Optical Sensor Based on Carbon Nanomaterials for UGLU Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Theoretical Framework
2.2. Biosensor Structure and Initial Parameters
3. Results and Discussions
3.1. Systems Considered in This Study
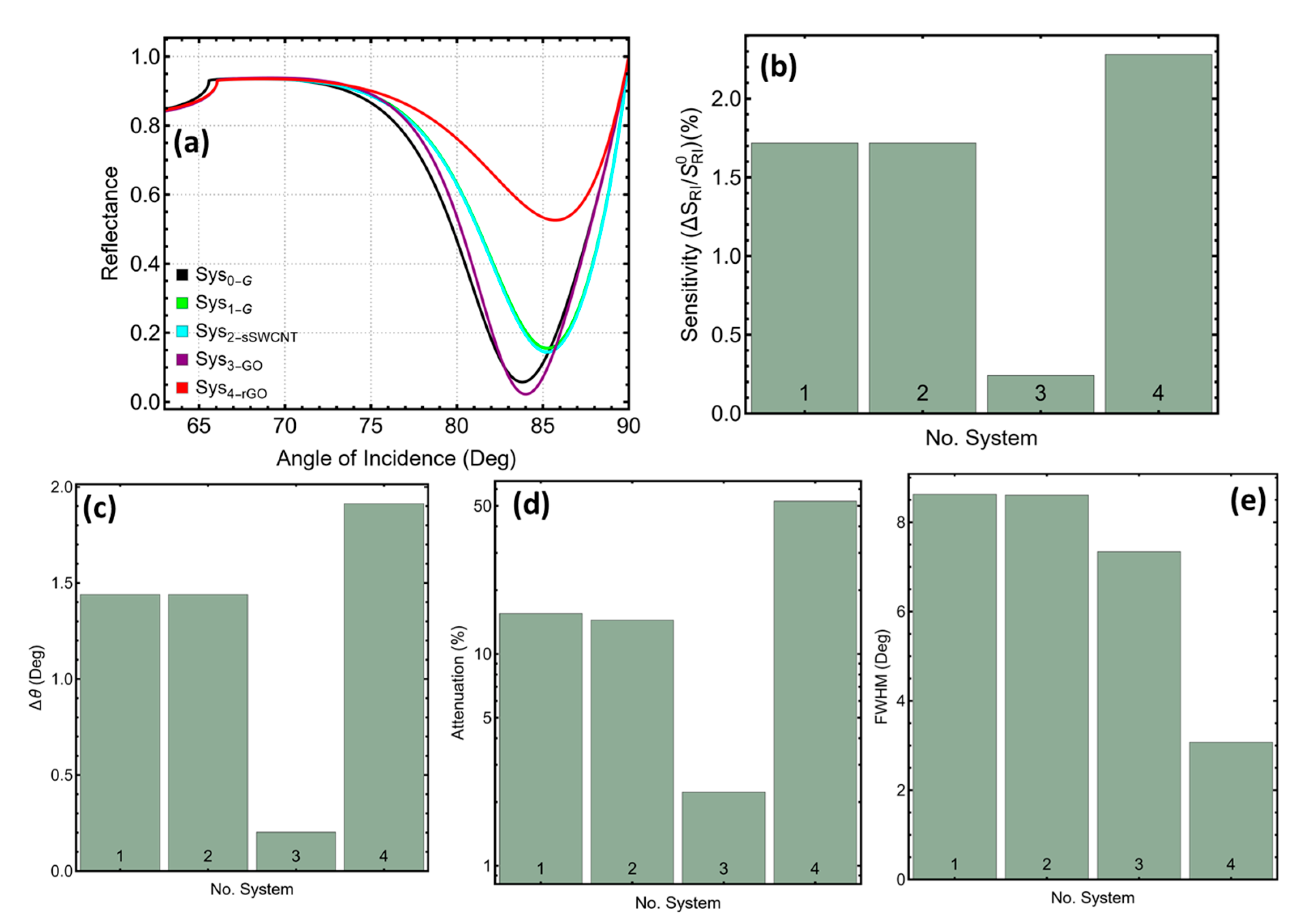
| Sys No. | Code | SPR Peak Position | Sensitivity Enhancement (%) | (Deg) | Attenuation (%) | FWHM |
|---|---|---|---|---|---|---|
| 1 | Sys1 | 85.25 | 1.71 | 1.44 | 15.52 | 8.62 |
| 2 | Sys2 | 85.25 | 1.71 | 1.44 | 14.42 | 8.60 |
| 3 | Sys3 | 84.01 | 0.24 | 0.20 | 2.22 | 7.33 |
| 4 | Sys4 | 85.72 | 2.28 | 1.91 | 52.60 | 3.07 |
3.2. Gold Thickness Optimization
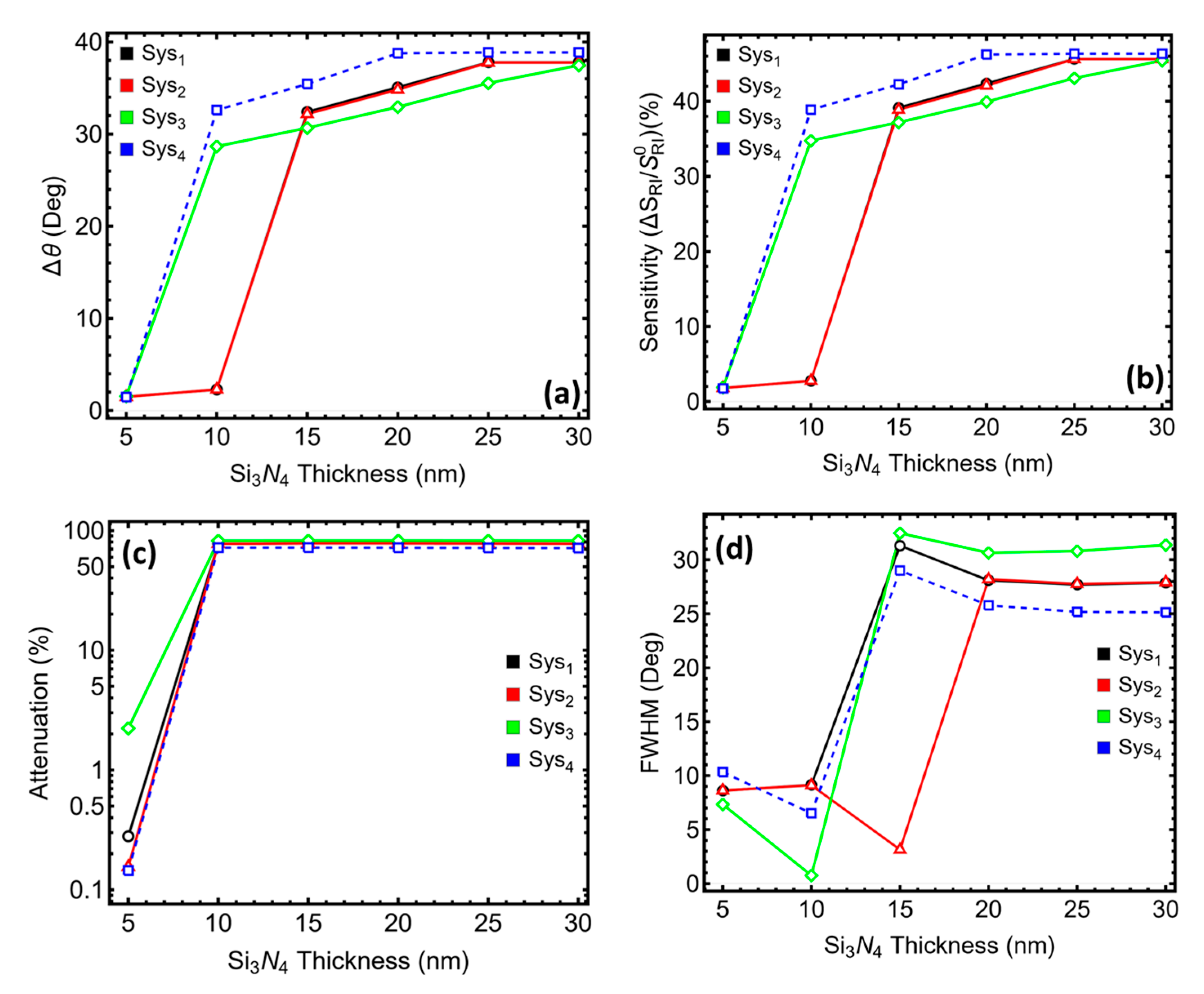
3.3. Silicon Nitride Thickness Optimization
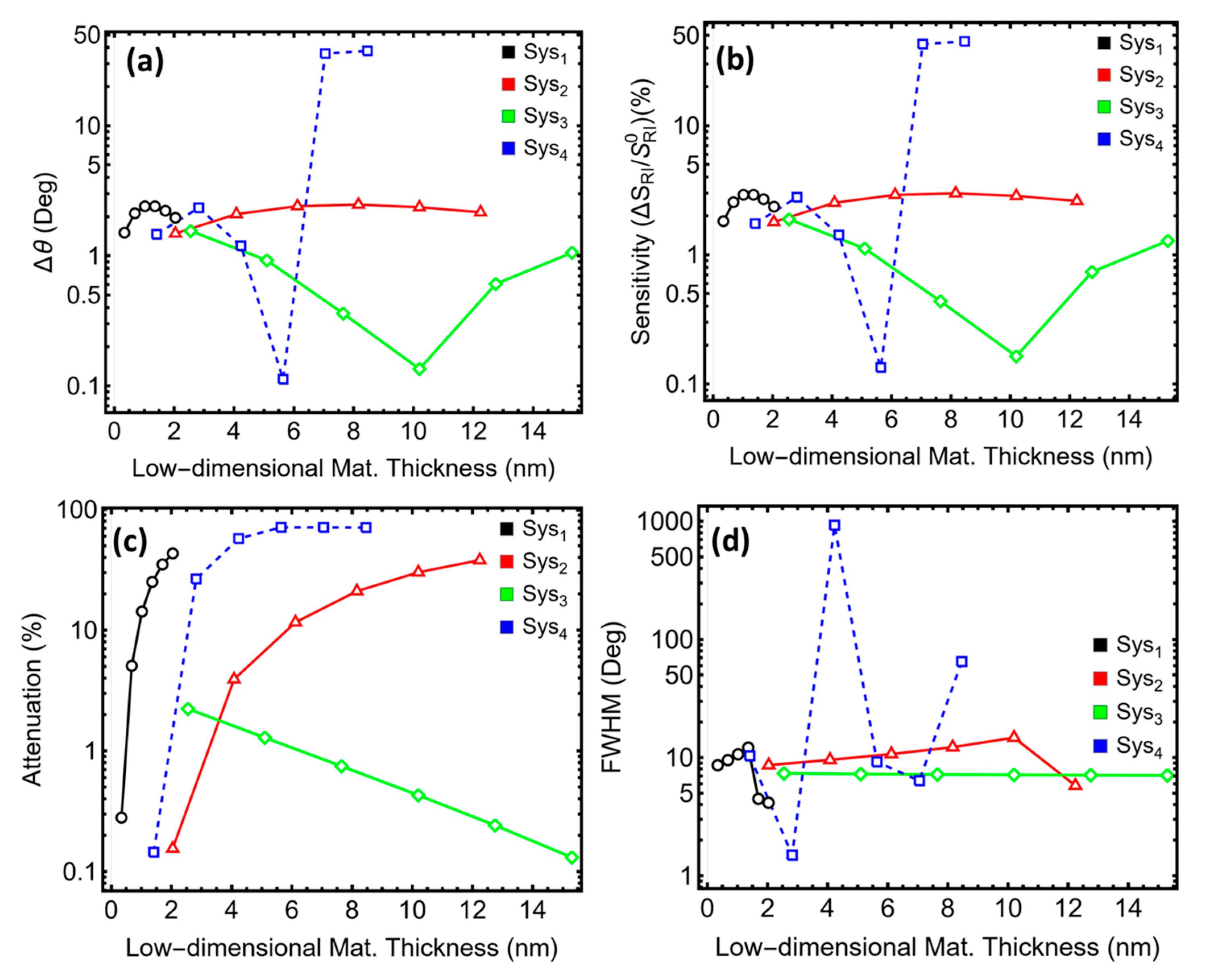
3.4. Low-Dimensional Thickness Layer Optimization
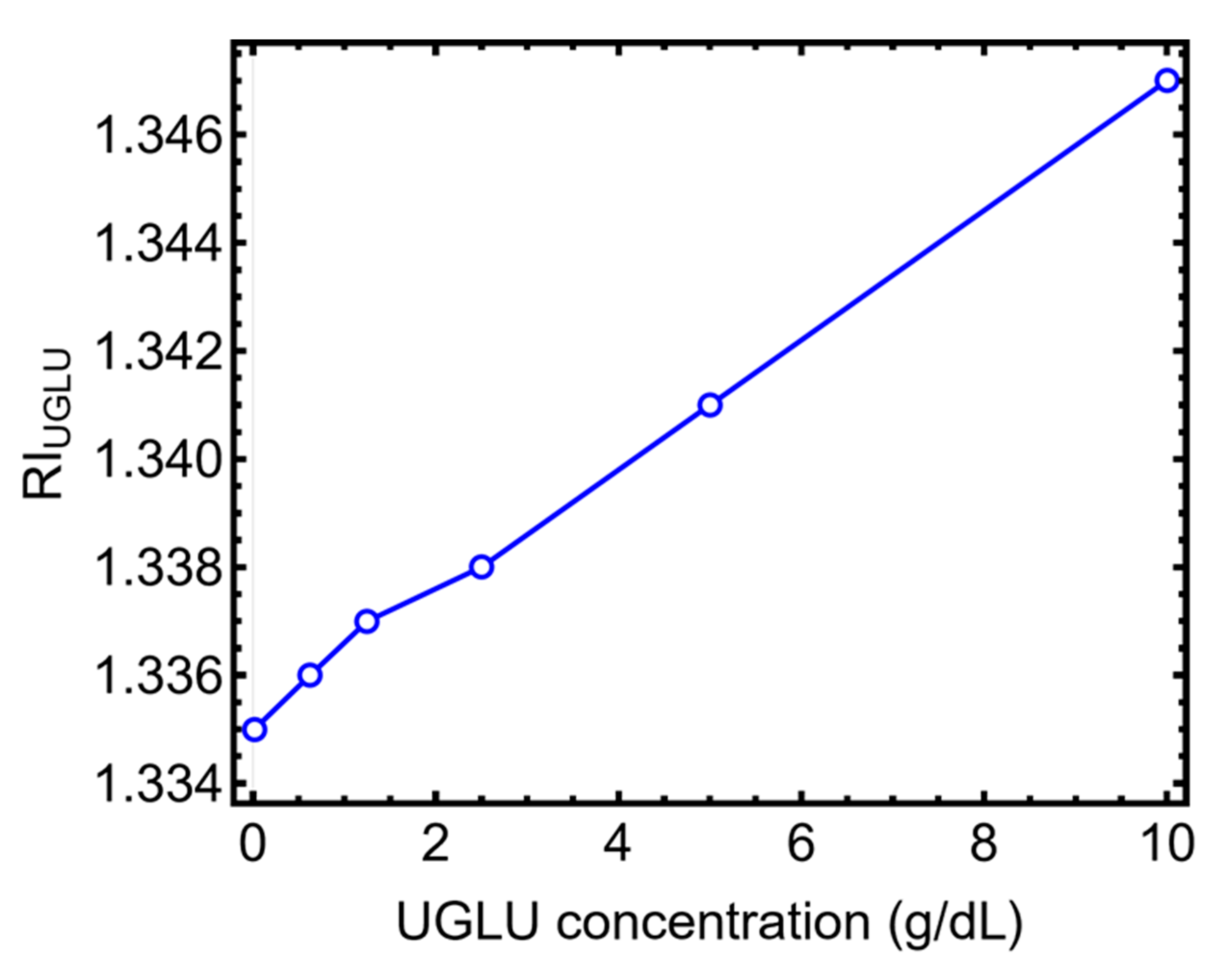
3.5. Glucose at Different Concentrations and Related Refractive Index
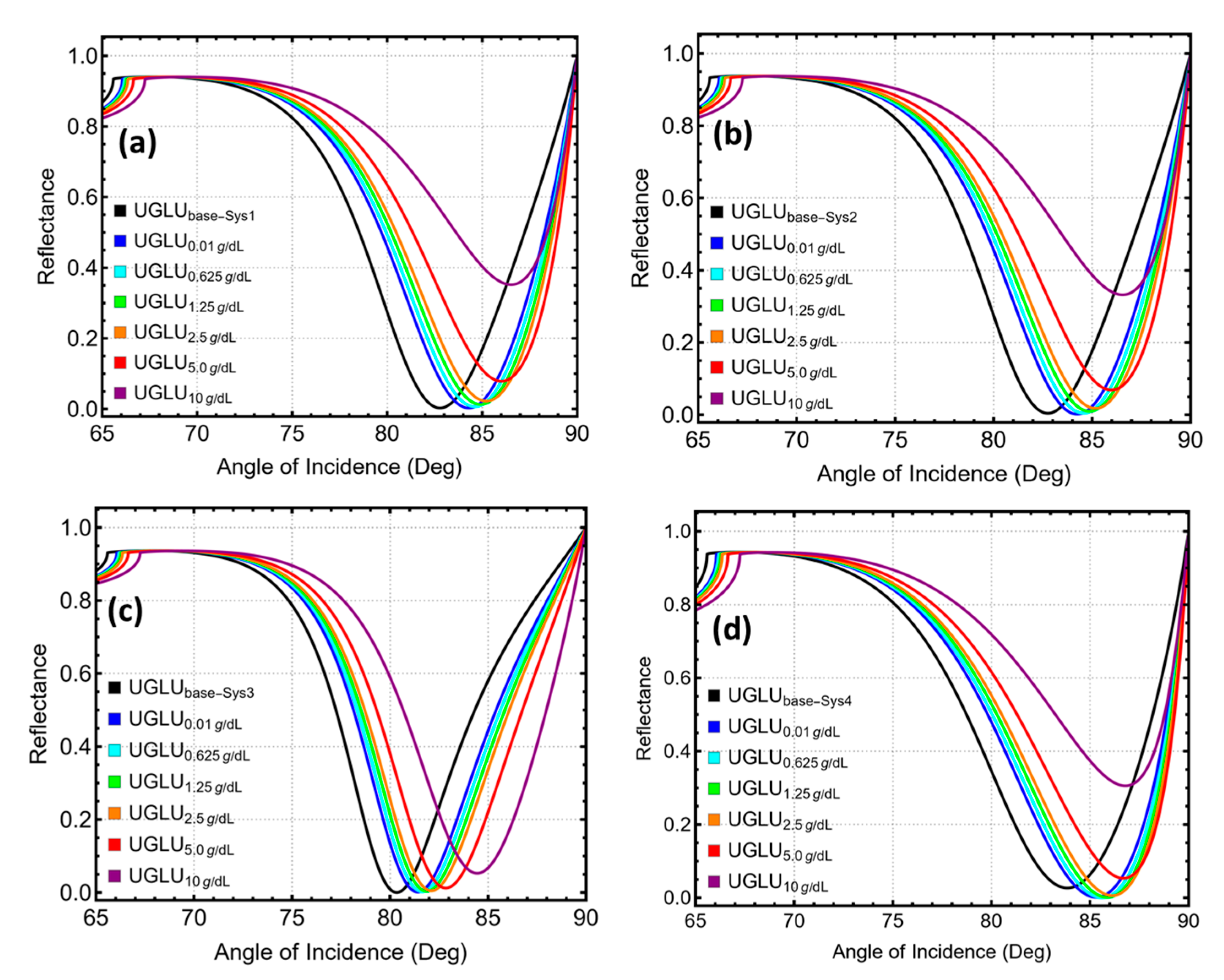
3.6. UGLU Sensing
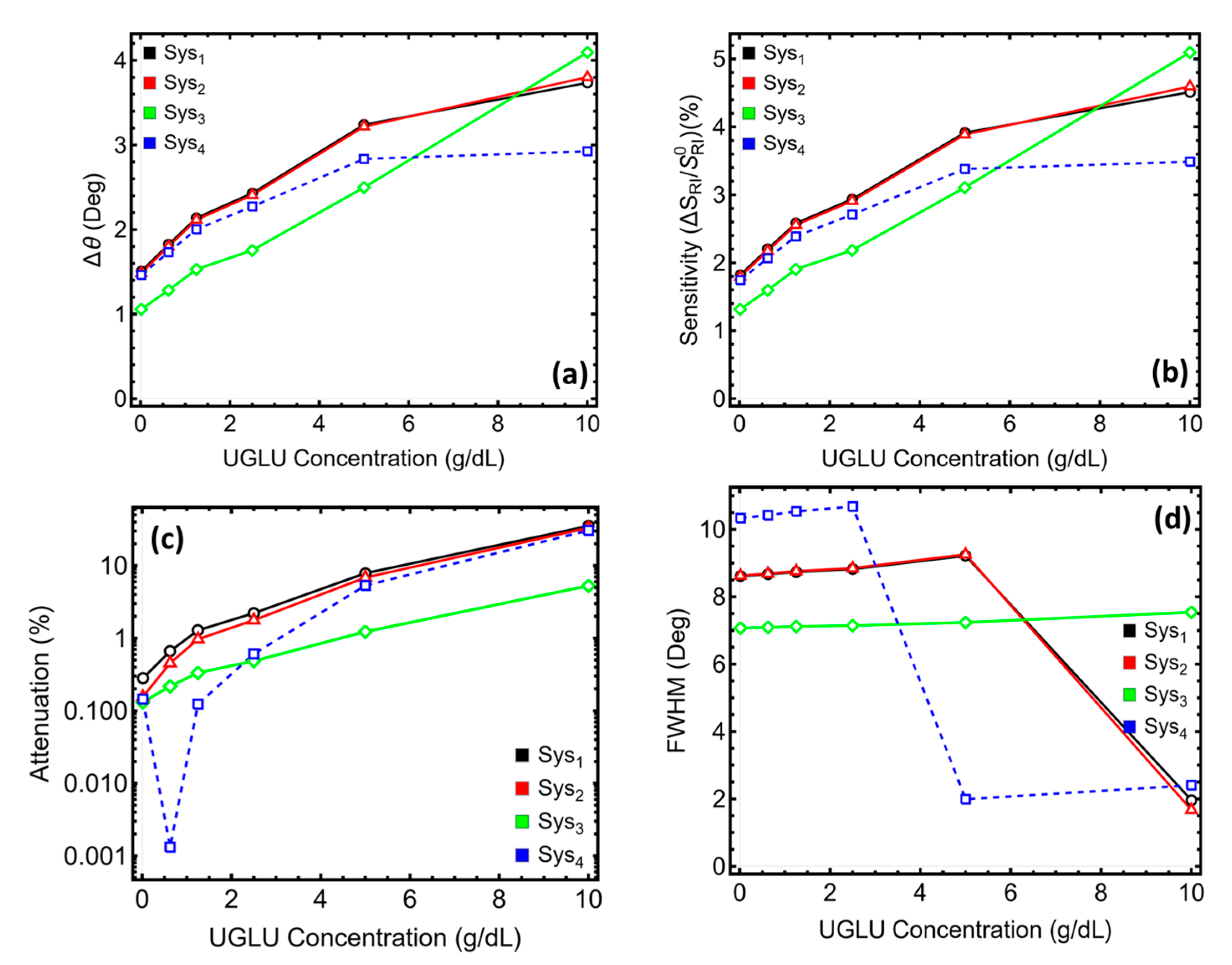
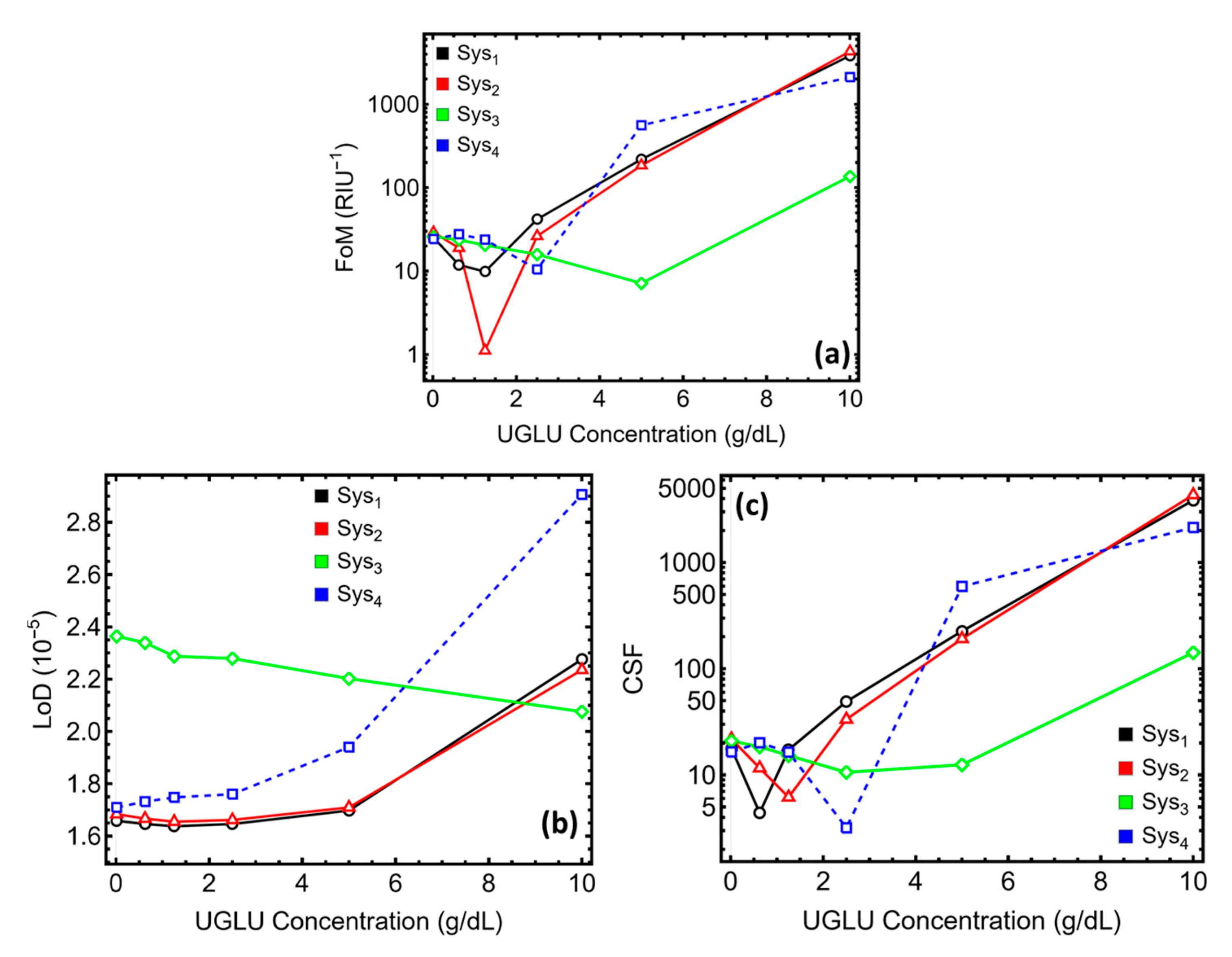
3.7. Performance Sensing Metrics
3.8. Evanescent-Field Distribution of the Optimized Stacks
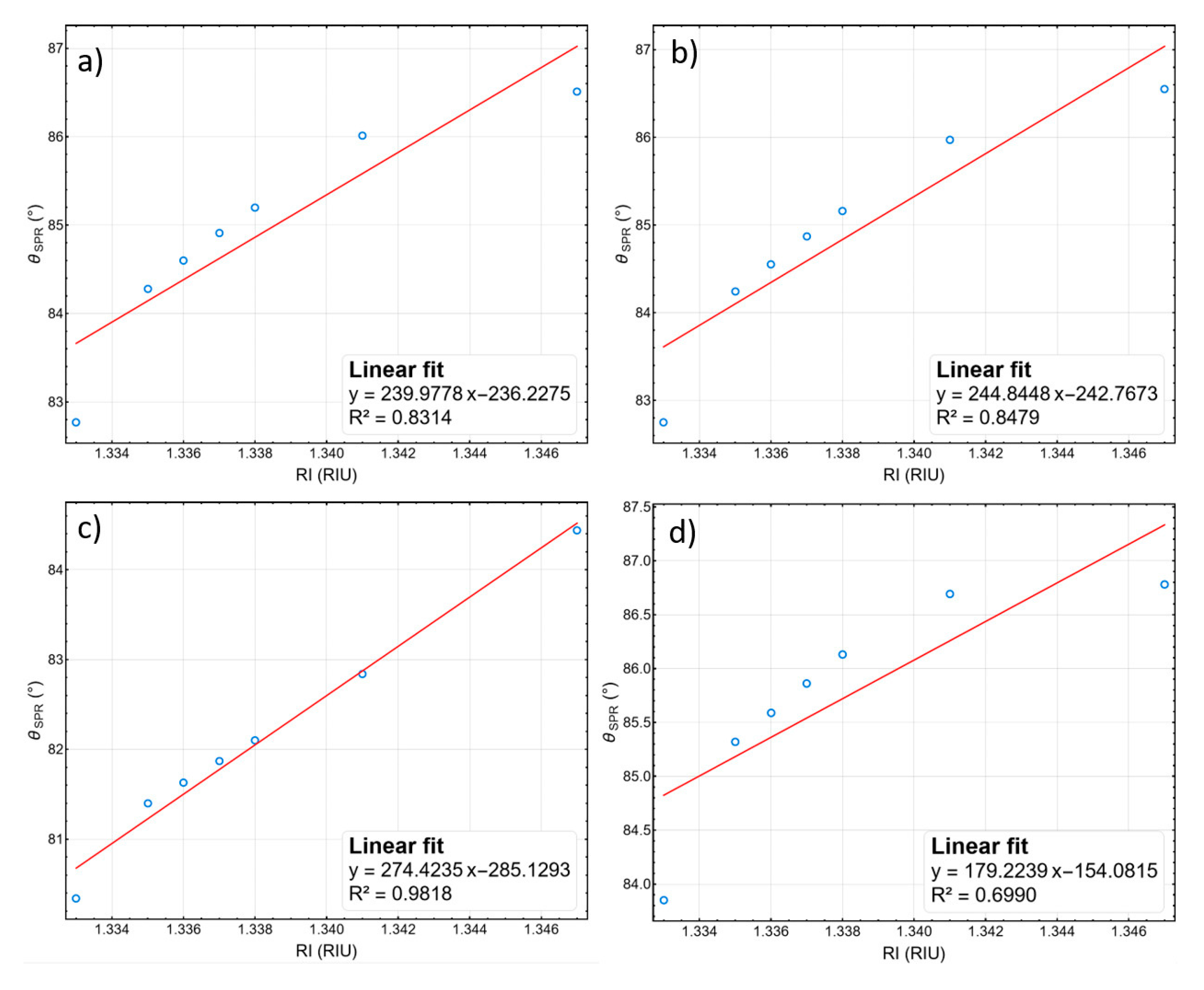
3.9. Linearity and Overall Sensitivity of the Optimized Stacks
4. Limitations and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes mellitus: Insights from epidemiology, biochemistry, risk factors, diagnosis, complications and comprehensive management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Ehtewish, H.; Arredouani, A.; El-Agnaf, O. Diagnostic, prognostic, and mechanistic biomarkers of diabetes mellitus-associated cognitive decline. Int. J. Mol. Sci. 2022, 23, 6144. [Google Scholar] [CrossRef]
- Yadav, S.D.; Singh, N. Mechanistic insight of innovative biomarkers for screening of type II diabetes mellitus. Curr. Indian Sci. 2024, 2, E2210299X257270. [Google Scholar] [CrossRef]
- Kumar, R.; Saha, P.; Kumar, Y.; Sahana, S.; Dubey, A.; Prakash, O. A review on diabetes mellitus: Type 1 & type 2. World J. Pharm. Pharm. Sci. 2020, 9, 17336. [Google Scholar] [CrossRef]
- Rukundo, B. Useful interventions in treating diabetes: A comprehensive review. IAA J. Appl. Sci. 2025, 13, 6–13. [Google Scholar] [CrossRef]
- Zakir, M.; Ahuja, N.; Surksha, M.A.; Sachdev, R.; Kalariya, Y.; Nasir, M.; Kashif, M.; Shahzeen, F.; Tayyab, A.; Khan, M.S.M.; et al. Cardiovascular complications of diabetes: From microvascular to macrovascular pathways. Cureus 2023, 15, e45835. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493–511. [Google Scholar] [CrossRef]
- Kight, E.C. Advancements in Point-of-Care Diagnostic Assays for Non-Invasive Samples in Resource Constrained Settings. Ph.D. Thesis, Vanderbilt University, Nashville, TN, USA, 12 May 2023. [Google Scholar]
- Dhindsa, S. Evaluating Saliva Based Glucose Testing for Accessible Diabetes Care in Tanzania. Master’s Thesis, Yale School of Public Health, New Haven, CT, USA, 2025. [Google Scholar]
- Eerdekens, G.-J.; Rex, S.; Mesotten, D. Accuracy of blood glucose measurement and blood glucose targets. J. Diabetes Sci. Technol. 2020, 14, 560–567. [Google Scholar] [CrossRef]
- Sacks, D.B.; Bruns, D.E.; Goldstein, D.E.; Maclaren, N.K.; McDonald, J.M.; Parrott, M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin. Chem. 2002, 48, 436–472. [Google Scholar] [CrossRef] [PubMed]
- Lepowsky, E.; Ghaderinezhad, F.; Knowlton, S.; Tasoglu, S. Paper-based assays for urine analysis. Biomicrofluidics 2017, 11, 051501. [Google Scholar] [CrossRef] [PubMed]
- Rehan, I.; Ullah, R.; Rehan, K.; Ullah, R. Noninvasive monitoring of urine glucose using phase lock-in rotating analyzer polarimeter. Laser Phys. 2025, 35, 086201. [Google Scholar] [CrossRef]
- Zhang, J.; Mai, X.; Hong, X.; Chen, Y.; Li, X. Optical fiber SPR biosensor with a solid-phase enzymatic reaction device for glucose detection. Sens. Actuators B Chem. 2022, 366, 131984. [Google Scholar] [CrossRef]
- Sheheryar, T. Next-generation hybrid multi-material surface plasmon resonance biosensor for non-invasive glucose detection with machine learning optimization. Plasmonics 2025, 1–17. [Google Scholar] [CrossRef]
- Zheng, W.; Han, B.; E, S.; Sun, Y.; Li, X.; Cai, Y.; Zhang, Y. Highly-sensitive and reflective glucose sensor based on optical fiber surface plasmon resonance. Microchem. J. 2020, 157, 105010. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.; Chu, S.; Qian, S.; Wang, F.; Masson, J.-F.; Han, X.; Peng, W. Fiber-optic surface plasmon resonance glucose sensor enhanced with phenylboronic acid modified Au nanoparticles. Biosens. Bioelectron. 2018, 117, 637–643. [Google Scholar] [CrossRef]
- Bužavaitė-Vertelienė, E. Strong Coupling of Hybrid Tamm and Surface Plasmon Polaritons States. Ph.D. Thesis, Vilnius University, Vilnius, Lithuania, 2022. [Google Scholar] [CrossRef]
- Karki, B.; Jha, A.; Pal, A.; Srivastava, V. Sensitivity enhancement of refractive index–based surface plasmon resonance sensor for glucose detection. Opt. Quantum Electron. 2022, 54, 595. [Google Scholar] [CrossRef]
- Rahman, K.M.; Nayan, F.; Ahmed, R.; Rahman, M. Design and development of high sensitive surface plasmon resonance biosensors for glucose detection. Plasmonics 2025, 20, 2305–2319. [Google Scholar] [CrossRef]
- Puiu, M.; Bala, C. SPR and SPR imaging: Recent trends in developing nanodevices for detection and real-time monitoring of biomolecular events. Sensors 2016, 16, 870. [Google Scholar] [CrossRef]
- Vinay, B.K.; Ganesan, S.; Suranjan, T.R. Portable and miniaturized optical surface plasmon resonance systems: Progress in label-free detection for on-site and real-time sensing. Plasmonics 2025, 1–14. [Google Scholar] [CrossRef]
- Butt, M.A. Surface plasmon resonance-based biodetection systems: Principles, progress and applications—A comprehensive review. Biosensors 2025, 15, 35. [Google Scholar] [CrossRef]
- Hojjat Jodaylami, M.; Masson, J.-F.; Badia, A. Surface plasmon resonance sensing. Nat. Rev. Methods Primers 2025, 5, 47. [Google Scholar] [CrossRef]
- Das, C.M.; Kong, K.V.; Yong, K.-T. Diagnostic plasmonic sensors: Opportunities and challenges. Chem. Commun. 2022, 58, 12031–12045. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Z.H.; Zhao, W.M.; Wang, L.; Yan, X.; Zhu, A.S.; Qiu, F.M.; Zhang, K.K. Research advances on surface plasmon resonance biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef]
- Manickam, P.; Senthil, R. Numerical investigation of side-polished SPR PCF sensor for urine analysis. Plasmonics 2022, 17, 2023–2030. [Google Scholar] [CrossRef]
- Yadav, P.K.; Kumar, A.; Upadhyay, S.; Kumar, A.; Srivastava, A.; Srivastava, M.; Srivastava, S.K. 2D material–based surface plasmon resonance biosensors for applications in different domains: An insight. Microchim. Acta 2024, 191, 373. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.S.; Raghuwanshi, S.K.; Kumar, S. Recent advances in two-dimensional materials-based Kretschmann configuration for SPR sensors: A review. IEEE Sens. J. 2022, 22, 1105–1119. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Hossain, M.I.; Ahsan, M.; Islam, M.F. Recent Developments in the Utilization of Nanomaterials for Sensing Platforms. In Recent Developments in Green Electrochemical Sensors: Design, Performance, and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2023; Volume 1437, Chapter 4; pp. 61–99. [Google Scholar] [CrossRef]
- Dutta, T.; Llamas-Garro, I.; Velázquez-González, J.S.; Bas, J.; Dubey, R.; Mishra, S.K. A New Generation of Satellite Sensors Based on Graphene and Carbon Nanotubes: A Review. IEEE Sens. J. 2024, 24, 31645–31657. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, A. Surface Plasmon Resonance [SPR]-Based Biosensor for Sugar Monitoring via Urine Samples. In Photonic Sensors for Biomedical Applications; Sharon, P., Gowda, P.B., Chaudhary, A., Eds.; Apple Academic Press: Waretown, NJ, USA, 2025; pp. 1–20. [Google Scholar] [CrossRef]
- Eddin, F.B.K.; Fan, H.; Liu, Z.; Donati, P.; Amin, Y.; Fen, Y.W.; Liang, J.; Pompa, P.P.; He, S. Progress in Surface Plasmon and Other Resonance Biosensors for Biomedical Applications. Adv. Mater. Technol. 2025, 10, 2500536. [Google Scholar] [CrossRef]
- Hinman, S.S.; McKeating, K.S.; Cheng, Q. Surface Plasmon Resonance: Material and Interface Design for Universal Accessibility. Anal. Chem. 2017, 90, 19–39. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Yi, R.; Guo, X.; Qi, Z. Single-layer graphene-based surface plasmon resonance sensor with dynamic evanescent field enhancement for biomarker study. J. Mod. Opt. 2020, 67, 671–681. [Google Scholar] [CrossRef]
- Wu, L.; Chu, H.S.; Koh, W.S.; Li, E.P. Highly sensitive graphene biosensors based on surface plasmon resonance. Opt. Express 2010, 18, 14395–14400. [Google Scholar] [CrossRef] [PubMed]
- Tene, T.; Guevara, M.; Caicedo, I.; Granizo Jara, J.L.; Borja, M.; Gahramanli, L.; Vacacela Gomez, C.; Bellucci, S. MoS2–Si3N4-Based SPR Biosensor for the Detection of Malaria at Different Stages: A Theoretical Insight. Biosens. Bioelectron. X 2025, 26, 100655. [Google Scholar] [CrossRef]
- Cheon, S.; Kihm, K.D.; Kim, H.G.; Lim, G.; Park, J.S.; Lee, J.S. How to Reliably Determine the Complex Refractive Index (RI) of Graphene by Using Two Independent Measurement Constraints. Sci. Rep. 2014, 4, 6364. [Google Scholar] [CrossRef]
- Mudgal, N.; Saharia, A.; Agarwal, A.; Ali, J.; Yupapin, P.; Singh, G. Modeling of Highly Sensitive Surface Plasmon Resonance (SPR) Sensor for Urine Glucose Detection. Opt. Quantum Electron. 2020, 52, 307. [Google Scholar] [CrossRef]
- Shoshi, M.S.; Huraiya, M.A.; Raj, V.R.; Jawad, A.; Kong, C.Y.; Tabata, H.; Ramaraj, S.G.; Razzak, S.M.A. Revolutionary barium titanate–BlueP/TMDCs SPR sensor: Ultra-sensitive detection of urine glucose levels. Talanta Open 2025, 11, 100401. [Google Scholar] [CrossRef]
- Rahman, K.M. Modeling of a BK7 prism/Ag/Ni/ZnSe/SM-based sensor for maintaining optimum performance in glucose level detection. Biosens. Bioelectron. X 2025, 24, 100604. [Google Scholar] [CrossRef]
- Mostufa, S.; Paul, A.K.; Chakrabarti, K. Detection of hemoglobin in blood and urine glucose level samples using a graphene-coated SPR based biosensor. OSA Contin. 2021, 4, 2164–2176. [Google Scholar] [CrossRef]
- El-Assar, M.; Taha, T.E.; El-Samie, F.E.A.; Fayed, H.A.; Aly, M.H. ZnSe-based highly-sensitive SPR biosensor for detection of different cancer cells and urine glucose levels. Opt. Quantum Electron. 2022, 55, 76. [Google Scholar] [CrossRef]
- Houari, F.; El Barghouti, M.; Mir, A.; Akjouj, A. Nanosensors Based on Bimetallic Plasmonic Layer and Black Phosphorus: Application to Urine Glucose Detection. Sensors 2024, 24, 5058. [Google Scholar] [CrossRef]
- Akib, T.B.A.; Rana, M.M.; Mehedi, I.M. Multi-Layer SPR Biosensor for In-Situ Amplified Monitoring of the SARS-CoV-2 Omicron (B.1.1.529) Variant. Biosens. Bioelectron. X 2024, 16, 100434. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Srivastava, S.K. Silicon Nitride–BP-Based Surface Plasmon Resonance Highly Sensitive Biosensor for Virus SARS-CoV-2 Detection. Plasmonics 2022, 17, 1065–1077. [Google Scholar] [CrossRef]
- Tene, T.; Svozilík, J.; Colcha, D.; Cevallos, Y.; Vinueza-Naranjo, P.G.; Vacacela Gomez, C.; Bellucci, S. The Tunable Parameters of Graphene-Based Biosensors. Sensors 2024, 24, 5049. [Google Scholar] [CrossRef]
- Song, B.; Liu, F.; Wang, H.; Miao, J.; Chen, Y.; Kumar, P.; Zhang, H.; Liu, X.; Gu, H.; Stach, E.A.; et al. Giant Gate-Tunability of Complex Refractive Index in Semiconducting Carbon Nanotubes. ACS Photonics 2020, 7, 2896–2905. [Google Scholar] [CrossRef]
- Xue, T.; Cui, X.; Chen, J.; Liu, C.; Wang, Q.; Wang, H.; Zheng, W. A Switch of the Oxidation State of Graphene Oxide on a Surface Plasmon Resonance Chip. ACS Appl. Mater. Interfaces 2013, 5, 2096–2103. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Huang, M. Direct CVD Growth of Twisted Graphene: Unveiling Mechanisms and Prospects for Future Applications. Small 2025, 21, 2500628. [Google Scholar] [CrossRef]
- Neupane, T.; Tabibi, B.; Seo, F.J. Spatial self-phase modulation in WS2 and MoS2 atomic layers. Opt. Mater. Express 2020, 10, 831–842. [Google Scholar] [CrossRef]




| Ref. | Structure | Conc. g dL−1 | Sensitivity (°/RIU) | QF | DA |
|---|---|---|---|---|---|
| Mudgal et al. [39] | BK7/Au/MoS2/h-BN/Graphene | 10 | 194.12 | 16.04 | 0.27 |
| Shoshi et al. [45] | BK7/Ag/BaTiO3/BlueP-WS2 | 10 | 435.00 | 82.70 | 0.19 |
| Rahman et al. [46] | BK7/Ag/Ni/ZnSe | 10 | 348.00 | 102.66 | 0.30 |
| Mostufa et al. [47] | BK7/Au/PtSe2/Graphene | 10 | 166.67 | --- | --- |
| El-Assar et al. [48] | BK7/Ag/ZnSe | 10 | 366.60 | 66.41 | 0.18 |
| Houari et al. [49] | BK7/TiO2/SiO2/Ag/Au/BP | 10 | 240.00 | 29.28 | 1.09 |
| This work | Sys1 | Overall Sensitivity | 239.98 | 9.30 | 0.15 |
| This work | Sys2 | Overall Sensitivity | 244.85 | 12.61 | 0.21 |
| This work | Sys3 | Overall Sensitivity | 274.42 | 10.37 | 0.17 |
| This work | Sys4 | Overall Sensitivity | 179.22 | 9.87 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tene, T.; Guevara, M.; López, S.; Mayorga, D.; Buñay Caizaguano, A.; Chimbo Pilco, J.C.; Vacacela Gomez, C. Optical Sensor Based on Carbon Nanomaterials for UGLU Detection. Photonics 2025, 12, 1089. https://doi.org/10.3390/photonics12111089
Tene T, Guevara M, López S, Mayorga D, Buñay Caizaguano A, Chimbo Pilco JC, Vacacela Gomez C. Optical Sensor Based on Carbon Nanomaterials for UGLU Detection. Photonics. 2025; 12(11):1089. https://doi.org/10.3390/photonics12111089
Chicago/Turabian StyleTene, Talia, Marco Guevara, Santiago López, Diego Mayorga, Alex Buñay Caizaguano, Juan Carlos Chimbo Pilco, and Cristian Vacacela Gomez. 2025. "Optical Sensor Based on Carbon Nanomaterials for UGLU Detection" Photonics 12, no. 11: 1089. https://doi.org/10.3390/photonics12111089
APA StyleTene, T., Guevara, M., López, S., Mayorga, D., Buñay Caizaguano, A., Chimbo Pilco, J. C., & Vacacela Gomez, C. (2025). Optical Sensor Based on Carbon Nanomaterials for UGLU Detection. Photonics, 12(11), 1089. https://doi.org/10.3390/photonics12111089







