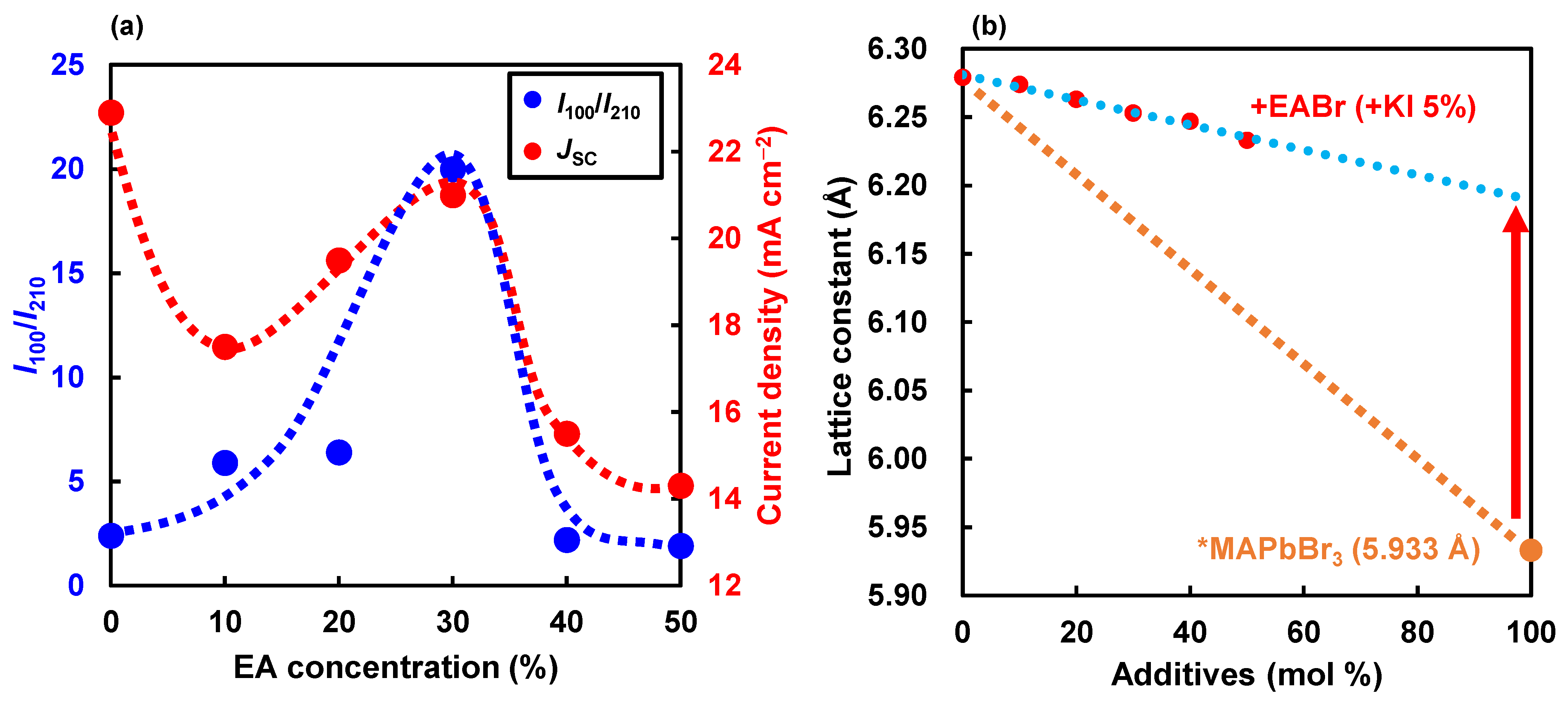
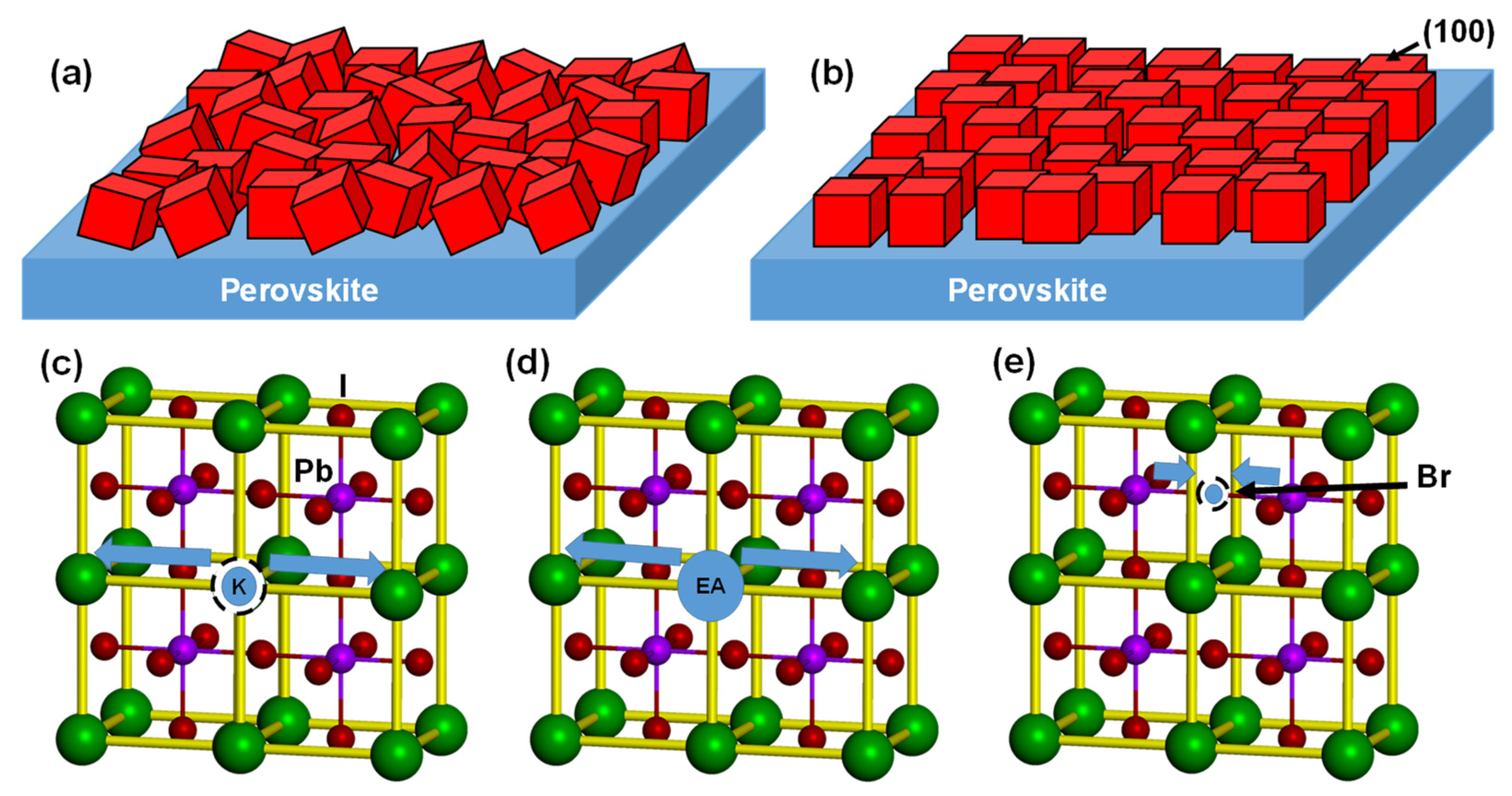
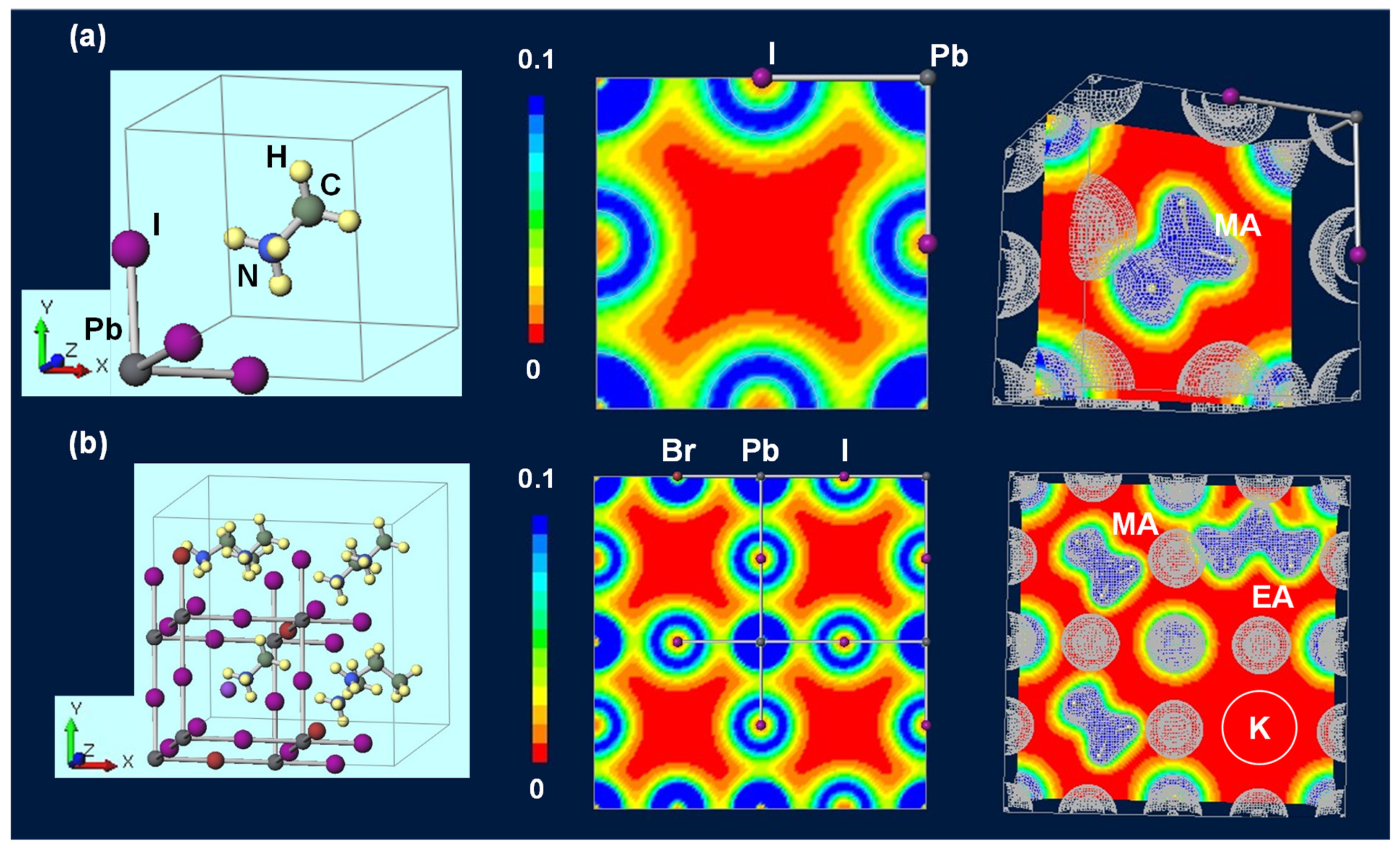

| Device | JSC (mA cm−2) | VOC (V) | FF | RS (Ω cm2) | RSh (Ω cm2) | η (%) | ηave (%) | HI | Eg (eV) | t | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KI (%) | EABr (%) | ||||||||||
| 0 | 0 | 22.9 | 0.789 | 0.516 | 7.29 | 277 | 9.33 | 8.11 | 0.014 | 1.556 | 0.912 |
| 5 | 10 | 17.5 | 0.830 | 0.593 | 5.52 | 249 | 8.63 | 6.76 | 0.084 | 1.565 | 0.919 |
| 5 | 20 | 19.5 | 0.864 | 0.665 | 4.24 | 1015 | 11.22 | 9.46 | 0.073 | 1.582 | 0.933 |
| 5 | 30 | 21.0 | 0.942 | 0.649 | 5.69 | 2209 | 12.88 | 11.11 | 0.076 | 1.600 | 0.947 |
| 5 | 40 | 15.5 | 0.815 | 0.586 | 6.14 | 437 | 7.38 | 6.04 | 0.063 | 1.613 | 0.961 |
| 5 | 50 | 14.3 | 0.757 | 0.440 | 9.99 | 163 | 4.77 | 3.25 | 0.187 | 1.640 | 0.975 |
| Device | Lattice Constant (Å) | Crystallite Size (Å) | Orientation I100/I210 | |
|---|---|---|---|---|
| KI (%) | EABr (%) | |||
| 0 | 0 | 6.279(1) | 868 | 2.4 |
| 5 | 10 | 6.274(0) | 596 | 5.9 |
| 5 | 20 | 6.263(2) | 663 | 6.4 |
| 5 | 30 | 6.253(3) | 1019 | 20.0 |
| 5 | 40 | 6.247(3) | 640 | 2.2 |
| 5 | 50 | 6.233(1) | 532 | 1.9 |
| Perovskite Crystal | Eg (eV) | Total Energy E (eV) | ΔE (eV cell−1) |
|---|---|---|---|
| MAPbI3 | 1.340 | −3504.0 | 0 |
| MA0.625EA0.25 K0.125PbI2.5Br0.5 | 1.377 | −3514.5 | −10.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terada, S.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Ethylammonium Bromide- and Potassium-Added CH3NH3PbI3 Perovskite Solar Cells. Photonics 2022, 9, 791. https://doi.org/10.3390/photonics9110791
Terada S, Oku T, Suzuki A, Okita M, Fukunishi S, Tachikawa T, Hasegawa T. Ethylammonium Bromide- and Potassium-Added CH3NH3PbI3 Perovskite Solar Cells. Photonics. 2022; 9(11):791. https://doi.org/10.3390/photonics9110791
Chicago/Turabian StyleTerada, Shuhei, Takeo Oku, Atsushi Suzuki, Masanobu Okita, Sakiko Fukunishi, Tomoharu Tachikawa, and Tomoya Hasegawa. 2022. "Ethylammonium Bromide- and Potassium-Added CH3NH3PbI3 Perovskite Solar Cells" Photonics 9, no. 11: 791. https://doi.org/10.3390/photonics9110791
APA StyleTerada, S., Oku, T., Suzuki, A., Okita, M., Fukunishi, S., Tachikawa, T., & Hasegawa, T. (2022). Ethylammonium Bromide- and Potassium-Added CH3NH3PbI3 Perovskite Solar Cells. Photonics, 9(11), 791. https://doi.org/10.3390/photonics9110791





