Carbon Dot-Decorated Polystyrene Microspheres for Whispering-Gallery Mode Biosensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CDs
2.2. Synthesis of Polystyrene Microspheres
2.3. Impregnation of CDs into Microspheres
2.4. WGM Sensing
2.5. Characterization
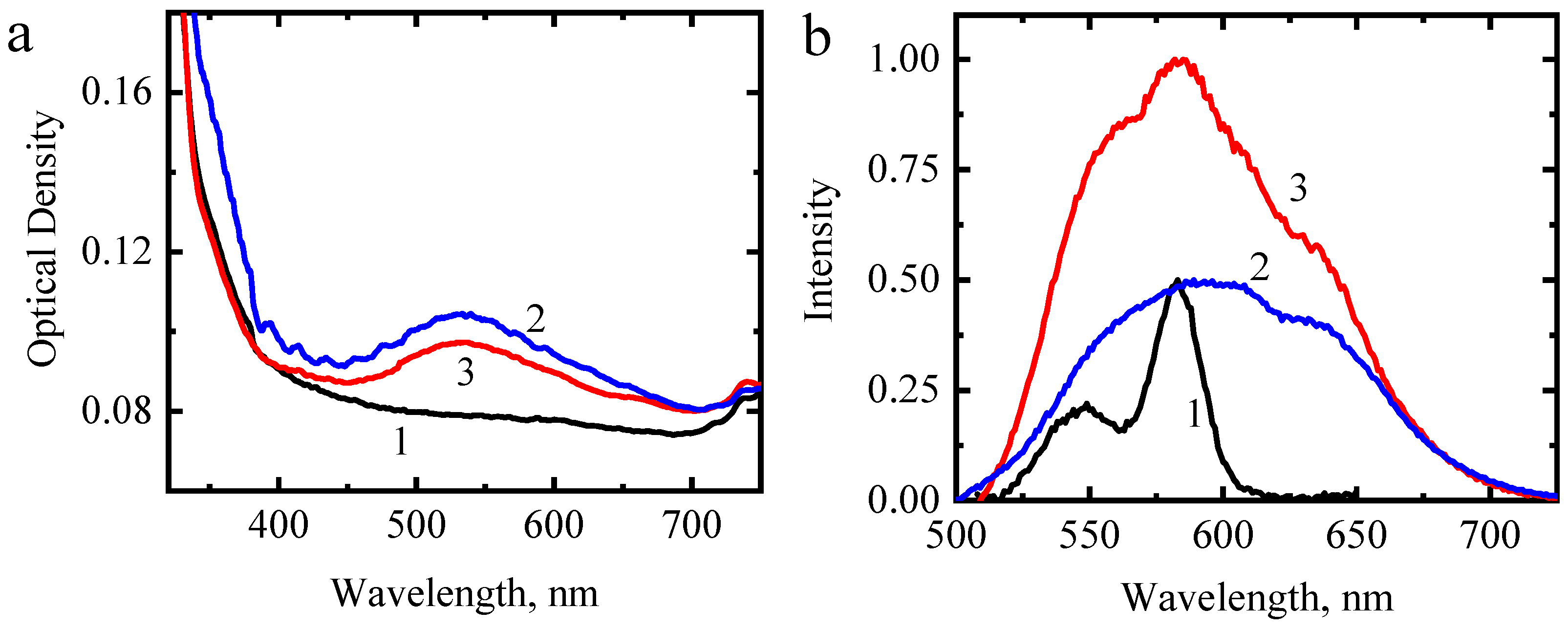
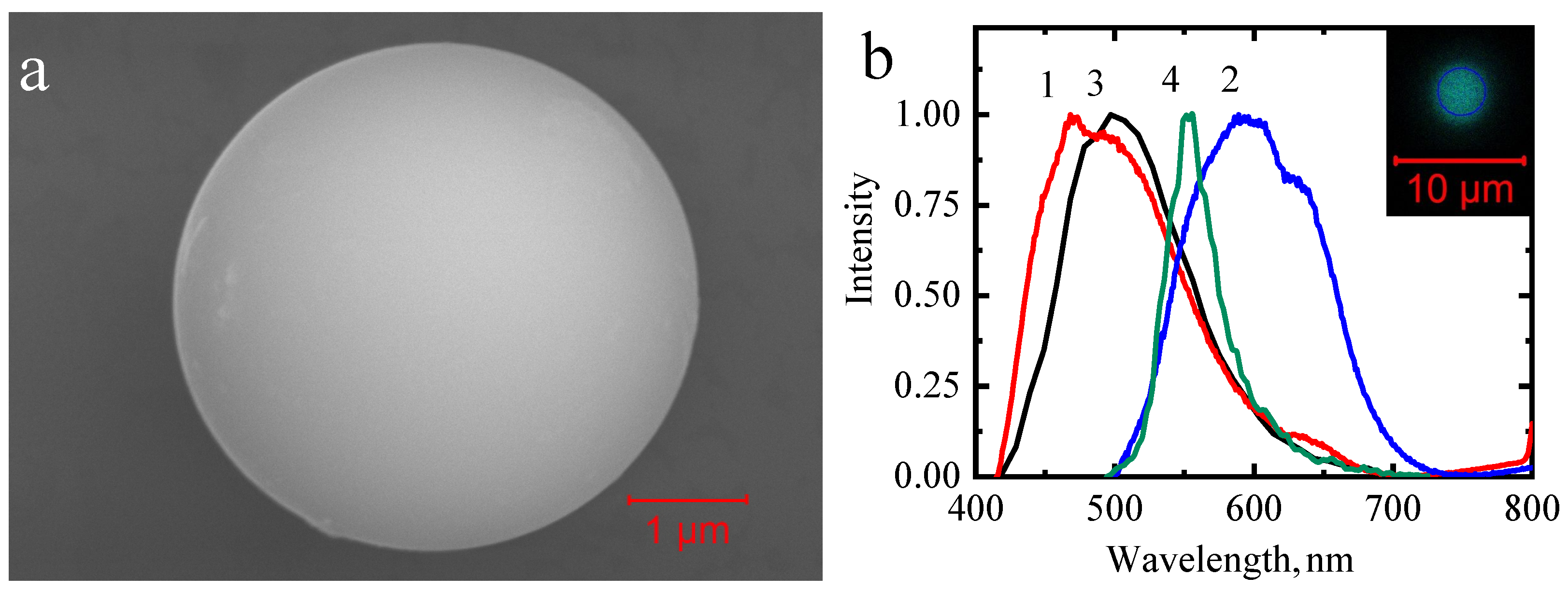
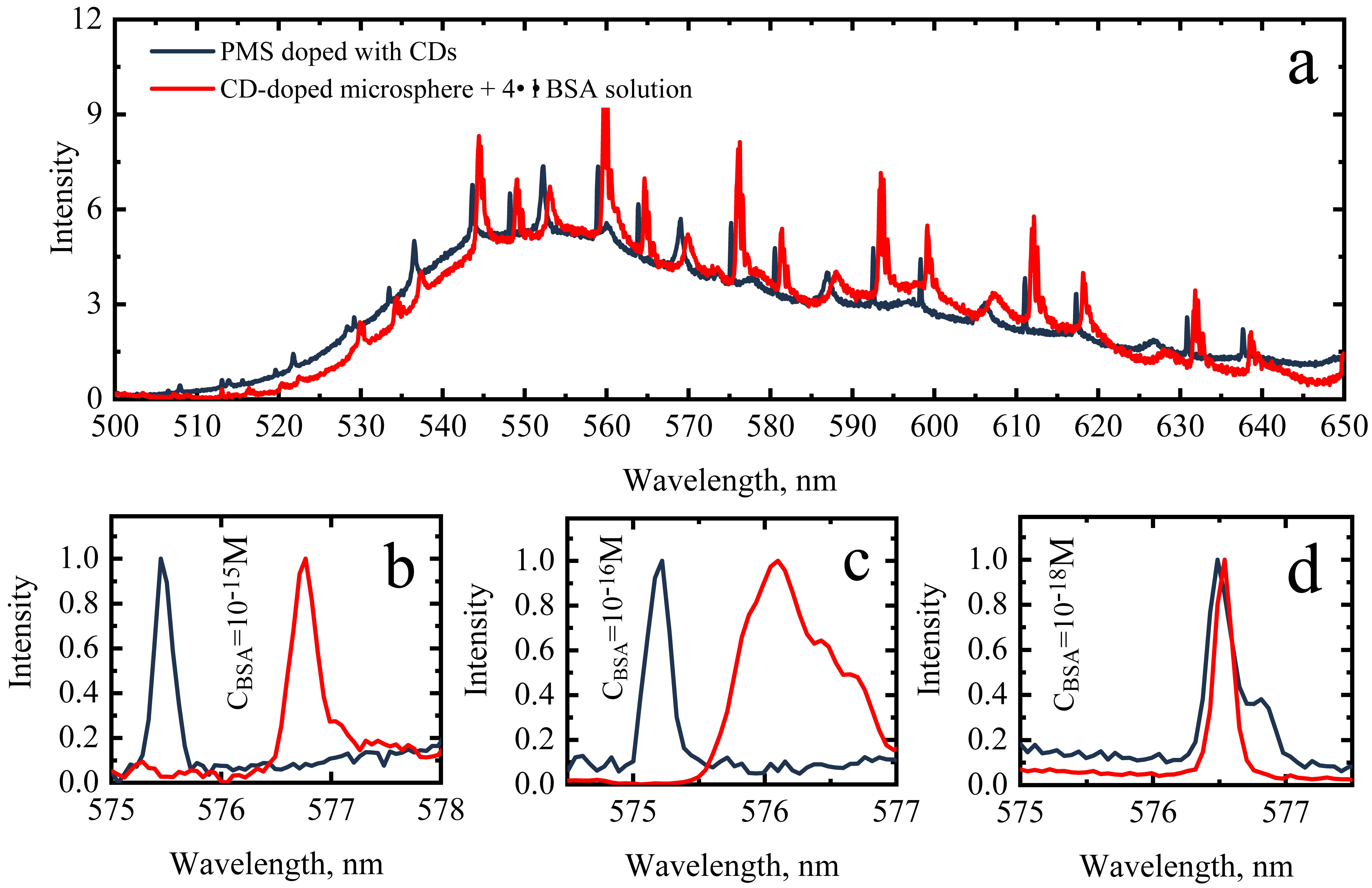
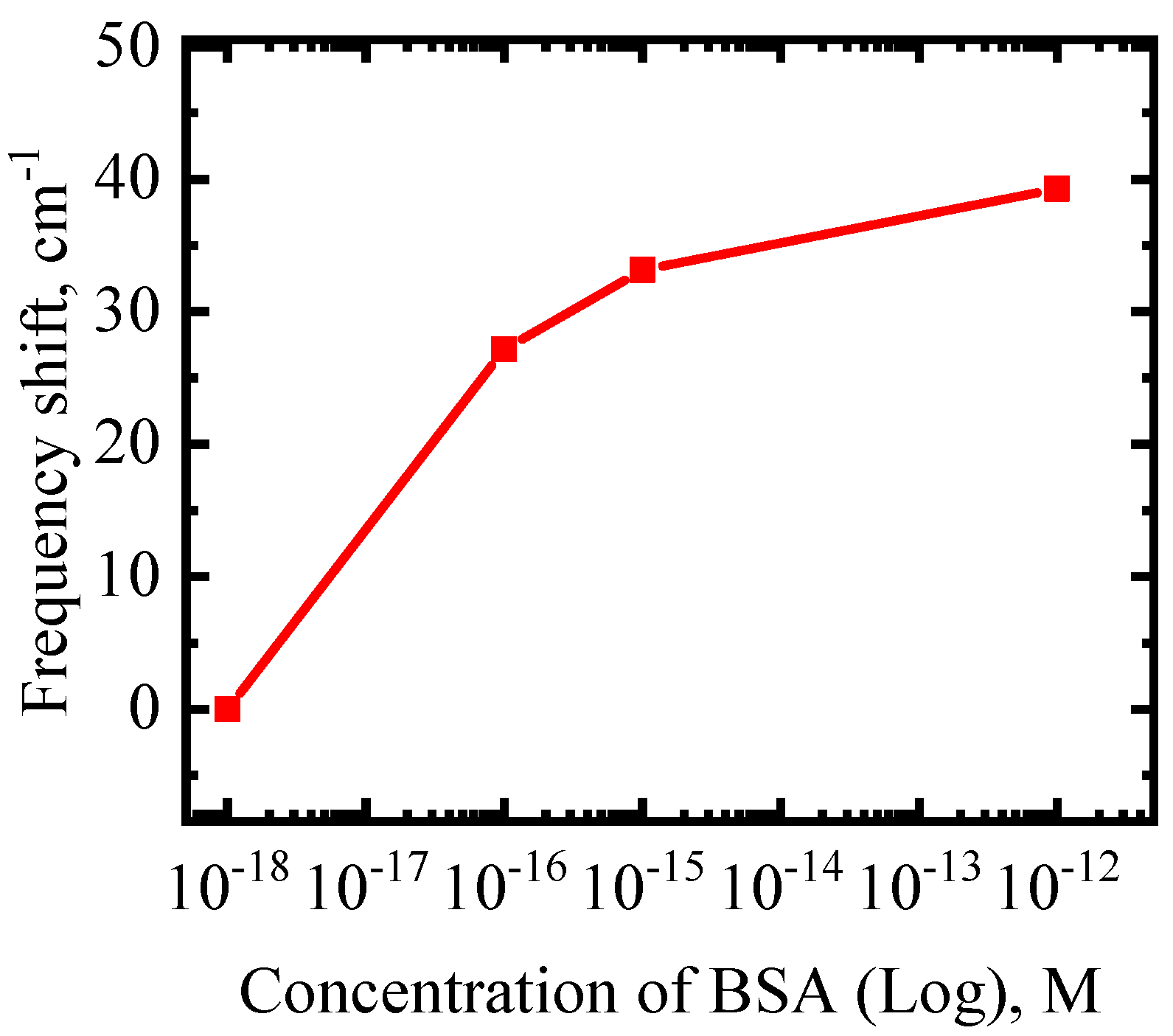
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDs | Carbon dots |
| WGM | Whispering gallery mode |
| BSA | Bovine serum albumin |
Appendix A
| Sample | Peak | , nm | FWHM, nm | Q-Factor |
|---|---|---|---|---|
| 1. CD-doped Microsphere | 1 | 561.54 | 0.289 | 1954 ± 100 |
| 2 | 577.89 | 0.312 | 1863 ± 96 | |
| 3 | 595.28 | 0.350 | 1711 ± 91 | |
| 1. CD-doped microsphere after adding BSA solution () | 1 | 564.77 | 0.375 | 1497 ± 78 |
| 2 | 581.21 | 0.405 | 1427 ± 74 | |
| 3 | 598.76 | 0.365 | 1631 ± 85 | |
| 2. CD-doped microsphere | 1 | 559.18 | 0.224 | 2496 ± 170 |
| 2 | 575.45 | 0.205 | 2807 ± 184 | |
| 3 | 592.82 | 0.2 | 2963 ± 194 | |
| 2. CD-doped microsphere after adding BSA solution () | 1 | 560.45 | 0.318 | 1762 ± 150 |
| 2 | 576.77 | 0.290 | 1989 ± 154 | |
| 3 | 594.14 | 0.273 | 2176 ± 169 | |
| 3. CD-doped microsphere | 1 | 558.95 | 0.211 | 2649 ± 80 |
| 2 | 575.22 | 0.214 | 2688 ± 82 | |
| 3 | 592.54 | 0.208 | 2849 ± 86 | |
| 3. CD-doped microsphere after adding BSA solution () | 1 | 559.87 | 0.415 | 1349 ± 140 |
| 2 | 576.26 | 0.324 | 1779 ± 176 | |
| 3 | 593.47 | 0.388 | 1530 ± 150 |
References
- Toropov, N.; Cabello, G.; Serrano, M.P.; Gutha, R.R.; Rafti, M.; Vollmer, F. Review of biosensing with whispering-gallery mode lasers. Light Sci. Appl. 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Houghton, M.C.; Kashanian, S.V.; Derrien, T.L.; Masuda, K.; Vollmer, F. Whispering-Gallery Mode Optoplasmonic Microcavities: From Advanced Single-Molecule Sensors and Microlasers to Applications in Synthetic Biology. ACS Photonics 2024, 11, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Baaske, M.D.; Vollmer, F. Optical observation of single atomic ions interacting with plasmonic nanorods in aqueous solution. Nat. Photonics 2016, 10, 733–739. [Google Scholar] [CrossRef]
- Duan, R.; Li, Y.; Li, H.; Yang, J. Detection of heavy metal ions using whispering gallery mode lasing in functionalized liquid crystal microdroplets. Biomed. Opt. Express 2019, 10, 6073–6083. [Google Scholar] [CrossRef] [PubMed]
- Toropov, N.A.; Houghton, M.C.; Yu, D.; Vollmer, F. Thermo-optoplasmonic single-molecule sensing on optical microcavities. bioRxiv 2023. [Google Scholar] [CrossRef]
- Yu, X.C.; Tang, S.J.; Liu, W.; Xu, Y.; Gong, Q.; Chen, Y.L.; Xiao, Y.F. Single-molecule optofluidic microsensor with interface whispering gallery modes. Proc. Natl. Acad. Sci. USA 2022, 119, e2108678119. [Google Scholar] [CrossRef] [PubMed]
- Garrett, C.G.B.; Kaiser, W.; Bond, W.L. Stimulated Emission into Optical Whispering Modes of Spheres. Phys. Rev. 1961, 124, 1807–1809. [Google Scholar] [CrossRef]
- Wang, H.; Xu, T.; Xu, T.; Wang, Z.; Wang, Z.; Liu, Y.; Chen, H.; Jiang, J.; Jiang, J.; Liu, T. Highly sensitive and label-free detection of biotin using a liquid crystal-based optofluidic biosensor. Biomed. Opt. Express 2023, 14, 3763–3774. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qin, Y.; Tan, X.; Chen, Y.C.; Chen, Q.; Weng, W.H.; Wang, X.; Fan, X. Ultrasound Modulated Droplet Lasers. ACS Photonics 2019, 6, 531–537. [Google Scholar] [CrossRef]
- Álvarez Freile, J.; Choukrani, G.; Zimmermann, K.; Bremer, E.; Dähne, L. Whispering Gallery Modes-based biosensors for real-time monitoring and binding characterization of antibody-based cancer immunotherapeutics. Sens. Actuators B 2021, 346, 130512. [Google Scholar] [CrossRef]
- Karl, M.; Dietrich, C.P.; Schubert, M.; Samuel, I.D.W.; Turnbull, G.A.; Gather, M.C. Single cell induced optical confinement in biological lasers. J. Phys. D Appl. Phys. 2017, 50, 084005. [Google Scholar] [CrossRef]
- Schubert, M.; Woolfson, L.; Barnard, I.R.M.; Dorward, A.M.; Casement, B.; Morton, A.; Robertson, G.B.; Appleton, P.L.; Miles, G.B.; Tucker, C.S.; et al. Monitoring contractility in cardiac tissue with cellular resolution using biointegrated microlasers. Nat. Photonics 2020, 14, 452–458. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Ushakova, E.V.; Fedorov, A.V.; Rogach, A.L. Applications of Carbon Dots in Optoelectronics. Nanomaterials 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Döring, A.; Ushakova, E.; Rogach, A.L. Chiral carbon dots: Synthesis, optical properties, and emerging applications. Light Sci. Appl. 2022, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.H.; Bardakci, F.; Akgöl, S.; Kusat, K.; Adnan, M.; Alam, M.J.; Gupta, R.; Sahreen, S.; Chen, Y.; Gopinath, S.C.B.; et al. Green Carbon Dots: Synthesis, Characterization, Properties and Biomedical Applications. J. Funct. Biomater. 2023, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Recent Developments of Carbon Dots in Biosensing: A Review. ACS Sens. 2020, 5, 2724–2741. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Waterhouse, G.I.N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Sci. 2022, 2, 2200012. [Google Scholar] [CrossRef]
- Vedernikova, A.A.; Miruschenko, M.D.; Arefina, I.A.; Xie, J.; Huang, H.; Koroleva, A.V.; Zhizhin, E.V.; Cherevkov, S.A.; Timin, A.S.; Mitusova, K.A.; et al. Green and Red Emissive N,O-Doped Chiral Carbon Dots Functionalized with l-Cysteine. J. Phys. Chem. Lett. 2024, 15, 113–120. [Google Scholar] [CrossRef]
- Han, Z.; Ni, Y.; Ren, J.; Zhang, W.; Wang, Y.; Xie, Z.; Zhou, S.; Yu, S.F. Highly efficient and ultra-narrow bandwidth orange emissive carbon dots for microcavity lasers. Nanoscale 2019, 11, 11577–11583. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wang, Y.; Mei, J.; Zhao, D. Whispering Gallery Mode Laser from Carbon Dot–NaCl Hybrid Crystals. ACS Appl. Mater. Interfaces 2017, 9, 18248–18253. [Google Scholar] [CrossRef] [PubMed]
- Kurassova, K.; Filatov, N.A.; Alexan, G.; Dadadzhanova, A.I.; Dadadzhanov, D.R.; Toropov, N.A.; Vartanyan, T.A. Microfluidic Fabrication of Polymeric Microspheres Doped with Quantum Dots for Biosensors. In Optical Sensors: Proceedings Optica Sensing Congress 2023, AIS, FTS, HISE, Sensors, ES 2023; Optica Publishing Group: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Venkatakrishnarao, D.; Sahoo, C.; Vattikunta, R.; Annadhasan, M.; Naraharisetty, S.R.G.; Chandrasekar, R. 2D Arrangement of Polymer Microsphere Photonic Cavities Doped with Novel N-Rich Carbon Quantum Dots Display Enhanced One- and Two-Photon Luminescence Driven by Optical Resonances. Adv. Opt. Mater. 2017, 5, 1700695. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Jahanban-Esfahlan, R.; Roufegarinejad, L.; Tabibiazar, M.; Amarowicz, R. Recent developments in the detection of bovine serum albumin. Int. J. Biol. Macromol. 2019, 138, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, O.; Szota, M.; Rakowski, K.; Prochownik, M.; Doveiko, D.; Chen, Y.; Jachimska, B. Bovine Serum Albumin as a Platform for Designing Biologically Active Nanocarriers—Experimental and Computational Studies. Int. J. Mol. Sci. 2023, 25, 37. [Google Scholar] [CrossRef] [PubMed]
- Duskey, J.T.; da Ros, F.; Ottonelli, I.; Zambelli, B.; Vandelli, M.A.; Tosi, G.; Ruozi, B. Enzyme Stability in Nanoparticle Preparations Part 1: Bovine Serum Albumin Improves Enzyme Function. Molecules 2020, 25, 4593. [Google Scholar] [CrossRef] [PubMed]
- Mafra, A.C.O.; Kopp, W.; Beltrame, M.B.; de Lima Camargo Giordano, R.; de Arruda Ribeiro, M.P.; Tardioli, P.W. Diffusion effects of bovine serum albumin on cross-linked aggregates of catalase. J. Mol. Catal. B Enzym. 2016, 133, 107–116. [Google Scholar] [CrossRef]
- Mardikasari, S.A.; Katona, G.; Sipos, B.; Ambrus, R.; Csóka, I. Preparation and Optimization of Bovine Serum Albumin Nanoparticles as a Promising Gelling System for Enhanced Nasal Drug Administration. Gels 2023, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Liu, J.; He, W.; Li, Z.; Luan, Y.; Song, Y.; Garg, S. Folic acid-grafted bovine serum albumin decorated graphene oxide: An efficient drug carrier for targeted cancer therapy. J. Colloid Interface Sci. 2017, 490, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, N.; Tomšík, E.; Laishevkina, S.; Iakobson, O.; Pankova, G. Cross-linked polyelectrolyte microspheres: Preparation and new insights into electro-surface properties. Soft Matter 2021, 17, 2290–2301. [Google Scholar] [CrossRef]
- Laishevkina, S.; Iakobson, O.; Saprykina, N.; Dobrodumov, A.; Chelibanov, V.; Tomšík, E.; Shevchenko, N. Hydrophilic polyelectrolyte microspheres as a template for poly(3,4-ethylenedioxythiophene) synthesis. Soft Matter 2023, 19, 4144–4154. [Google Scholar] [CrossRef]
- Song, S.A.; Jung, K.Y.; Oh, J.Y.; Chang, Y.W.; Kim, K.; Lim, S.N.; Jeong, Y.C. Enhancement of cell performance using nano polystyrene beads in photoelectrodes for dye-sensitized solar cells. J. Taiwan Inst. Chem. Eng. 2017, 78, 195–199. [Google Scholar] [CrossRef]
- Nabiullina, R.D.; Nikitin, I.Y.; Soloveva, E.O.; Gladskikh, I.A.; Starovoytov, A.A. Optical properties of nanoporous aluminum oxide activated by molecular clusters of pseudoisocyanine dye. In Proceedings of SPIE, Nanophotonics IX; SPIE: Bellingham, WA, USA, 2022; Volume 12131, pp. 170–174. [Google Scholar] [CrossRef]
- Ðorđević, L.; Arcudi, F.; D’Urso, A.; Cacioppo, M.; Micali, N.; Bürgi, T.; Purrello, R.; Prato, M. Design principles of chiral carbon nanodots help convey chirality from molecular to nanoscale level. Nat. Commun. 2018, 9, 3442. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Yang, X.; Han, X.; Jiao, Y.; Wei, T.; Yang, D.; Xu, H.; Nie, G. Highly Fluorescent Chiral N-S-Doped Carbon Dots from Cysteine: Affecting Cellular Energy Metabolism. Angew. Chem. Int. Ed. 2018, 57, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
- Vedernikova, A.A.; Miruschenko, M.D.; Arefina, I.A.; Babaev, A.A.; Stepanidenko, E.A.; Cherevkov, S.A.; Spiridonov, I.G.; Danilov, D.V.; Koroleva, A.V.; Zhizhin, E.V.; et al. Dual-Purpose Sensing Nanoprobe Based on Carbon Dots from o-Phenylenediamine: PH and Solvent Polarity Measurement. Nanomaterials 2022, 12, 3314. [Google Scholar] [CrossRef] [PubMed]
- Zulfajri, M.; Sudewi, S.; Ismulyati, S.; Rasool, A.; Adlim, M.; Huang, G.G. Carbon Dot/Polymer Composites with Various Precursors and Their Sensing Applications: A Review. Coatings 2021, 11, 1100. [Google Scholar] [CrossRef]
- Kar, D.K.; Praveenkumar, V.; Si, S.; Panigrahi, H.; Mishra, S. Carbon Dots and Their Polymeric Nanocomposites: Insight into Their Synthesis, Photoluminescence Mechanisms, and Recent Trends in Sensing Applications. ACS Omega 2024, 9, 11050–11080. [Google Scholar] [CrossRef]
- Mohammad-Jafarieh, P.; Akbarzadeh, A.; Salamat-Ahangari, R.; Pourhassan-Moghaddam, M.; Jamshidi-Ghaleh, K. Solvent effect on the absorption and emission spectra of carbon dots: Evaluation of ground and excited state dipole moment. BMC Chem. 2021, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Foell, C.A.; Schelew, E.; Qiao, H.; Abel, K.A.; Hughes, S.; van Veggel, F.C.J.M.; Young, J.F. Saturation behaviour of colloidal PbSe quantum dot exciton emission coupled into silicon photonic circuits. Opt. Express 2012, 20, 10453–10469. [Google Scholar] [CrossRef]
- Arnold, S.; Khoshsima, M.; Teraoka, I.; Holler, S.; Vollmer, F. Shift of whispering-gallery modes in microspheres by protein adsorption. Opt. Lett. 2003, 28, 272–274. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starovoytov, A.A.; Soloveva, E.O.; Kurassova, K.; Bogdanov, K.V.; Arefina, I.A.; Shevchenko, N.N.; Vartanyan, T.A.; Dadadzhanov, D.R.; Toropov, N.A. Carbon Dot-Decorated Polystyrene Microspheres for Whispering-Gallery Mode Biosensing. Photonics 2024, 11, 480. https://doi.org/10.3390/photonics11050480
Starovoytov AA, Soloveva EO, Kurassova K, Bogdanov KV, Arefina IA, Shevchenko NN, Vartanyan TA, Dadadzhanov DR, Toropov NA. Carbon Dot-Decorated Polystyrene Microspheres for Whispering-Gallery Mode Biosensing. Photonics. 2024; 11(5):480. https://doi.org/10.3390/photonics11050480
Chicago/Turabian StyleStarovoytov, Anton A., Evgeniia O. Soloveva, Kamilla Kurassova, Kirill V. Bogdanov, Irina A. Arefina, Natalia N. Shevchenko, Tigran A. Vartanyan, Daler R. Dadadzhanov, and Nikita A. Toropov. 2024. "Carbon Dot-Decorated Polystyrene Microspheres for Whispering-Gallery Mode Biosensing" Photonics 11, no. 5: 480. https://doi.org/10.3390/photonics11050480
APA StyleStarovoytov, A. A., Soloveva, E. O., Kurassova, K., Bogdanov, K. V., Arefina, I. A., Shevchenko, N. N., Vartanyan, T. A., Dadadzhanov, D. R., & Toropov, N. A. (2024). Carbon Dot-Decorated Polystyrene Microspheres for Whispering-Gallery Mode Biosensing. Photonics, 11(5), 480. https://doi.org/10.3390/photonics11050480









