Abstract
Alveolar bone repair is a complex and extremely important process, so that functions such as the mastication, occlusion and osseointegration of implants can be properly reestablished. Therefore, in order to optimize this process, many procedures have been used, such as grafting with biomaterials and the application of platelet-rich fibrin (PRF). Another method that has been studied is the use of photobiomodulation (PBM) with the use of low-level laser therapy (LLLT), which, through the absorption of photons by the tissue, triggers photochemical mechanisms in the cells so that they start to act in the search for homeostasis of the affected region. Therefore, the objective of this review was to analyze the use of LLLT as a possible auxiliary tool in the alveolar bone repair process. A search was carried out in scientific databases (PubMed/MEDLINE, Web of Science, Scopus and Cochrane) regarding the following descriptors: “low-level laser therapy AND alveolar bone repair” and “photobiomodulation AND alveolar bone repair”. Eighteen studies were selected for detailed analysis, after excluding duplicates and articles that did not meet predetermined inclusion or non-inclusion criteria. According to the studies, it has been seen that LLLT promotes the acceleration of alveolar repair due to the stimulation of ATP production, activation of transcription and growth factors, attenuation of the inflammatory process and induction of angiogenesis. These factors depend on the laser application protocol, and the Gallium Aluminum Arsenide—GaAlAs laser, with a wavelength of 830 nm, was the most used and, when applications of different energy densities were compared, the highest dosages showed themselves to be more efficient. Thus, it was possible to conclude that PBM with LLLT has beneficial effects on the alveolar bone repair process due to its ability to reduce pain, the inflammatory process, induce vascular sprouting and, consequently, accelerate the formation of a new bone matrix, favoring the maintenance or increase in height and/or thickness of the alveolar bone ridge.
1. Introduction
Loss of alveolar bone can occur for several reasons, such as resorption due to periodontal diseases which can be caused by excessive smoking, with consequent gingival recession and loss of alveolar bone due to vasoconstriction of periodontal tissues [1,2]. In addition, alveolar losses occur due to oral surgeries involving tumors in the maxilla and mandible [3] and due to factors linked to advancing age, which leads to an imbalance between the processes of osteogenesis and bone resorption [4,5]. The alveolar bone tissue is also influenced by hormonal imbalances, such as estrogenic decline, which leads to increased activity of osteoclasts [6] or inflammatory processes, which interfere with bone homeostasis and accelerate the reabsorption of bone tissue. However, the main cause of bone loss or atrophy of the alveolar ridge is tooth extractions [7].
Alveolar bone repair after tooth extraction, under normal conditions, occurs following a four-stage physiological process: cell proliferation, connective tissue development, maturation of the formed connective tissue and bone differentiation or mineralization [8]. Initially, there is the formation of a clot in the alveolar cavity, which is gradually reabsorbed as endothelial cells and fibroblasts migrate to the site, forming an immature connective tissue filled with inflammatory cells resulting from clot formation. Subsequently, the differentiation of osteoprogenitor cells into osteoblasts occurs through bone-modulating protein molecules (BMP) [9].
Thus, there is the production of more BMP molecules so that there is a proliferation of osteoblasts in the tissue, in which the development of connective tissue occurs through the synthesis of the organic matrix composed of collagen, proteoglycans and glycoproteins. After the formation of the organic matrix, the osteoblasts produce Alp (Alkaline Phosphatase) vesicles, which receive calcium and phosphate from the blood vessels, enabling the synthesis of hydroxyapatite crystals—a compound that promotes the mineralization of bone tissue [10,11]. This process of alveolar repair is important, as problems related to loss or damage to alveolar bone can lead to several negative consequences, such as difficulty in dental occlusion, masticatory function, maintenance of prosthetic function and aesthetic impairment [12].
In order to optimize this process, procedures have been used, such as bone grafting with biomaterials [13], the application of platelet-rich fibrin (PRF) [14], and the application of plasma rich in growth factors (PRGF) [15]. The most commonly used bone grafts are natural or synthetic polymers, which have osteogenic, osteoinductive and osteoconductive characteristics, widely used in bone regeneration applications [16]. The use of PRF and PRGF is due to the secretion of growth factors by platelets and leukocytes, which stimulate the process of bone regeneration [17].
Another method for optimizing alveolar bone repair that has been studied and used in clinical dental practice is local low-level laser therapy (LLLT). The use of LLLT, or currently called photobiomodulation therapy (PBM), consists of applying light from a low-energy laser (with a wavelength spectrum of 600–1110 nm) [18], which is absorbed by the irradiated tissue. This promotes chemical changes (photobiostimulation), resulting in the production of a series of growth factors which act on the proliferation of molecules and cells necessary for bone repair, such as collagen, fibroblasts, molecules involved in angiogenesis and osteoblasts [19].
The restorative tissue effects promoted by the use of LLLT were initially investigated in 1967 by the researcher Endre Mester, who described the biostimulatory action of lasers in the tissue repair process [20]. From the year 1980, after advances in scientific experimentation, there was a gradual expansion of the use of LLLT in several cases, such as in repair processes of nervous tissue, bone tissue, respiratory tract tissues and tissues affected by burns, among others [21]. This biostimulatory effect is due to the induction of cell proliferation and growth factors essential to the tissue repair process, in addition to promoting a decrease in the density of inflammatory cells and stimulating collagen synthesis [22,23].
Physical stimulation is considered as the most popular among non-conventional techniques that can improve bone formation mechanisms. Technologies include photonic, magnetic, electrical and mechanical stimulatory techniques, with photonics being the most popular and promising technique in the mechanisms of new bone formation, with mitochondrial stimulation through a biochemical effect. Photonics include static laser therapy, pulsed laser therapy techniques and LLLT [24,25,26].
In dentistry, LLLT can be used for both soft and hard tissue [27]. Studies use complementary or adjuvant techniques in order to favor the rehabilitation or prevention of diseases that affect structures of the stomatognathic system—for example, the use of ozone therapy alone or in association with LLLT [28,29], medication-related osteonecrosis of the jaw (MRONJ) [30], antimicrobial therapies (photodynamic therapy, PDT) [31], acute and chronic pain [32], acceleration of the orthodontic tooth movement [33] and other things [34].
Laser irradiation is effective when applied to healing sites such as fractures or bone defects and tooth extraction sites. In this way, osteoblasts can be recruited along the bone borders of undifferentiated precursor cells, and LLLT can potentially stimulate osteoblast recruitment and/or maturation [35,36]. There is also stimulation of collagen synthesis, with type I collagen mRNA being increased by LLLT during healing. Because type I collagen is the major bone matrix protein, laser PBM stimulation of the collagen level supports a stimulatory effect on bone formation [37]. Another event in repair is the sprouting of new blood vessels, which provides important elements for this process [38].
Considering the importance of the osteogenic, anti-inflammatory and biostimulating capacity of photobiomodulation, with low-level laser therapy, and in order to compare scientific studies in the area, the objective of this review was to analyze the use of PBM as a possible therapy that improves the alveolar repair process in the maxilla and mandible.
2. Materials and Methods
We accessed and selected manuscripts from 4 databases, PubMed/MEDLINE, Web of Science, Scopus (Elsevier) and Cochrane, without a search period limit relative to the year of publication and using the keywords: “low-level laser therapy AND alveolar bone repair” and “photobiomodulation AND alveolar bone repair”. With the intersection of keywords, a detailed analysis of the results was carried out, with the title of the scientific paper and its abstract being important criteria for selection. Subsequently, the manuscripts were separated into included and non-included according to the eligibility criteria we stipulated. When selecting studies for detailed analysis, two independent reviewers scanned the manuscripts, considering the selection criteria, with the aim of minimizing bias.
The criteria used for inclusion were studies carried out in both humans and animals, in vivo studies, publications in English that allowed access to the full text and sufficient data for the understanding of the photobiomodulation protocol. The following were not included: duplicate articles, when the manuscript was not directly related to the purpose of this review; cases in which photobiomodulation with a low-level laser was not used or a high-intensity laser was used; languages other than English; when we did not have access to the full text; articles such as letters to the editor, reviews, commentaries, conference abstracts or dissertations and theses from repositories.
The studies that presented a title and abstract related to the chosen topic were initially selected based on the focus of this review: low-level laser therapy and photobiomodulation, as used in the alveolar bone repair process. The next step used was to exclude duplicated articles in the databases consulted and also to remove studies that did not follow the eligibility criteria through careful reading of the texts. This step was carried out paying special attention to the methodology used in the study, ensuring that the procedures used were, in fact, related to the theme proposed here.
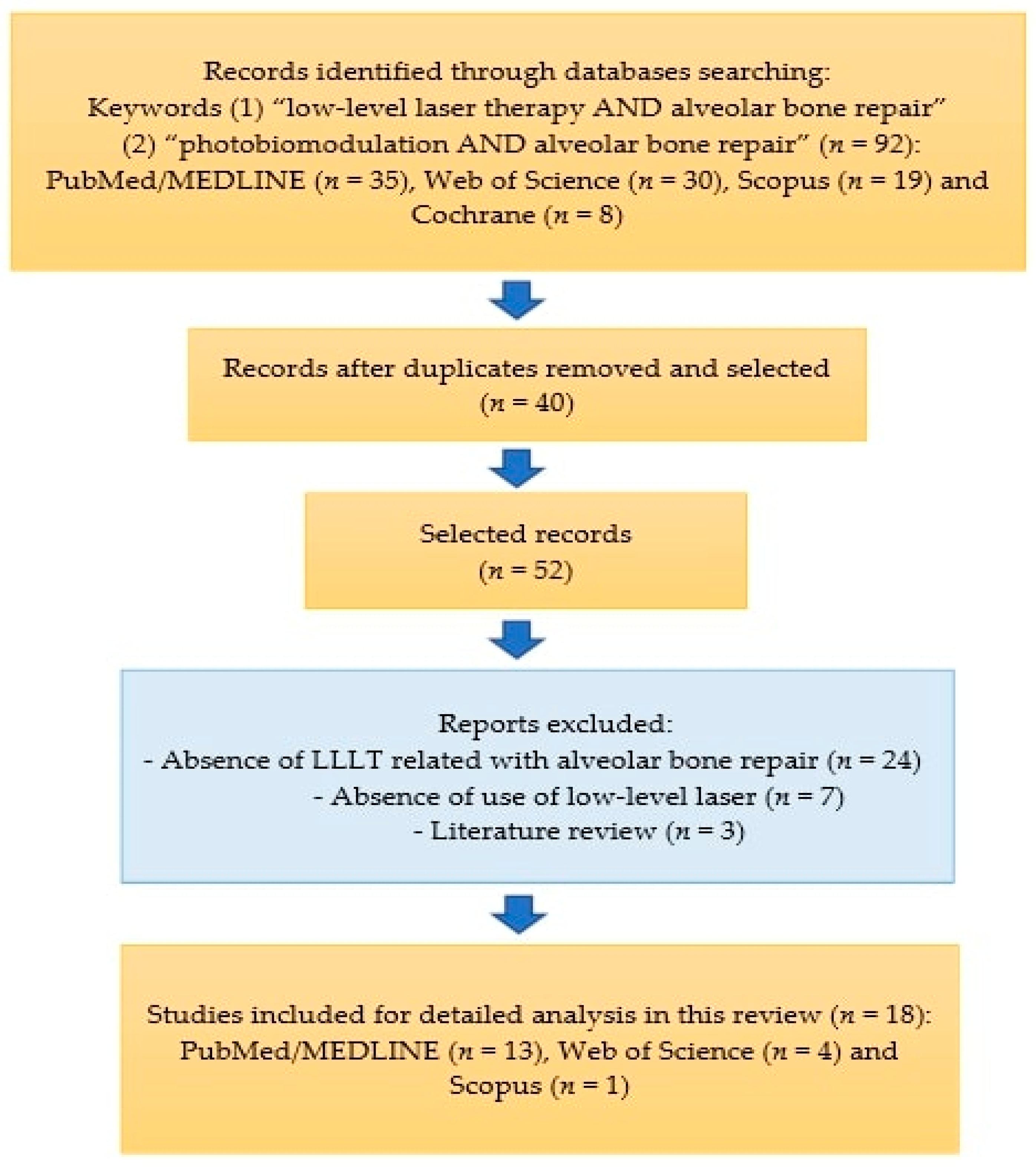
The article selection scheme is shown in Figure 1.
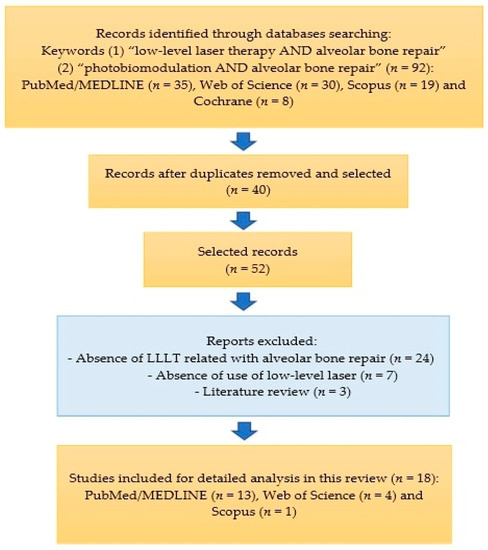
Figure 1.
Diagram showing the selection of review articles.
3. Results
In the search and scanning carried out in the bibliographic databases, we found 35 articles in PubMed/MEDLINE, of which 22 were excluded because they were duplicates or due to the inclusion/exclusion criteria. We also found thirty articles on Web of Science and selected four articles, nineteen articles on Scopus and one article was selected and eight articles on Cochrane and no articles were selected, totaling eighteen articles for qualitative and detailed analysis.
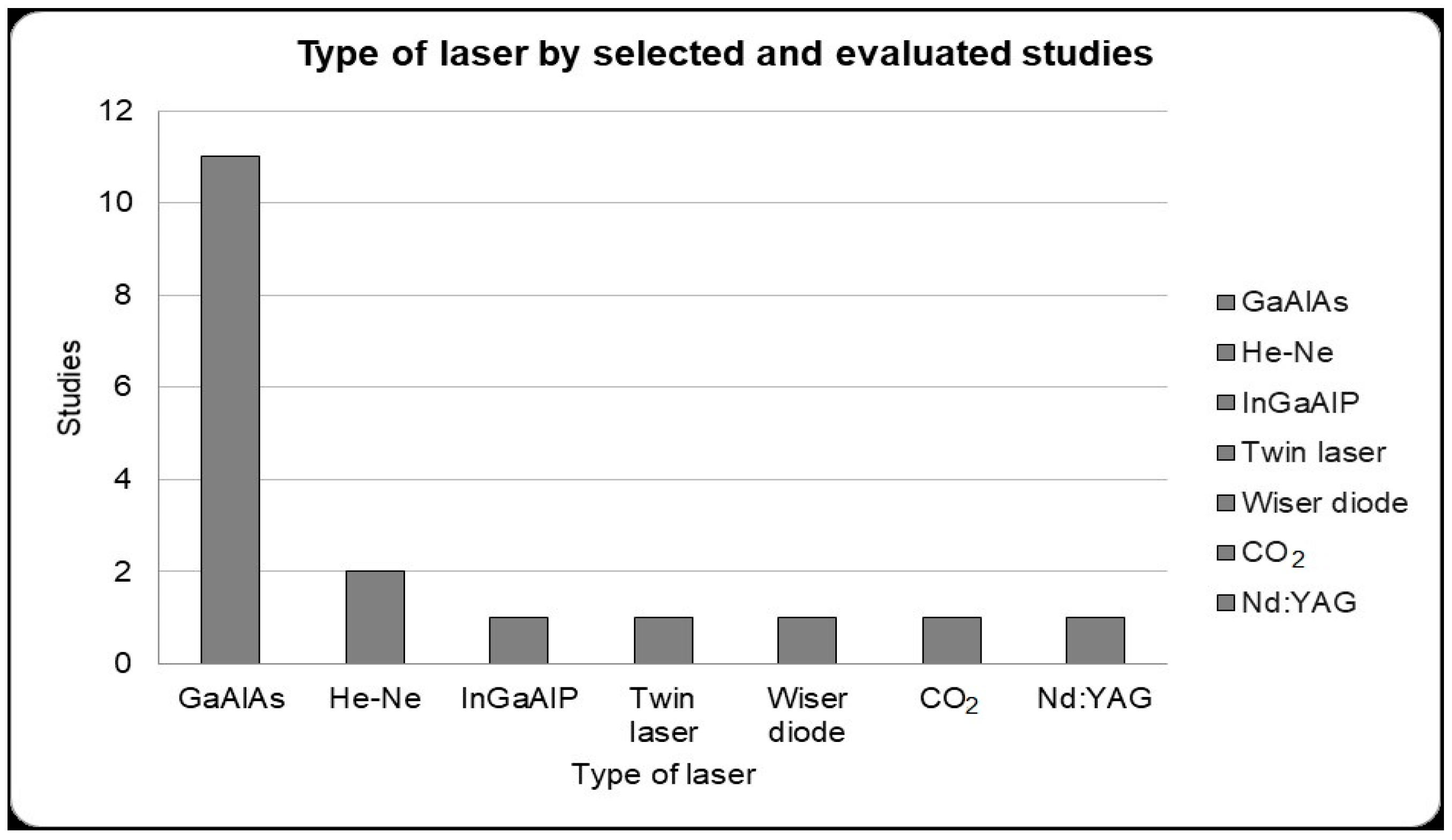
From the studies selected for detailed, we can see that eleven studies used a gallium–aluminum–arsenide laser (GaAlAs), two used helium–neon (He-Ne), one used indium gallium aluminum phosphorus (InGaAlP); one used a twin laser, one used a Wiser wireless diode laser, one used a CO2 laser and one used neodymium-doped yttrium aluminum garnet (Nd;YAG) (Figure 2).
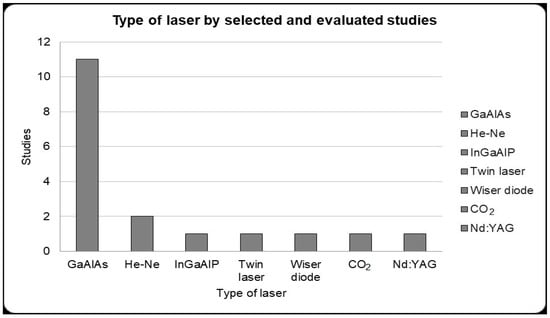
Figure 2.
Type of laser used for photobiomodulation therapy presented by the selected studies for detailed analysis. Gallium–aluminum–arsenide (GaAlAs) laser that presented greater use in the selected studies in alveolar bone repair (11 studies).
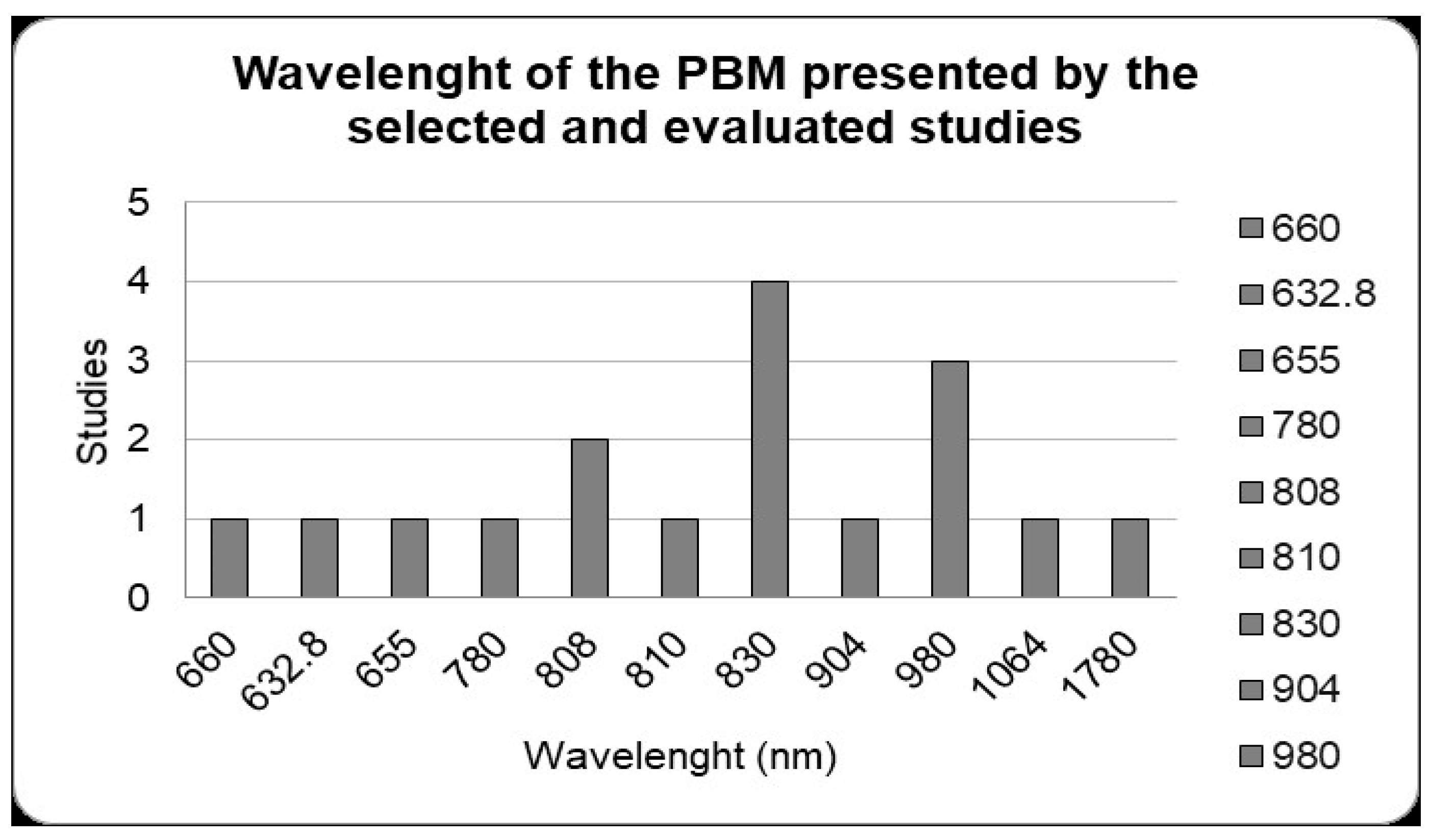
In the photobiomodulation protocols of the selected studies, when the wavelengths were analyzed the most used was 830 nm in four studies. Then, the next most was 980 nm in three studies; 808 nm in two studies; and 660 nm, 632.8 nm, 780 nm, 810 nm, 904 nm, 1064 nm and 1780 nm with one study each (Figure 3).
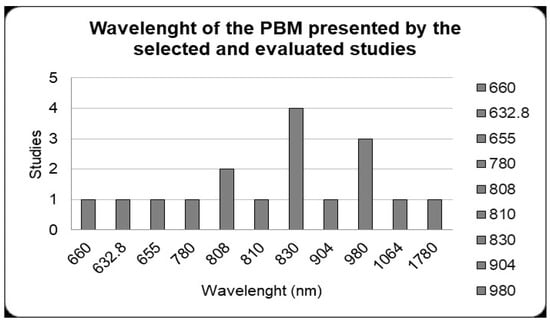
Figure 3.
Photobiomodulation protocols. Wavelength (nm) used by the studies included. The 830 nm wavelength was the most used in studies with alveolar bone repair (four studies). Studies that used different wavelengths were considered separately in the data shown in the figure.
Table 1 shows the selected studies that were carried out in animal models and, in Table 2, the studies in humans. In the layout of the columns in Table 1 and Table 2 of this integrative review, the references, elements of the PBM protocol and PICO strategy (P: patient or problem; I: intervention; C: control or comparison; O: outcome) were inserted [39].

Table 1.
Articles that were selected for detailed analysis—animal studies.

Table 2.
Articles that were selected for detailed analysis—human studies.
4. Discussion
In view of the increase in research on methods that improve and accelerate alveolar bone repair after tooth extraction, and also the findings on the beneficial effects of low-level laser therapy (LLLT) on tissue repair, this integrative review aimed to evaluate, based on the scientific literature, the effects of LLLT or photobiostimulation in the repair of maxillary or mandibular alveolar bone. It was possible to describe that there is scientific evidence attesting to the fact that treatment with LLLT collaborates in reducing repair time and improves the quality of the process of neoformation of alveolar bone.
The biological bases of the alveolar repair process were first described by Euler who, after histological and radiographic analysis, was able to discriminate the alterations that occurred in dental sockets of dogs after extraction [57,58]. Subsequently, the repair process of bone alveolar tissue was defined by Carvalho et al. (1997) who, using histometric analysis in rats, were able to determine the set of reactions triggered within the alveolus after tooth extraction [59].
Experiments to analyze alveolar repair have already been conducted in several animal species, such as rats [60,61], dogs [62], rabbits [63,64], guinea pigs [65] and humans [66], since the repair stages are the same in all these beings, however, the duration of the process varies according to the specimen studied. In our study, it was possible to observe that the rat was the animal model most used to carry out experiments on the dental alveolar repair process because these animals, like rabbits, are good models due to their ease of use, creation, maintenance and ethical aspects. However, unlike experiments carried out in dogs, which have greater bone compatibility with humans, it is not possible to fully transfer the results obtained in rats and rabbits to clinical situations in humans [63,67].
The chronology of events involving bone tissue repair after tooth extraction in rats has already been widely studied and can be summarized as follows: 24 h postoperatively: the site is filled with blood clots and fibrin bundles; 3–5 days: the proliferation of fibroblasts, blood capillaries and the beginning of the replacement of the clot by granulation tissue with an increase in inflammatory cells is observed; 6–7 days: an increase in the amount of osteoblasts in the periphery of the socket and the formation of bone trabeculae in the apical and middle thirds; 9–10 days: the presence of more organized connective tissue, vascularized and rich in fibroblasts, in addition to thickening of bone trabeculae; 13–21 days: there is an intensification of osteoblast activity with the formation of osteoid tissue while the central portion remains filled with granulation tissue; 24–28 days: the occupation of all thirds and central portion of the alveolus by thickened bone trabeculae and gradual decrease of the intertrabecular spaces, determining the beginning of bone remodeling; over 28 days: bone remodeling of the socket and alveolar process [68,69,70].
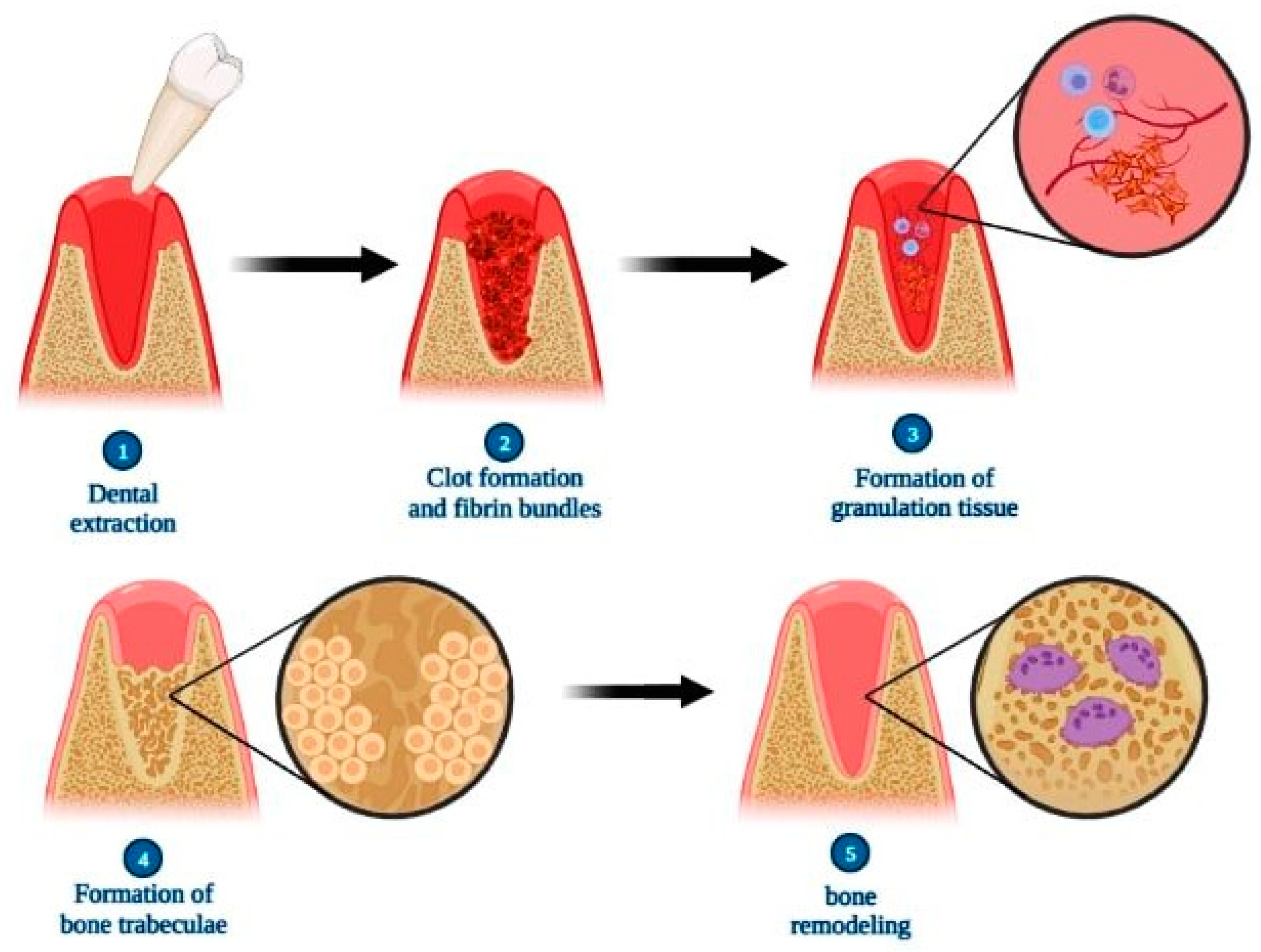
Comparatively, alveolar tissue repair in humans follows the same steps as that presented by rats, but the chronology differs so that only between 12 and 16 weeks postoperatively does the complete filling of the dental alveolus by bone tissue occur, and only after the 17th week are the bone remodeling of the socket and alveolar process verified [71] (Figure 4).
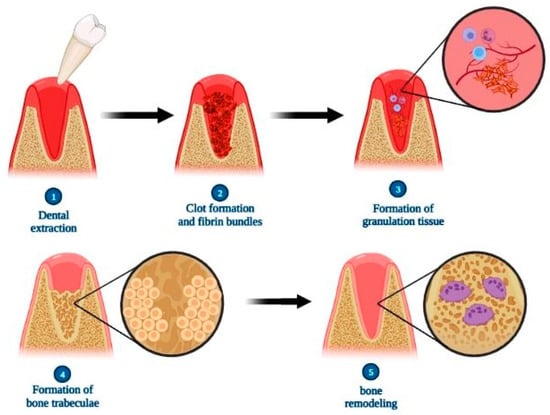
Figure 4.
Scheme illustrating the steps of alveolar bone repair after tooth extraction. Briefly, the repair process is characterized initially by the formation of the clot and fibrin bundles, followed by the proliferation of fibroblasts, blood capillaries and inflammatory cells resulting in granulation tissue. In sequence, there is an increase in the amount of osteoblasts at the site, producing bone trabeculae that replace the granulation tissue. Finally, there is a gradual decrease in intertrabecular spaces in the process of bone remodeling, resulting in repaired bone tissue. Created with BioRender.com (accessed on 6 April 2023).
Lasers (light amplification by stimulated emission of radiation) are devices that emit a type of electromagnetic radiation, characterized by being relatively uniform in parameters related to wavelength, phase and polarization. Theodore Maiman initially studied these factors in 1960, when he carried out his research using ruby laser [72]. Lasers can be divided into two main classifications: high-power lasers (HLLT), which are indicated for surgical procedures, such as cuts and cauterization, and have an ablation effect [73]; and low-level lasers (LLLT), which are more used for therapeutic purposes and biostimulators [74]. Low-level laser is a type of laser whose action does not take the form of heat but through the absorption of photons by the tissue, which triggers photochemical mechanisms at the cellular level, promoting various effects in the biological system and resulting in the biomodulation of the cell so that it works in search of the normalization state of the affected region [75].
Since the last decade, studies involving the therapeutic properties of low-level laser have been increasing in such a way that many beneficial effects related to the application of this device in the field of dentistry have already been discovered. We can highlight properties such as the reduction of pain, inflammation and edema, assisting in the healing process and accelerating the tissue repair process [76,77]. Regarding this last property, studies have already demonstrated that LLLT is already widely used in the area of rehabilitation and regenerative medicine to accelerate the regeneration of different types of tissue in the human body, such as nervous tissue [78,79,80,81]; muscles [82]; bone tissue [83,84,85,86]; respiratory tract tissue [87]; tissues affected by burns [88] and other body tissue types [89]. Metin et al. (2018) obtained positive results when using LLLT in soft and hard tissues after endodontic surgery, such that the laser group presented better results in relation to tissue repair, increase in bone volume and density and decrease in postoperative pain [90].
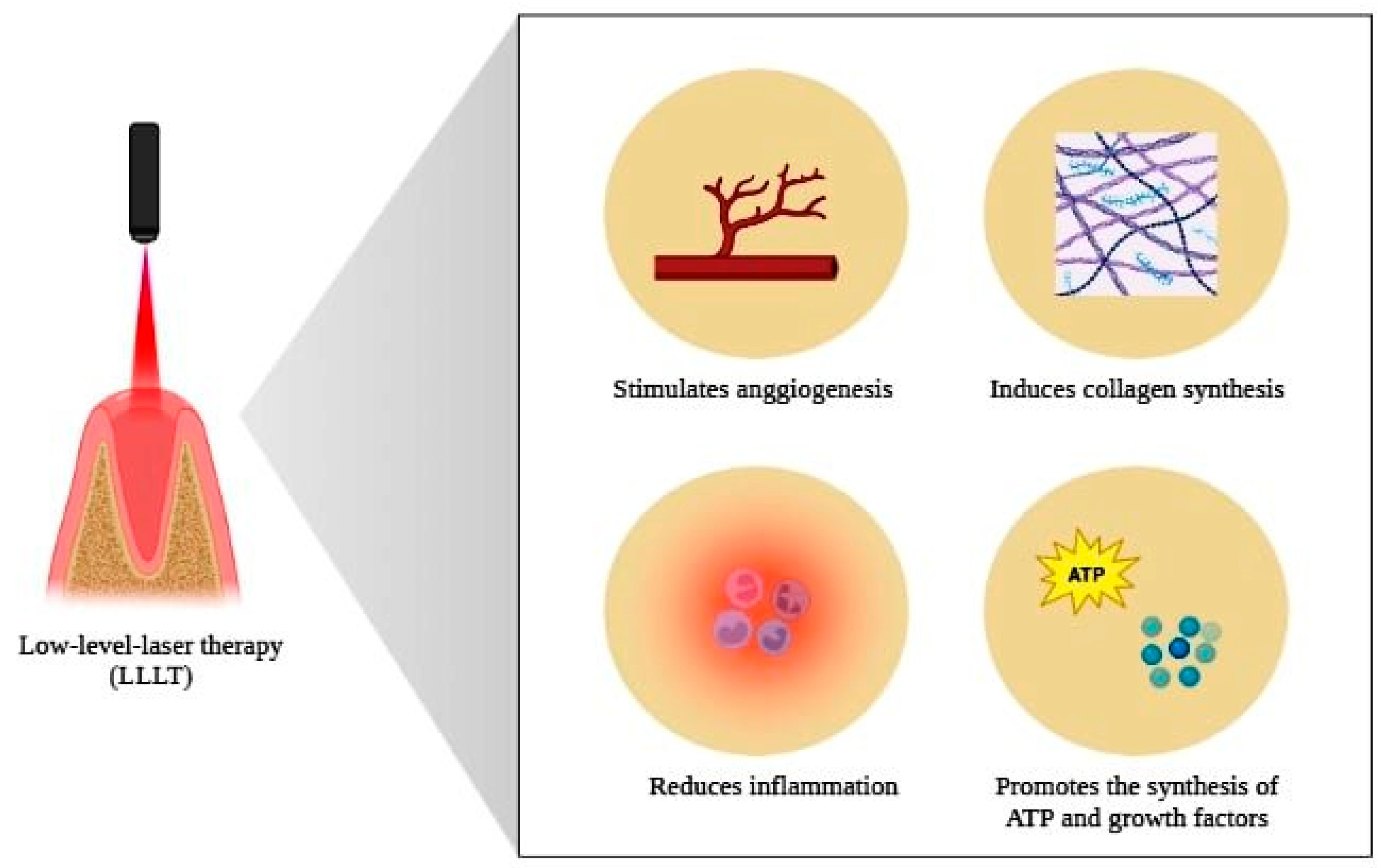
This property on tissue repair is due to factors such as the stimulation of ATP production, the activation of transcription factors, the activation of genes responsible for the synthesis of growth factors, the inhibition of factors responsible for cell death, the attenuation of the inflammatory process resulting from the extraction and the promotion of greater blood supply to the site to be repaired [91,92] (Figure 5).
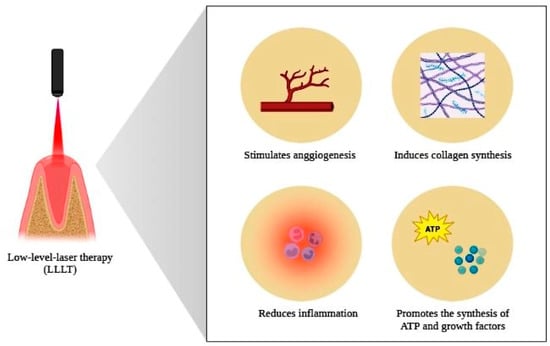
Figure 5.
Scheme illustrating the beneficial properties of low-level laser therapy on body tissues. Photobiomodulation, through the application of LLLT, stimulates angiogenesis, induces collagen synthesis, promotes the attenuation of the inflammatory process and stimulates the synthesis of ATP, as well as growth factors, properties that determine the accelerating potential of tissue repair provided for the use of this therapy. Created with BioRender.com (accessed on 6 April 2023).
According to our study, it was possible to analyze that the use of low-level lasers in the dental socket generated a significant increase in the expression of genes related to the neoformation of bone tissue, such as genes responsible for the synthesis of the receptor activator of nuclear factor kappa (RANK), the receptor activator of nuclear factor kappa B ligand (RANKL), osteoprotegerin (OPG), runt-related transcription factor 2 (RUNX-2), type 1 collagen and osteocalcin (OCN) and essential factors for the bone tissue deposition process [40,41,42,47,93].
It was also possible to observe a significant reduction in the inflammatory process after tooth extraction and an increase in vascularization in the region, which benefits new bone formation due to the greater contribution of minerals to the region [11,43,45,46,52,53,94]. Given these facts, LLLT accelerated the production of bone trabeculae and promoted an increase in the density of the formed bone [49,95].
These entire laser properties depend on the application protocol, which includes factors such as the wavelength used, energy density, irradiated area, time and frequency of laser application, in addition to the type of laser used [75,96]. Low-level lasers can be obtained from various sources such as GaAlAs (gallium–aluminum–arsenide); He-Ne (helium–neon); twin laser; Wiser wireless; CO2; InGaAlP (indium gallium aluminum phosphorus); Nd;YAG (neodymium-doped yttrium aluminum garnet), but it was possible to observe that the most used in dentistry in alveolar repair were the GaAlAs laser and the He-Ne laser (Figure 2). When comparing these two types of laser, the He-Ne laser and GaAlAs laser, GaAlAs proved to be more efficient due to its greater ability to penetrate tissue [43].
Regarding the laser energy density, it can be seen that the studies warn about care so that its intensity is not so low in such a way that it does not promote beneficial effects, nor so high that it can result in tissue damage that worsens the bone tissue regeneration [97]. The parameters are still not standardized, varying a lot between experiments, from 6 J/cm2 to 180 J/cm2 [43,56]. Furthermore, it was observed that when comparing two values of energy density of the same type of laser (6 J/cm2 or 10 J/cm2), the higher dosage of 10 J/cm2 proved to be more efficient in stimulating the tissue repair [43].
LLLT is not only used alone in an attempt to accelerate alveolar bone repair but is also used as a possible adjuvant in the action of other repair methods, such as mineral trioxide aggregate (MTA), bovine bone graft, collagen membrane and Bio-Oss® (Geistlich Pharma AG, Wolhusen, Switzerland). Mineral trioxide aggregate is a biomaterial that has the ability to induce osteogenesis by stimulating calcium deposition in the connective tissue [45]. According to the experiment, it was observed that the association of MTA with LLLT is more effective than the use of MTA alone, as a greater intensity of trabecular bone deposition was obtained. However, even though this association promotes good results, it was observed that the use of LLLT alone obtained better results, since a greater intensification of trabecular bone deposition was observed [45,98].
Bovine bone graft is widely used as an osteoconductor in the repair of bone alveolar tissue after tooth extraction and is often associated with the application of a collagen membrane to surround the graft (guided tissue regeneration—GTR). According to the experiment, grafting associated with the LLLT protocol proved more efficient in relation to the use of the isolated graft, as together the presence of greater bone volume was detected during tissue regeneration [31].
Bio-Oss® collagen is a bone substitute widely used in reconstructive dentistry due to its osteoconductive properties, in addition to being recommended for the reconstruction of alveolar bone after tooth extraction. According to the experiment, it was observed that the association of bone substitute with LLLT proved to be more efficient than the use of Bio-Oss alone, since both the association and the use of LLLT alone presented a higher mineralization index in relation to the use of Bio-Oss® alone [50]. However, the mechanism of the effect of the combination of LLLT and Bio-Oss® is not yet clear and needs to be further studied.
Visible light has wavelengths in the range of approximately 400 nm to 780 nm. When the wavelength is above this range we have infrared radiation, and when it is below we have ultraviolet radiation (UV) [99]. The wavelengths most used in dentistry are included in the red and infrared range, close to the electromagnetic spectrum, and this range of non-ionizing energy is safer for clinical application [100]. We can mention the use of UV irradiation, the management of polymicrobial biofilms in periodontal and peri-implant microbiomes, or endodontic infections and inflammation in root canals, contributing to the destruction of microorganisms and the release of cytokines, chemokines and biomarkers in tissues [101]. Longer wavelengths (>1110 nm), such as erbium lasers (2780 nm and 2940 nm) and carbon dioxide (CO2) lasers (9300 nm and 10,600 nm), are high-power lasers that, in dentistry, the erbium is used for caries prevention, cavity preparations, surface treatment of ceramics and the CO2 laser indicated for soft tissues [102].
LLLT uses low-power lasers with wavelengths from 660 nm to 1110 nm, a range that is more indicated to have good effects in the initial stages of bone repair [18]. In this review, it was observed that the most used wavelength was 830 nm, which demonstrated satisfactory results [18]. In addition, regarding the laser application protocol, different application intervals during and after tooth extraction were analyzed; however, it was observed that the laser has more visible and efficient results in the initial period of the alveolar bone repair process, and after this period the action of the laser is not as noticeable when compared to the natural repair process [51,103].
Most of the laser systems studied in this review are continuous-wave (CW) lasers. CW lasers are light sources that continuously pump and emit light [104]. Ultrashort pulse lasers offer innovative opportunities for material processing. The most common forms of laser technology are the nanosecond, picosecond and femtosecond lasers [105]. Picosecond and femtosecond lasers with reduced pulse durations have significant advantages in the higher-precision ultra-fast laser ablation industry [106]. Femtosecond lasers can be used in dental surgery and minimally invasive treatments of carious tissue. Femtosecond laser ablation offers a tool for generating cavities without cracking tooth tissue [107].
For brief context, it is possible to state that low-level laser therapy is beneficial to the alveolar bone repair process due to its biostimulatory effects, which are angiogenesis and collagen matrix synthesis inducers, inflammation, pain and edema inhibitors [108,109]. The difficulty in comparing the different protocols for the use of LLLT for alveolar bone repair can be considered a limitation of this review, in view of the use of very different protocols between one study and another.
Still, another difficulty to be reported, after consulting the PubMed/Medline, Web of Science, Scopus and Cochrane databases with the crossing of the keywords determined by us, was the existence of few articles with a randomized clinical trial, so that the findings regarding the benefits of LLLT in alveolar bone repair are not, in fact, much used in clinical research for this purpose. In this way, the expectation is created that more studies should be carried out in order to seek the standardization of the LLLT protocols and that more clinical experiments should be carried out so that LLLT has its use in the repair of alveolar bone consolidated and used in a way routine in dentistry. In addition, we can describe as future objectives, new studies on other materials or techniques that contribute to improving bone regeneration, such as the use of ozone or other methods of physical stimulation [110,111,112].
5. Conclusions
This integrative review was designed with the objective of analyzing studies that used low-level laser therapy associated with alveolar bone repair. The use of LLLT is mainly due to the fact that it is considered an auxiliary therapy in the tissue repair process, since studies show its biostimulating effects such as stimulation of collagen synthesis, growth factors and ATP; decreased inflammation, pain, and swelling; and induction of angiogenesis among other factors that help in the process of tissue repair. We also observed that these factors depend on the laser application protocol, with the GaAlAs (gallium–aluminum–arsenide)-type laser with a wavelength of 830 nm being the most prevalent, and when comparing applications of different energy densities, higher dosages proved to be more efficient.
However, there is difficulty in standardizing the application protocols due to the diversity of equipment and their forms of use after tooth extraction. Future studies that evaluate other complementary therapies and that aim to improve bone formation, such as ozone therapy and physical stimulation, should be carried out to contribute to clinical practice in dentistry and other areas of health.
Author Contributions
Conceptualization, R.G.R. and R.L.B.; methodology, R.G.R.; validation, D.V.B. and P.C.C.; formal analysis, R.G.R. and R.L.B.; investigation, R.G.R. and R.L.B.; data curation, D.V.B.; writing—original draft preparation, R.G.R.; writing—review and editing, R.G.R. and R.L.B.; visualization, D.V.B. and P.C.C.; supervision, R.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tatullo, M.; Gentile, S.; Paduano, F.; Santacroce, L.; Marrelli, M. Crosstalk between oral and general health status in e-smokers. Medicine 2016, 95, e5589. [Google Scholar] [CrossRef] [PubMed]
- Penoni, D.C.; Leão, A.T.T.; Fernandes, T.M.; Torres, S.R. Possible links between osteoporosis and periodontal disease. Rev. Bras. Reumatol. (Engl. Ed.) 2017, 57, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G. Non-Hodgkin lymphoma affecting the tongue: Unusual intra-oral location. Head Neck Oncol. 2011, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Pawinska, M.; Kondrat, A.; Jamiolkowski, J.; Paszynska, E. Dental Status and Oral Health Behaviors of Selected 45–74-Year-Old Men from Northeastern Poland. Int. J. Environ. Res. Public Health 2023, 20, 6005. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, M.; Anliker, N.; Sabatini, G.P.; De Paula, M.S.; Weber, A.R.; Molinero-mourelle, P. Assessment and Improvement of Masticatory Performance in Frail Older People: A Narrative Review. J. Clin. Med. 2023, 12, 3760. [Google Scholar] [CrossRef] [PubMed]
- Kalu, D.N. The ovariectomized rat model of postmenopa bone loss. Bone Miner. 1991, 15, 175–192. [Google Scholar] [CrossRef]
- Daigo, Y.; Daigo, E.; Hasegawa, A.; Fukuoka, H.; Ishikawa, M.; Takahashi, K. Utility of High-Intensity Laser Therapy Combined with Photobiomodulation Therapy for Socket Preservation after Tooth Extraction. Photobiomodulation Photomed. Laser Surg. 2020, 38, 75–83. [Google Scholar] [CrossRef]
- Kawasaki, K.; Shimizu, N. Effects of Low-Energy Laser Irradiation on Bone Remodeling during Experimental Tooth Movement in Rats. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2000, 26, 282–291. [Google Scholar] [CrossRef]
- Vieira, A.E.; Repeke, C.E.; De Barros Ferreira, S.; Colavite, P.M.; Biguetti, C.C.; Oliveira, R.C.; Assis, G.F.; Taga, R.; Trombone, A.P.F.; Garlet, G.P. Intramembranous bone healing process subsequent to tooth extraction in mice: Micro-computed tomography, histomorphometric and molecular characterization. PLoS ONE 2015, 10, e0128021. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Rosero, K.A.V.; Sampaio, R.M.F.; Deboni, M.C.Z.; Corrêa, L.; Marques, M.M.; Ferraz, E.P.; da Graça Naclério-Homem, M. Photobiomodulation as an adjunctive therapy for alveolar socket preservation: A preliminary study in humans. Lasers Med. Sci. 2020, 35, 1711–1720. [Google Scholar] [CrossRef]
- Horowitz, R.; Holtzclaw, D.; Rosen, P.S. A review on alveolar ridge preservation following tooth extraction. J. Evid. Based. Dent. Pract. 2012, 12, 149–160. [Google Scholar] [CrossRef]
- Stumbras, A.; Kuliesius, P.; Januzis, G.; Juodzbalys, G. Alveolar Ridge Preservation after Tooth Extraction Using Different Bone Graft Materials and Autologous Platelet Concentrates: A Systematic Review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Q.; Hou, J.; Wu, Y.; Liu, Y.; Li, R.; Pan, Y.; Zhang, D. Effect of platelet-rich fibrin on alveolar ridge preservation: A systematic review. J. Am. Dent. Assoc. 2019, 150, 766–778. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef]
- Sun, T.; Liu, M.; Yao, S.; Ji, Y.; Xiong, Z.; Tang, K.; Chen, K.; Yang, H.; Guo, X. Biomimetic composite scaffold containing small intestinal submucosa and mesoporous bioactive glass exhibits high osteogenic and angiogenic capacity. Tissue Eng.-Part A 2018, 24, 1044–1056. [Google Scholar] [CrossRef]
- Reis, C.H.B.; Buchaim, D.V.; Ortiz, A.d.C.; Fideles, S.O.M.; Dias, J.A.; Miglino, M.A.; Teixeira, D.d.B.; Pereira, E.d.S.B.M.; da Cunha, M.R.; Buchaim, R.L. Application of Fibrin Associated with Photobiomodulation as a Promising Strategy to Improve Regeneration in Tissue Engineering: A Systematic Review. Polymers 2022, 14, 2022. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Goissis, G.; Andreo, J.C.; Roque, D.D.; Roque, J.S.; Buchaim, D.V.; Rodrigues, A.d.C. Biocompatibility of anionic collagen matrices and its influence on the orientation of cellular growth. Braz. Dent. Sci. 2007, 10, 12–20. [Google Scholar] [CrossRef]
- Pires Oliveira, D.A.A.; De Oliveira, R.F.; Zangaro, R.A.; Soares, C.P. Evaluation of low-level laser therapy of osteoblastic cells. Photomed. Laser Surg. 2008, 26, 401–404. [Google Scholar] [CrossRef]
- Razzaghi, M.R.; Ghazimoradi, M.H.; Afzali, S.; Kamani, E.; Mohajerani, E.; Shirkavand, A.; Farivar, S. Effect of a Low-Level Laser on Liposomal Doxorubicin Efficacy in a Melanoma Cell Line. J. Lasers Med. Sci. 2021, 12, e28. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Chen, J.W.; Zhang, K. Effects of Low-Energy Gallium-Aluminum-Arsenide Laser Irradiation on Cultured Fibroblasts and Keratinocytes. Lasers Surg. Med. 1997, 20, 426–432. [Google Scholar] [CrossRef]
- Fiório, F.B.; Albertini, R.; Leal-Junior, E.C.P.; De Carvalho, P.D.T.C. Effect of low-level laser therapy on types i and III collagen and inflammatory cells in rats with induced third-degree burns. Lasers Med. Sci. 2014, 29, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.D.S.D.S.D.; Clark, R.M.D.O.; Ferreira, M.L. Efeitos da laserterapia de baixa potência na cicatrização de feridas cutâneas. Rev. Col. Bras. Cir. 2014, 41, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, K.; Hatefi, S.; Alizargar, J.; Abou-El-Hossein, K. Design of laser-assisted automatic continuous distraction osteogenesis device for oral and maxillofacial reconstruction applications. Majlesi J. Electr. Eng. 2019, 13, 135–145. [Google Scholar]
- Hatefi, S.; Etemadi Sh, M.; Alizargar, J.; Behdadipour, V.; Abou-El-Hossein, K. Two-Axis Continuous Distractor for Mandibular Reconstruction. Bioengineering 2022, 9, 371. [Google Scholar] [CrossRef]
- Jafarpour, T.; Smith, F. Low-level Laser Therapy Device for Assisting Distraction Osteogenesis in Maxillofacial Reconstruction Applications. Majlesi J. Electr. Eng. 2023, 17, 97–108. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Venugopal, A.; Marya, A.; Scribante, A. Evaluation of Adjuvant Systems in Non-Surgical Peri-Implant Treatment: A Literature Review. Healthcare 2022, 10, 886. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Soleo, R.; Di Fonso, F.; Politi, L.; Venugopal, A.; Marya, A.; Butera, A. Management of Periodontal Disease with Adjunctive Therapy with Ozone and Photobiomodulation (PBM): A Randomized Clinical Trial. Photonics 2022, 9, 138. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Luraghi, G.; Scribante, A. Ozonized water administration in peri-implant mucositis sites: A randomized clinical trial. Appl. Sci. 2021, 11, 7812. [Google Scholar] [CrossRef]
- Scribante, A.; Ghizzoni, M.; Pellegrini, M.; Pulicari, F.; Spadari, F. Laser Devices and Autologous Platelet Concentrates in Prevention and Treatment of Medication-Related Osteonecrosis of the Jaws: A Systematic Review. Medicine 2023, 59, 972. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Natoli, V.; Bruni, A.; Coscione, C.; Magliano, G.; Giacobbo, G.; Morelli, A.; Moressa, S.; Scribante, A. Bio-inspired systems in nonsurgical periodontal therapy to reduce contaminated aerosol during COVID-19: A comprehensive and bibliometric review. J. Clin. Med. 2020, 9, 3914. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Patwardhan, A.; Gilbraith, K.B.; Moutal, A.; Yang, X.; Chew, L.A.; Largent-Milnes, T.; Malan, T.P.; Vanderah, T.W.; Porreca, F.; et al. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain 2017, 158, 347–360. [Google Scholar] [CrossRef]
- Almpani, K.; Kantarci, A. Nonsurgical Methods for the Acceleration of the Orthodontic Tooth Movement. Front. Oral Biol. 2015, 18, 80–91. [Google Scholar] [CrossRef]
- Pomini, K.T.; Andreo, J.C.; De Rodrigues, A.C.; De Gonçalves, J.B.O.; Daré, L.R.; German, I.J.S.; Rosa, G.M.; Buchaim, R.L. Effect of low-intensity pulsed ultrasound on bone regeneration biochemical and radiologic analyses. J. Ultrasound Med. 2014, 33, 713–717. [Google Scholar] [CrossRef]
- Saito, S.; Shimizu, N. Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 525–532. [Google Scholar] [CrossRef]
- Facchin, F.; Canaider, S.; Tassinari, R.; Zannini, C.; Bianconi, E.; Taglioli, V.; Olivi, E.; Cavallini, C.; Tausel, M.; Ventura, C. Physical energies to the rescue of damaged tissues. World J. Stem Cells 2019, 11, 297–321. [Google Scholar] [CrossRef]
- Farzan, A.; Khaleghi, K.; Pirayesh, Z. Effect of Low-Level Laser Therapy on Bone Formation in Rapid Palatal Expansion: A Systematic Review. J. Lasers Med. Sci. 2022, 13, e13. [Google Scholar] [CrossRef]
- Berni, M.; Brancato, A.M.; Torriani, C.; Bina, V.; Annunziata, S.; Cornella, E.; Trucchi, M.; Jannelli, E.; Mosconi, M.; Gastaldi, G.; et al. The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. Int. J. Mol. Sci. 2023, 24, 7094. [Google Scholar] [CrossRef]
- Santos, C.M.d.C.; Pimenta, C.A.d.M.; Nobre, M.R.C. The PICO strategy for the research question construction and evidence search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Özyurt, A.; Elmas, Ç.; Seymen, C.M.; Peker, V.T.; Altunkaynak, B.; Güngör, M.N. Effects of Low-Level Laser Therapy With a Herbal Extract on Alveolar Bone Healing. J. Oral Maxillofac. Surg. 2018, 76, 287.e1–287.e10. [Google Scholar] [CrossRef]
- Ribeiro, L.N.S.; Monteiro, P.M.; Barretto, G.D.; Luiz, K.G.; Alves, S.Y.F.; Stuani, M.B.S. The effect of cigarette smoking and low-level laser irradiation in RANK/RANKL/OPG expression. Braz. Dent. J. 2020, 31, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.S.; de Figueiredo, F.A.T.; da Silva Mira, P.C.; Arnez, M.F.M.; Matsumoto, M.A.N.; de Menezes, L.M.; Küchler, E.C.; Stuani, M.B.S. Low-level laser therapy (LLLT) improves alveolar bone healing in rats. Lasers Med. Sci. 2022, 37, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Çırak, E.; Özyurt, A.; Peker, T.; Ömeroğlu, S.; Güngör, M.N. Comparative evaluation of various low-level laser therapies on bone healing following tooth extraction: An experimental animal study. J. Cranio-Maxillofac. Surg. 2018, 46, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Kang, K.L. Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: A pilot study. Lasers Med. Sci. 2012, 27, 223–230. [Google Scholar] [CrossRef]
- Oliveira, E.A.; de Oliveira, V.G.M.; Pires, J.A.; Barreto, A.L.S.; Ribeiro, M.A.G.; Pinheiro, A.L.B.; Marques, A.M.C.; de Melo, C.M.; de Albuquerque, R.L.C. Effect of low-level laser therapy and mineral trioxide aggregate on alveolar bone repair. Braz. J. Oral Sci. 2008, 7, 1657–1661. [Google Scholar]
- Pretel, H.; Lizarelli, R.F.Z.; Ramalho, L.T.O. Effect of low-level laser therapy on bone repair: Histological study in rats. Lasers Surg. Med. 2007, 39, 788–796. [Google Scholar] [CrossRef]
- Park, J.B.; Ahn, S.J.; Kang, Y.G.; Kim, E.C.; Heo, J.S.; Kang, K.L. Effects of increased low-level diode laser irradiation time on extraction socket healing in rats. Lasers Med. Sci. 2015, 30, 719–726. [Google Scholar] [CrossRef]
- Abdel Hamid, M.A.; Zaied, A.A.; Zayet, M.K.; Abdelmageed, H.; Hassan, E.A.; Amaroli, A. Efficacy of Flat-Top Hand-Piece Using 980 nm Diode Laser Photobiomodulation on Socket Healing after Extraction: Split-Mouth Experimental Model in Dogs. Photochem. Photobiol. 2021, 97, 627–633. [Google Scholar] [CrossRef]
- Fukuoka, H.; Daigo, Y.; Enoki, N.; Taniguchi, K.; Sato, H. Influence of carbon dioxide laser irradiation on the healing process of extraction sockets. Acta Odontol. Scand. 2010, 69, 33–40. [Google Scholar] [CrossRef]
- Rochkind, S.; Kogan, G.; Luger, E.G.; Salame, K.; Karp, E.; Graif, M.; Weiss, J. Molecular Structure of the Bony Tissue after Experimental Trauma to the Mandibular Region followed by Laser Therapy. Photomed. Laser Surg. 2004, 22, 249–253. [Google Scholar] [CrossRef]
- Luca, R.E.; Giuliani, A.; Mănescu, A.; Heredea, R.; Hoinoiu, B.; Constantin, G.D.; Duma, V.F.; Todea, C.D. Osteogenic potential of bovine bone graft in combination with laser photobiomodulation: An ex vivo demonstrative study in wistar rats by cross-linked studies based on synchrotron microtomography and histology. Int. J. Mol. Sci. 2020, 21, 778. [Google Scholar] [CrossRef]
- Forte, C.P.F.; Matos, A.P.; Mendes, F.H.; Dias, C.C.; Ferreira, A.E.C.; Bezerra, T.P.; Sousa, F.B.; Barros Silva, P.G. De Photobiomodulation Therapy Reduces the Inflammatory Process without Inhibiting Bone Deposition in Rats in an Extraction Model. Photobiomodulation Photomed. Laser Surg. 2020, 38, 673–678. [Google Scholar] [CrossRef]
- Statkievicz, C.; Toro, L.F.; de Mello-Neto, J.M.; de Sá, D.P.; Casatti, C.A.; Issa, J.P.M.; Cintra, L.T.A.; de Almeida, J.M.; Nagata, M.J.H.; Garcia, V.G.; et al. Photomodulation multiple sessions as a promising preventive therapy for medication-related osteonecrosis of the jaws after tooth extraction in rats. J. Photochem. Photobiol. B Biol. 2018, 184, 7–17. [Google Scholar] [CrossRef]
- Mergoni, G.; Vescovi, P.; Sala, R.; Merigo, E.; Passerini, P.; Maestri, R.; Corradi, D.; Govoni, P.; Nammour, S.; Bianchi, M.G. The effect of laser therapy on the expression of osteocalcin and osteopontin after tooth extraction in rats treated with zoledronate and dexamethasone. Support. Care Cancer 2016, 24, 807–813. [Google Scholar] [CrossRef]
- Romão, M.M.A.; Marques, M.M.; Cortes, A.R.G.; Horliana, A.C.R.T.; Moreira, M.S.; Lascala, C.A. Micro-computed tomography and histomorphometric analysis of human alveolar bone repair induced by laser phototherapy: A pilot study. Int. J. Oral Maxillofac. Surg. 2015, 44, 1521–1528. [Google Scholar] [CrossRef]
- Mozzati, M.; Martinasso, G.; Cocero, N.; Pol, R.; Maggiora, M.; Muzi, G.; Canuto, R.A. Superpulsed laser therapy on healing process after tooth extraction in patients waiting for liver transplantation. Lasers Med. Sci. 2012, 27, 353–359. [Google Scholar] [CrossRef]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Euler, H. Die Heilung von Extraktionswunden. Dtsch. Monatschr. Zahnh 1923, 41, 655. [Google Scholar]
- Carvalho, T.L.; Bombonato, K.F.B.L. Histometric analysis of rat alveolar wound healing. Braz Dent J. 1997, 8, 9–12. [Google Scholar]
- Zhao, Y.; Gong, Y.; Liu, X.; He, J.; Zheng, B.; Liu, Y. The Experimental Study of Periodontal Ligament Stem Cells Derived Exosomes with Hydrogel Accelerating Bone Regeneration on Alveolar Bone Defect. Pharmaceutics 2022, 14, 2189. [Google Scholar] [CrossRef]
- Pitol-Palin, L.; Batista, F.R.d.S.; Gomes-Ferreira, P.H.S.; Mulinari-Santos, G.; Ervolino, E.; Souza, F.Á.; Matsushita, D.H.; Okamoto, R. Different stages of alveolar bone repair process are compromised in the type 2 diabetes condition: An experimental study in rats. Biology 2020, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Wang, C.Y.; Wan, P.; Wang, S.G.; Wang, X.M. Comparison of bone regeneration in alveolar bone of dogs on mineralized collagen grafts with two composition ratios of nanohydroxyapatite and collagen. Regen. Biomater. 2016, 3, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Andersson, L.; Tolba, R.; Al-Asfour, A.; Bartella, A.K.; Gremse, F.; Rosenhain, S.; Hölzle, F.; Kessler, P.; Lethaus, B. Bone regeneration using composite non-demineralized xenogenic dentin with beta-tricalcium phosphate in experimental alveolar cleft repair in a rabbit model. J. Transl. Med. 2017, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.C.; Wang, H.; Li, J.; Zhang, D.; Yin, L.Q.; Yan, Y.F.; Ma, X. Repair of alveolar cleft bone defects by bone collagen particles combined with human umbilical cord mesenchymal stem cells in rabbit. BioMed. Eng. OnLine 2020, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Torres, H.; Mauricio, F.; Mendoza, R.; Alvítez-Temoche, D.; Medina, J.; Mayta-Tovalino, F. Histological Comparison of Post-extraction Alveolar Bone Repair Treated with Melatonin and Calcium Sulfate: An In Vivo Study in Cavia porcellus. J.Contemp. Dent. Pract. 2021, 22, 739–744. [Google Scholar] [CrossRef]
- Mandarino, D.; Luz, D.; Moraschini, V.; Rodrigues, D.M.; Alveolar, E.S.P.B. Alveolar ridge preservation using a non-resorbable membrane: Randomized clinical trial with biomolecular analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 1465–1473. [Google Scholar] [CrossRef]
- Bigham-sadegh, A.; Oryan, A. Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering fracture healing, bone graft substitutes and bone tissue regeneration. Connect. Tissue Res. 2015, 56, 175–194. [Google Scholar] [CrossRef]
- Al-obaidi, M.M.J.; Al-bayaty, F.H.; Al, R.; Hassandarvish, P.; Rouhollahi, E. ScienceDirect Protective effect of ellagic acid on healing alveolar bone after tooth extraction in rat—A histological and immunohistochemical study. Arch. Oral Biol. 2014, 59, 987–999. [Google Scholar] [CrossRef]
- Ervolino, E.; Statkievicz, C.; Felipe, L.; De Mello-Neto, J.M.; Priscila, T.; Paulo, J.; Issa, M.; Cássia, R.; Dornelles, M.; Milanezi, J.; et al. Antimicrobial photodynamic therapy improves the alveolar repair process and prevents the occurrence of osteonecrosis of the jaws after tooth extraction in senile rats treated with zoledronate. Bone 2019, 120, 101–113. [Google Scholar] [CrossRef]
- Panzarini, R.; Sonoda, C.K.; Tomiko, C.; Hamata, M. Histological and immunohistochemical analyses of the chronology of healing process after immediate tooth replantation in incisor rat teeth. Dent. Traumatol. 2013, 29, 15–22. [Google Scholar] [CrossRef]
- Van der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef]
- Farivar, S.; Malekshahabi, T.; Shiari, R. Biological Effects of Low Level Laser Therapy. J. Lasers Med. Sci. 2014, 5, 58–62. [Google Scholar]
- Jaeger, F.; Chiavaioli, G.M.d.O.; de Toledo, G.L.; Freire-Maia, B.; Amaral, M.B.F.; de Abreu, M.H.N.G.; de Arruda, J.A.A.; Mesquita, R.A. Efficacy and safety of diode laser during circumvestibular incision for Le Fort I osteotomy in orthognathic surgery: A triple-blind randomized clinical trial. Lasers Med. Sci. 2020, 35, 395–402. [Google Scholar] [CrossRef]
- Basso, F.G.; Oliveira, C.F.; Kurachi, C.; Hebling, J.; Costa, C.A.D.S. Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med. Sci. 2013, 28, 367–374. [Google Scholar] [CrossRef]
- Cavalcanti, T.M.; Quirino De Almeida-Barros, R.; Chaves De Vasconcelos Catão, M.H.; Patrícia, A.; Feitosa, A.; Diógenes, R.; Lins, A.U. Knowledge of the physical properties and interaction of laser with biological tissue in dentistry. An. Bras. De Dermatol. 2011, 86, 955–960. [Google Scholar] [CrossRef]
- Amitha, K.; Paramashivaiah, R.; Laxmaiah, M.; Prabhuji, V.; Subramanya, A.P.; Assiry, A.A.; Peeran, S.W.; Fageeh, H.; Bhavikatti, S.K.; Scardina, G.A. Clinical Assessment of the Effects of Low-Level Laser Therapy on Coronally Advanced Flap Procedure in the Management of Isolated Gingival Recession. Photonics 2022, 9, 932. [Google Scholar] [CrossRef]
- Pasquale, C.; Utyuzh, A.; Mikhailova, M.V.; Colombo, E.; Amaroli, A. Recovery from Idiopathic Facial Paralysis (Bell’ s Palsy) Using Photobiomodulation in Patients Non-Responsive to Standard Treatment: A Case Series Study. Photonics 2021, 8, 341. [Google Scholar] [CrossRef]
- Poiani, G.d.C.R.; Zaninotto, A.L.; Carneiro, A.M.C.; Zangaro, R.A.; Salgado, A.S.I.; Parreira, R.B.; de Andrade, A.F.; Teixeira, M.J.; Paiva, W.S. Photobiomodulation using low-level laser therapy (LLLT) for patients with chronic traumatic brain injury: A randomized controlled trial study protocol. Trials 2018, 19, 17. [Google Scholar] [CrossRef]
- Rodrigo, C.; Bueno, D.S.; Clara, M.; Tonin, C.; Buchaim, D.V.; Barraviera, B.; Seabra, R.; Junior, F.; Paulo, S.; Santos, S.; et al. Morphofunctional Improvement of the Facial Nerve and Muscles with Repair Using Heterologous Fibrin Biopolymer and Photobiomodulation. Pharmaceuticals 2023, 16, 653. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Rodrigues, A.C.; Buchaim, R.L.; Barraviera, B.; Junior, R.S.F.; Junior, G.M.R.; Bueno, C.R.S.; Roque, D.D.; Dias, D.V.; Dare, L.R.; et al. The new heterologous fibrin sealant in combination with low-level laser therapy (LLLT) in the repair of the buccal branch of the facial nerve. Lasers Med. Sci. 2016, 31, 965–972. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Andreo, J.C.; Barraviera, B.; Ferreira Junior, R.S.; Buchaim, D.V.; Rosa Junior, G.M.; De Oliveira, A.L.R.; De Castro Rodrigues, A. Effect of low-level laser therapy (LLLT) on peripheral nerve regeneration using fibrin glue derived from snake venom. Injury 2015, 46, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Lovisetto, R.; Malavazzi, T.C.D.S.; Andreo, L.; Rodrigues, M.F.S.D.; Bussadori, S.K.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A. Photobiomodulation Using Different Infrared Light Sources Promotes Muscle Precursor Cells Migration and Proliferation. Photonics 2022, 9, 469. [Google Scholar] [CrossRef]
- Pomini, K.T.; Buchaim, D.V.; Bighetti, A.C.C.; Hamzé, A.L.; Reis, C.H.B.; Duarte, M.A.H.; Alcalde, M.P.; Barraviera, B.; Júnior, R.S.F.; de Souza, A.T.; et al. Tissue Bioengineering with Fibrin Scaffolds and Deproteinized Bone Matrix Associated or Not with the Transoperative Laser Photobiomodulation Protocol. Molecules 2023, 28, 407. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.H.B.; Buchaim, R.L.; Pomini, K.T.; Hamzé, A.L.; Zattiti, I.V.; Duarte, M.A.H.; Alcalde, M.P.; Barraviera, B.; Ferreira Júnior, R.S.; Pontes, F.M.L.; et al. Effects of a Biocomplex Formed by Two Scaffold Biomaterials, Hydroxyapatite/Tricalcium Phosphate Ceramic and Fibrin Biopolymer, with Photobiomodulation, on Bone Repair. Polymers 2022, 14, 2075. [Google Scholar] [CrossRef]
- Angeletti, P.; Pereira, M.D.; Gomes, H.C.; Hino, C.T.; Ferreira, L.M. Effect of low-level laser therapy (GaAlAs) on bone regeneration in midpalatal anterior suture after surgically assisted rapid maxillary expansion. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e38–e46. [Google Scholar] [CrossRef]
- Neto, F.C.J.; Martimbianco, A.L.C.; de Andrade, R.P.; Bussadori, S.K.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S. Effects of photobiomodulation in the treatment of fractures: A systematic review and meta-analysis of randomized clinical trials. Lasers Med. Sci. 2020, 35, 513–522. [Google Scholar] [CrossRef]
- De Souza, G.H.M.; Ferraresi, C.; Moreno, M.A.; Pessoa, B.V.; Damiani, A.P.M.; Filho, V.G.; dos Santos, G.V.; Zamunér, A.R. Acute effects of photobiomodulation therapy applied to respiratory muscles of chronic obstructive pulmonary disease patients: A double-blind, randomized, placebo-controlled crossover trial. Lasers Med. Sci. 2020, 35, 1055–1063. [Google Scholar] [CrossRef]
- Alsharnoubi, J.; Shoukry, K.E.S.; Fawzy, M.W.; Mohamed, O. Evaluation of scars in children after treatment with low-level laser. Lasers Med. Sci. 2018, 33, 1991–1995. [Google Scholar] [CrossRef]
- Guncay, T.; Oyanedel, M.; Lemus, M.; Weinstein, A.; Ardiles, Á.O.; Marcos, J.; Fernandes, A.; Renato, Z.; Muñoz, P. The Transcranial Light Therapy Improves Synaptic Plasticity in the Alzheimer ’ s Disease Mouse Model. Brain Sci. 2022, 12, 1272. [Google Scholar] [CrossRef]
- Metin, R.; Tatli, U.; Evlice, B. Effects of low-level laser therapy on soft and hard tissue healing after endodontic surgery. Lasers Med. Sci. 2018, 33, 1699–1706. [Google Scholar] [CrossRef]
- De Oliveira Rosso, M.P.; Buchaim, D.V.; Pomini, K.T.; Della Coletta, B.B.; Bertoni Reis, C.H.; Galletti Pilon, J.P.; Duarte Júnior, G.; Buchaim, R.L. Photobiomodulation therapy (PBMT) applied in bone reconstructive surgery using bovine bone grafts: A systematic review. Materials 2019, 12, 4051. [Google Scholar] [CrossRef]
- Rosso, M.; Buchaim, D.; Kawano, N.; Furlanette, G.; Pomini, K.; Buchaim, R. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef]
- Bosco, A.F.; Faleiros, P.L.; Carmona, L.R.; Garcia, V.G.; Theodoro, L.H.; de Araujo, N.J.; Nagata, M.J.H.; de Almeida, J.M. Effects of low-level laser therapy on bone healing of critical-size defects treated with bovine bone graft. J. Photochem. Photobiol. B 2016, 163, 303–310. [Google Scholar] [CrossRef]
- He, W.L.; Yu, F.Y.; Li, C.J.; Pan, J.; Zhuang, R.; Duan, P.J. A systematic review and meta-analysis on the efficacy of low-level laser therapy in the management of complication after mandibular third molar surgery. Lasers Med. Sci. 2015, 30, 1779–1788. [Google Scholar] [CrossRef]
- Fallahnezhad, S.; Piryaei, A.; Tabeie, F.; Nazarian, H.; Darbandi, H.; Amini, A.; Mostafavinia, A.; Ghorishi, S.K.; Jalalifirouzkouhi, A.; Bayat, M. Low-level laser therapy with helium–neon laser improved viability of osteoporotic bone marrow-derived mesenchymal stem cells from ovariectomy-induced osteoporotic rats. J. Biomed. Opt. 2016, 21, 098002. [Google Scholar] [CrossRef]
- Sterczała, B.; Grzech-Lésniak, K.; Michel, O.; Trzeciakowski, W.; Dominiak, M.; Jurczyszyn, K. Assessment of human gingival fibroblast proliferation after laser stimulation in vitro using different laser types and wavelengths (1064, 980, 635, 450, and 405 nm)—Preliminary report. J. Pers. Med. 2021, 11, 98. [Google Scholar] [CrossRef]
- Ebrahimi, T.; Moslemi, N.; Rokn, A.; Heidari, M.; Nokhbatolfoghahaie, H.; Fekrazad, R. The influence of low-intensity laser therapy on bone healing. J. Dent. 2012, 9, 238–248. [Google Scholar]
- Palczewska-Komsa, M.; Kaczor-Wiankowska, K.; Nowicka, A. New bioactive calcium silicate cement mineral trioxide aggregate repair high plasticity (Mta hp)— a systematic review. Materials 2021, 14, 4573. [Google Scholar] [CrossRef]
- Mild, K.H.; Lundström, R.; Wilén, J. Non-ionizing radiation in swedish health care—Exposure and safety aspects. Int. J. Environ. Res. Public Health 2019, 16, 1186. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Tenore, G.; Luzi, M.C.; Palaia, G.; Mohsen, A.; Pergolini, D.; Romeo, U. Laser photobiomodulation (Pbm)—A possible new frontier for the treatment of oral cancer: A review of in vitro and in vivo studies. Healthcare 2021, 9, 134. [Google Scholar] [CrossRef]
- Luchian, I.; Budală, D.G.; Baciu, E.R.; Ursu, R.G.; Diaconu-Popa, D.; Butnaru, O.; Tatarciuc, M. The Involvement of Photobiology in Contemporary Dentistry—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 3985. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.; Zhao, I.S.; Gutknecht, N.; Chu, C.H. Use of carbon dioxide lasers in dentistry. Lasers Dent. Sci. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- De Oliveira Rosso, M.P.; Oyadomari, A.T.; Pomini, K.T.; Della Coletta, B.B.; Shindo, J.V.T.C.; Júnior, R.S.F.; Barraviera, B.; Cassaro, C.V.; Buchaim, D.V.; Teixeira, D.D.B.; et al. Photobiomodulation therapy associated with heterologous fibrin biopolymer and bovine bone matrix helps to reconstruct long bones. Biomolecules 2020, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.S.; Wu, J.; Xu, H.; Wang, Z. Emerging Light-Emitting Materials for Photonic Integration. Adv. Mater. 2021, 33, 2003733. [Google Scholar] [CrossRef] [PubMed]
- Pou-Álvarez, P.; Riveiro, A.; Nóvoa, X.R.; Fernández-Arias, M.; del Val, J.; Comesaña, R.; Boutinguiza, M.; Lusquiños, F.; Pou, J. Nanosecond, picosecond and femtosecond laser surface treatment of magnesium alloy: Role of pulse length. Surf. Coat. Technol. 2021, 427, 127802. [Google Scholar] [CrossRef]
- Hamad, A.; Li, L.; Liu, Z. A comparison of the characteristics of nanosecond, picosecond and femtosecond lasers generated Ag, TiO2 and Au nanoparticles in deionised water. Appl. Phys. A Mater. Sci. Process. 2015, 120, 1247–1260. [Google Scholar] [CrossRef]
- Serbin, J.; Bauer, T.; Fallnich, C.; Kasenbacher, A.; Arnold, W.H. Femtosecond lasers as novel tool in dental surgery. Appl. Surf. Sci. 2002, 197–198, 737–740. [Google Scholar] [CrossRef]
- Saito, C.T.M.H.; Gulinelli, J.L.; Panzarini, S.R.; Garcia, V.G.; Okamoto, R.; Okamoto, T.; Sonoda, C.K.; Poi, W.R. Effect of low-level laser therapy on the healing process after tooth replantation: A histomorphometrical and immunohistochemical analysis. Dent. Traumatol. 2011, 27, 30–39. [Google Scholar] [CrossRef]
- Freitas, N.R.; Guerrini, L.B.; Esper, L.A.; Sbrana, M.C.; Dalben, G.D.S.; Soares, S.; Almeida, A.L.P.F. Evaluation of photobiomodulation therapy associated with guided bone regeneration in critical size defects. In vivo study. J. Appl. Oral Sci. 2018, 26, e20170244. [Google Scholar] [CrossRef]
- Colombo, M.; Gallo, S.; Garofoli, A.; Poggio, C.; Arciola, C.R.; Scribante, A. Ozone gel in chronic periodontal disease: A randomized clinical trial on the anti-inflammatory effects of ozone application. Biology 2021, 10, 625. [Google Scholar] [CrossRef]
- Hatefi, S.; Alizargar, J.; Le Roux, F.; Hatefi, K.; Etemadi Sh, M.; Davids, H.; Hsieh, N.C.; Smith, F.; Abou-El-Hossein, K. Review of physical stimulation techniques for assisting distraction osteogenesis in maxillofacial reconstruction applications. Med. Eng. Phys. 2021, 91, 28–38. [Google Scholar] [CrossRef]
- Huang, X.; Das, R.; Patel, A.; Duc Nguyen, T. Physical Stimulations for Bone and Cartilage Regeneration. Regen. Eng. Transl. Med. 2018, 4, 216–237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).