Chemical Dosimetry Using Bisbenzimidazoles: Solvent-Dependent Fluorescence Response of Hoechst 33258 to Radiation Exposure
Abstract
1. Introduction
2. Materials and Methods
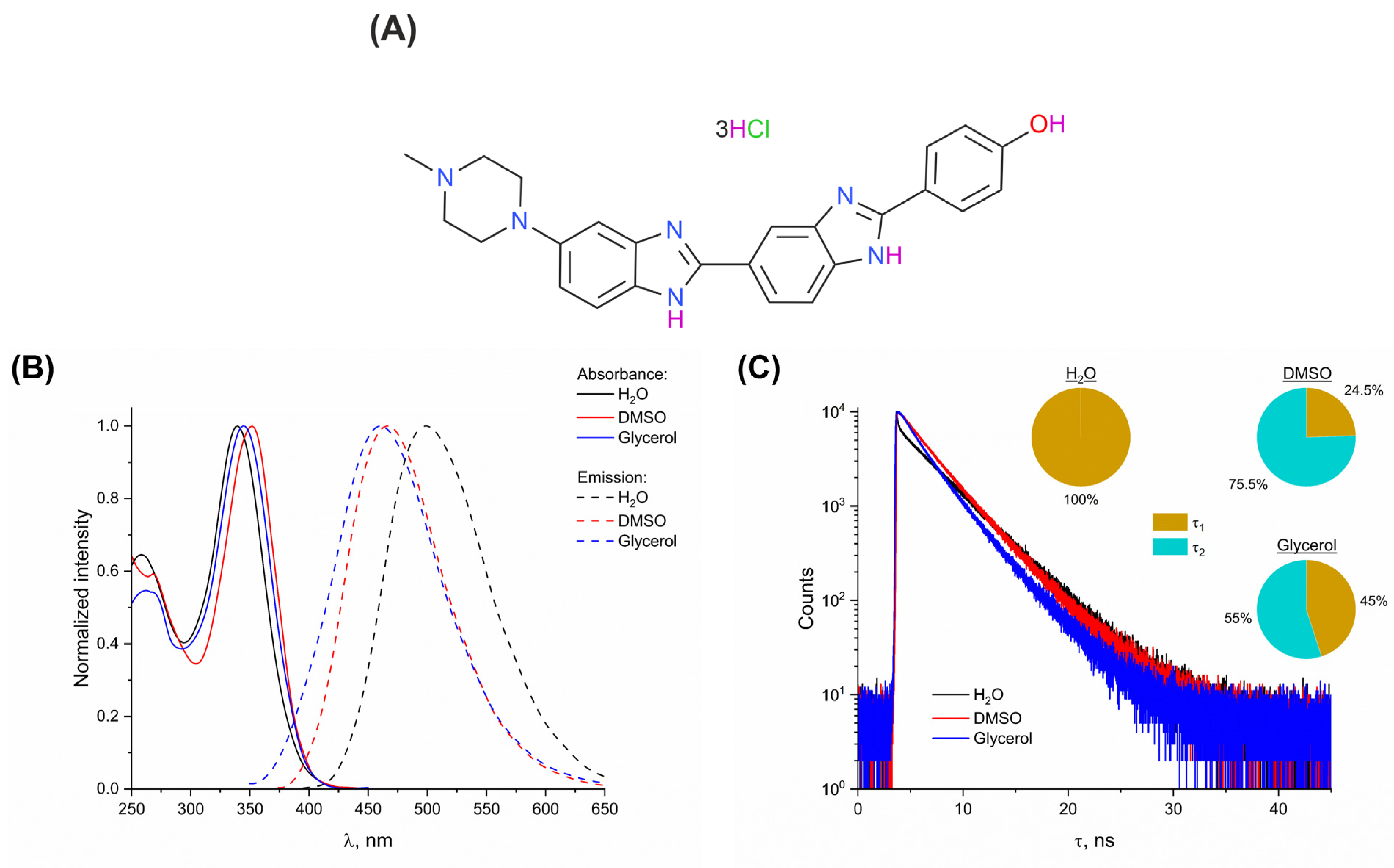
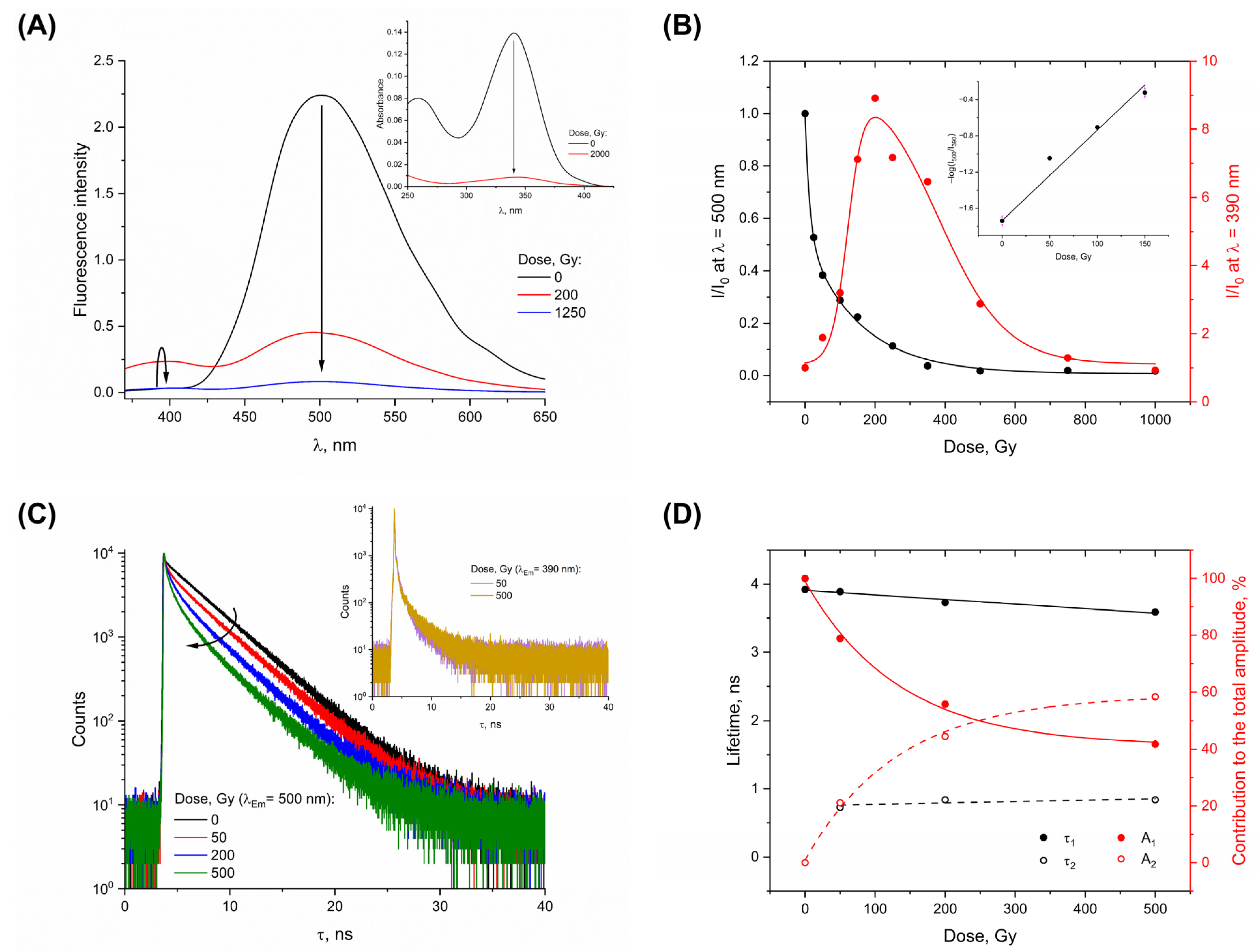
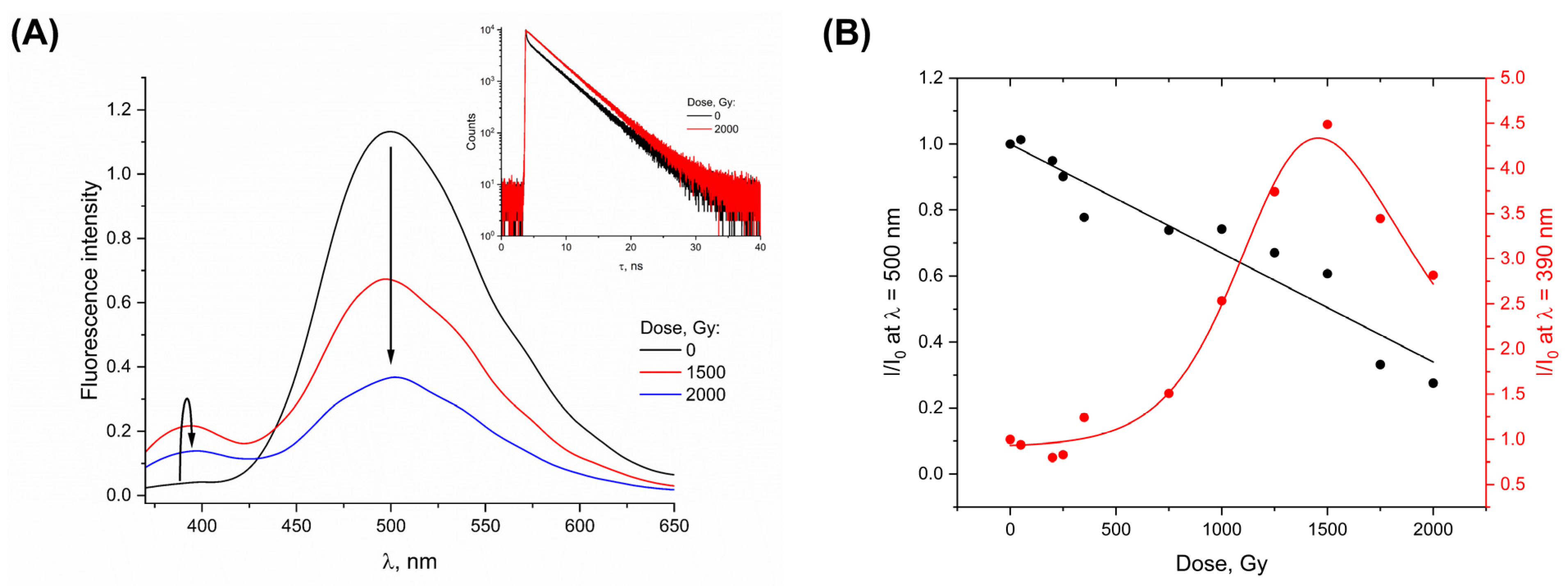
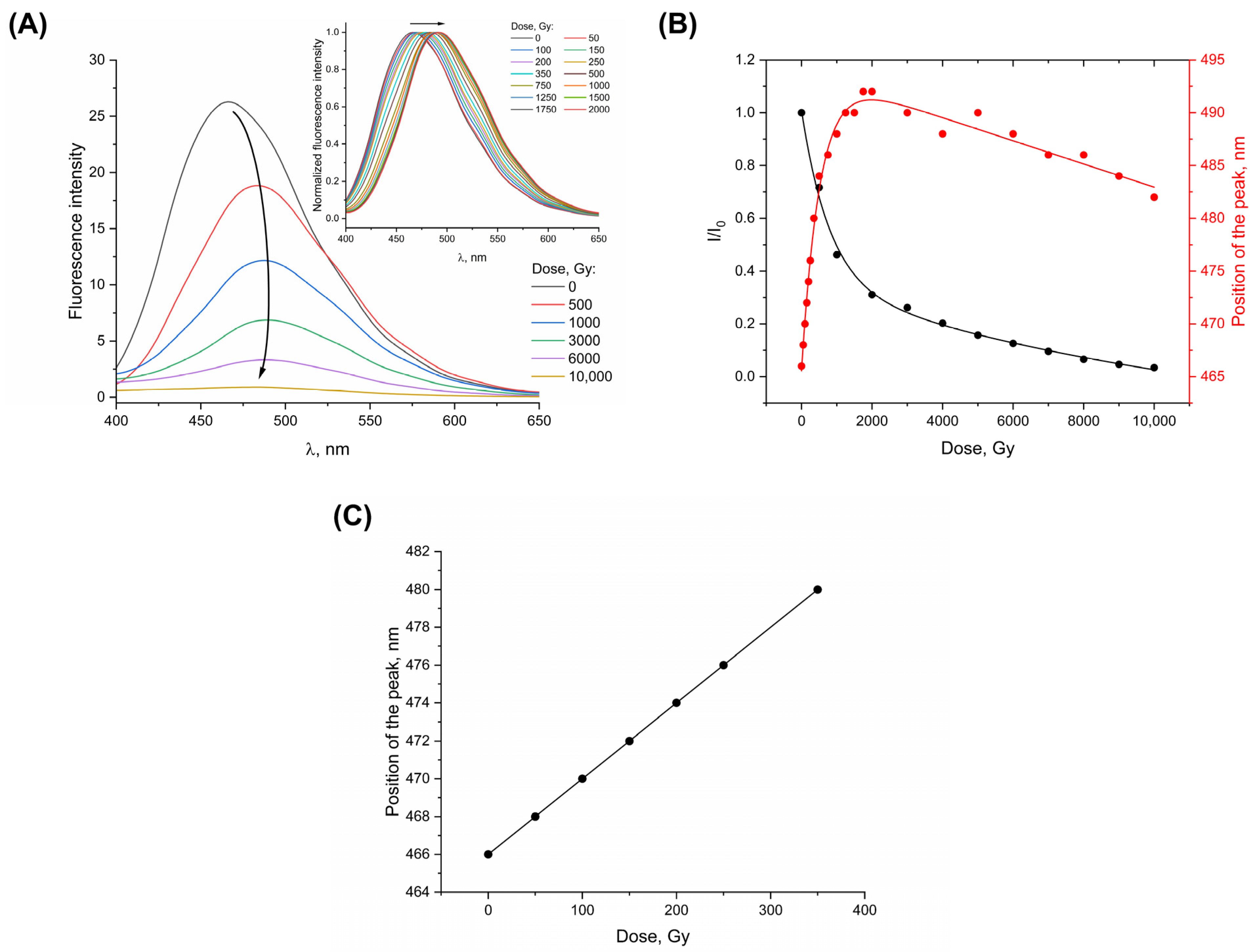
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallico, E.; Camerino, B. Azione riducente dei raggi X su soluzioni di bleu di metilene. Experientia 1948, 4, 109–110. [Google Scholar] [CrossRef]
- Day, M.J.; Stein, G. The action of ionizing radiations on aqueous solutions of methylene blue. Radiat. Res. 1957, 6, 666–679. [Google Scholar] [CrossRef]
- El-Assy, N.B.; Chen, Y.D.; Walker, M.L.; Al-Sheikhly, M.; McLaughlin, W.L. Anionic triphenylmethane dye solutions for low-dose food irradiation dosimetry. Radiat. Phys. Chem. 1995, 46, 1189–1197. [Google Scholar] [CrossRef]
- Khan, H.M.; Anwer, M.; Chaudhry, Z.S. Dosimetric characterisation of aqueous solution of brilliant green for low-dose food irradiation dosimetry. Radiat. Phys. Chem. 2002, 63, 713–717. [Google Scholar] [CrossRef]
- Soliman, Y.S.; Basfar, A.A.; Msalam, R.I. A radiochromic film based on leucomalachite green for high-dose dosimetry applications. Radiat. Meas. 2014, 62, 45–51. [Google Scholar] [CrossRef]
- Al Zahrany, A.A.; Rabaeh, K.A.; Basfar, A.A. Radiation-induced color bleaching of methyl red in polyvinyl butyral film dosimeter. Radiat. Phys. Chem. 2011, 80, 1263–1267. [Google Scholar] [CrossRef]
- Kayed, S.F. Metal complexes of azo compounds: Synthesis, characterization, molecular modeling and degradation study by gamma radiation. Inorg. Chem. Commun. 2022, 142, 109634. [Google Scholar] [CrossRef]
- Ashawa, S.C.; Kini, U.R.; Madhvanath, U. The aqueous coumarin system as a low range chemical dosimeter. Int. J. Appl. Radiat. Isot. 1979, 30, 7–10. [Google Scholar] [CrossRef]
- Collins, A.K.; Makrigiorgos, G.M.; Svensson, G.K. Coumarin chemical dosimeter for radiation therapy. Med. Phys. 1994, 21, 1741–1747. [Google Scholar] [CrossRef]
- Asai, K.; Koshimizu, M.; Fujimoto, Y.; Asai, K. Isomerization behavior of spiropyran-based compounds upon X-ray irradiation. Radiat. Meas. 2017, 106, 166–169. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.S.A.; Abdel-Rahim, F.; El-Assy, N.B. Styrylcyanine dye solutions for radiation dosimetry. J. Radioanal. Nucl. Chem. 1984, 81, 67–75. [Google Scholar] [CrossRef]
- Rauf, M.A.; Salman Ashraf, S. Radiation induced degradation of dyes—An overview. J. Hazard. Mater. 2009, 16, 6–16. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, W.L. Solid-phase chemical dosimeters. In Technical Developments and Prospects of Sterilization by Ionizing Radiation; Gaughran, E.R.L., Goudie, A.J., Eds.; Multiscience Publications Ltd.: Montréal, CA, USA, 1974; pp. 219–252. [Google Scholar]
- Whittaker, B. A new PMMA dosimeter for low doses and low temperatures. Int. J. Radiat. Appl. Instrum. C 1990, 35, 699–702. [Google Scholar] [CrossRef]
- Adamovics, J.; Maryanski, M.J. Characterisation of PRESAGE: A new 3-D radiochromic solid polymer dosemeter for ionising radiation. Radiat. Prot. Dosimetry 2006, 120, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Beshir, W.B.; Eid, S.; Gafar, S.M.; Ebraheem, S. Application of solutions of Rhodamine B in dosimetry. Appl. Radiat. 2014, 89, 13–17. [Google Scholar] [CrossRef]
- Barr, N.F.; Stark, M.B. Chemical dosimetry with fluorescent compounds: The destruction of the fluorescence of quinine by gamma rays. Radiat. Res. 1960, 12, 1–4. [Google Scholar] [CrossRef]
- Khan, H.M.; McLaughlin, W.L. Gamma-ray dosimetry by spectrofluorimetry of phenylacetic acid solution. Radiat. Phys. Chem. 1992, 39, 243–249. [Google Scholar] [CrossRef]
- Stodilka, R.Z.; Carson, J.J.L.; Yu, K.; Zaman, B.; Li, C.; Wilkinson, D. Optical degradation of CdSe/ZnS quantum dots upon gamma-ray irradiation. J. Phys. Chem. C 2009, 113, 2580–2585. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, W.; Cai, Z.; Zhang, G.; Zhang, D. A facile and convenient fluorescence detection of gamma-ray radiation based on the aggregation-induced emission. J. Mater. Chem. 2011, 21, 14487–14491. [Google Scholar] [CrossRef]
- Han, J.M.; Xu, M.; Wang, B.; Wu, N.; Yang, X.; Yang, H.; Salter, B.J.; Zang, L. Low dose detection of γ radiation via solvent assisted fluorescence quenching. J. Am. Chem. Soc. 2014, 136, 5090–5096. [Google Scholar] [CrossRef]
- Jiang, L.; Li, W.; Nie, J.; Wang, R.; Chen, X.; Fan, W.; Hu, L. Fluorescent nanogel sensors for X-ray dosimetry. ACS Sens. 2021, 6, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.P.; Bateman, S.A.; Hook, R.J.; Martin, R.F.; Reum, M.E.; Rose, M.; Whittaker, A.R.D. DNA binding compounds. VI. Synthesis and characterization of 2,5’-disubstituted bibenzimidazoles related to the DNA minor groove binder Hoechst-33258. Aust. J. Chem. 1994, 47, 1751–1769. [Google Scholar] [CrossRef]
- Sing, A.K.; Lown, J.W. Synthesis of analogs of Hoechst 33258 designed for altered base and sequence recognition. Synth. Commun. 2000, 30, 923–939. [Google Scholar] [CrossRef]
- Gromyko, A.V.; Popov, K.V.; Mosoleva, A.P.; Streltsov, S.A.; Grokhovsky, S.L.; Oleinikov, V.A.; Zhuze, A.L. DNA sequence-specific ligands: XII. Synthesis and cytological studies of dimeric Hoechst 33258 molecules. Russ. J. Bioorg. Chem. 2005, 31, 344–351. [Google Scholar] [CrossRef]
- Bucevičius, J.; Lukinavičius, G.; Gerasimaitė, R. The use of Hoechst dyes for DNA staining and beyond. Chemosensors 2018, 6, 18. [Google Scholar] [CrossRef]
- Nimesh, H.; Tiwari, V.; Yang, C.; Gundala, S.R.; Chuttani, K.; Hazari, P.P.; Mishra, A.K.; Sharma, A.; Lal, J.; Katyal, A.; et al. Preclinical evaluation of DMA, a bisbenzimidazole, as radioprotector: Toxicity, pharmacokinetics, and biodistribution studies in Balb/c mice. Mol. Pharmacol. 2015, 88, 768–778. [Google Scholar] [CrossRef]
- Yasui, L.S.; Chen, K.; Wang, K.; Jones, T.P.; Caldwell, J.; Guse, D.; Kassis, A.I. Using Hoechst 33342 to target radioactivity to the cell nucleus. Radiat. Res. 2007, 167, 167–175. [Google Scholar] [CrossRef]
- Koval, V.S.; Arutyunyan, A.F.; Salyanov, V.I.; Klimova, R.R.; Kushch, A.A.; Rybalkina, E.Y.; Susova, O.Y.; Zhuze, A.L. DNA sequence-specific ligands. XVII. Synthesis, spectral properties, virological and biochemical studies of fluorescent dimeric bisbenzimidazoles DBA(n). Bioorg. Med. Chem. 2018, 26, 2302–2309. [Google Scholar] [CrossRef]
- Hao, X.; Han, S.; Zhu, J.; Hu, Y.; Chang, L.Y.; Pao, C.W.; Chen, J.L.; Chen, J.M.; Hawe, S.C. A bis-benzimidazole PMO ratiometric fluorescence sensor exhibiting AIEE and ESIPT for sensitive detection of Cu2+. RSC Adv. 2019, 9, 13567–13575. [Google Scholar] [CrossRef]
- Żurek-Biesiada, D.; Waligórski, P.; Dobrucki, J.W. UV-induced spectral shift and protonation of DNA fluorescent dye Hoechst 33258. J. Fluoresc. 2014, 24, 1791–1801. [Google Scholar] [CrossRef]
- Bazhulina, N.P.; Nikitin, A.M.; Rodin, S.A.; Surovaya, A.N.; Kravatsky, Y.V.; Pismensky, V.F.; Archipova, V.S.; Martin, R.; Gursky, G.V. Binding of Hoechst 33258 and its derivatives to DNA. J. Biomol. Struct. Dyn. 2009, 26, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Le Caër, S. Water radiolysis: Influence of oxide surfaces on H2 production under ionizing radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef]
- Haskins, A.H.; Buglewicz, D.J.; Hirakawa, H.; Fujimori, A.; Aizawa, Y.; Kato, T.A. Palmitoyl ascorbic acid 2-glucoside has the potential to protect mammalian cells from high-LET carbon-ion radiation. Sci. Rep. 2018, 8, 13822. [Google Scholar] [CrossRef]
- Eberhardt, M.K.; Colina, R. The reaction of OH radicals with dimethyl sulfoxide. A comparative study of Fenton’s reagent and the radiolysis of aqueous dimethyl sulfoxide solutions. J. Org. Chem. 1988, 53, 1071–1074. [Google Scholar] [CrossRef]
- Babbs, C.F.; Griffin, D.W. Scatchard analysis of methane sulfinic acid production from dimethyl sulfoxide: A method to quantify hydroxyl radical formation in physiologic systems. Free Radic. Biol. Med. 1989, 6, 493–503. [Google Scholar] [CrossRef]
- Görner, H. Direct and sensitized photoprocesses of bis-benzimidazole dyes and the effects of surfactants and DNA. Photochem. Photobiol. 2001, 73, 339–348. [Google Scholar] [CrossRef]
- Barooah, N.; Mohanty, J.; Pal, H.; Sarkar, S.K.; Mukherjee, T.; Bhasikuttan, A.C. pH and temperature dependent relaxation dynamics of Hoechst-33258: A time resolved fluorescence study. Photochem. Photobiol. Sci. 2011, 10, 35–41. [Google Scholar] [CrossRef]
- Buxton, G.V. An overview of the radiation chemistry of liquids. In Radiation Chemistry: From Basics to Applications in Material and Life Sciences; Spotheim-Maurizot, M., Mostafavi, M., Douki, T., Belloni, J., Eds.; EDP Sciences: Les Ulis, France, 2008; pp. 3–16. [Google Scholar]
- Barakat, M.F.; El-Banna, M. Use of some organic dyes in gamma irradiation dose determination. J. Radioanal. Nucl. Chem. 2011, 287, 367–375. [Google Scholar] [CrossRef]
- Benevides, C.A.; de Menezes, F.D.; de Araujo, R.E. Evaluation of fluorescent dye degradation indirectly induced by x-ray ionizing radiation. Appl. Opt. 2015, 54, 6935–6939. [Google Scholar] [CrossRef]
- Ergun, E. A new fluorometric dosimetry for low-medium gamma radiation doses. J. Fluoresc. 2021, 31, 941–950. [Google Scholar] [CrossRef]
- Kolyvanova, M.A.; Klimovich, M.A.; Koshevaya, E.D.; Bushmanov, Y.A.; Belousov, A.V.; Kuzmin, V.A.; Morozov, V.N. Cholesteric liquid-crystalline DNA—A new type of chemical detector of ionizing radiation. Liq. Cryst. 2022, 49, 1359–1366. [Google Scholar] [CrossRef]
- Kolyvanova, M.A.; Klimovich, M.A.; Belousov, A.V.; Kuzmin, V.A.; Morozov, V.N. A principal approach to the detection of radiation-induced DNA damage by circular dichroism spectroscopy and its dosimetric application. Photonics 2022, 9, 787. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolyvanova, M.A.; Klimovich, M.A.; Koshevaya, E.D.; Nikitin, E.A.; Lifanovsky, N.S.; Tyurin, V.Y.; Belousov, A.V.; Trofimov, A.V.; Kuzmin, V.A.; Morozov, V.N. Chemical Dosimetry Using Bisbenzimidazoles: Solvent-Dependent Fluorescence Response of Hoechst 33258 to Radiation Exposure. Photonics 2023, 10, 671. https://doi.org/10.3390/photonics10060671
Kolyvanova MA, Klimovich MA, Koshevaya ED, Nikitin EA, Lifanovsky NS, Tyurin VY, Belousov AV, Trofimov AV, Kuzmin VA, Morozov VN. Chemical Dosimetry Using Bisbenzimidazoles: Solvent-Dependent Fluorescence Response of Hoechst 33258 to Radiation Exposure. Photonics. 2023; 10(6):671. https://doi.org/10.3390/photonics10060671
Chicago/Turabian StyleKolyvanova, Maria A., Mikhail A. Klimovich, Ekaterina D. Koshevaya, Evgeny A. Nikitin, Nikita S. Lifanovsky, Vladimir Y. Tyurin, Alexandr V. Belousov, Aleksei V. Trofimov, Vladimir A. Kuzmin, and Vladimir N. Morozov. 2023. "Chemical Dosimetry Using Bisbenzimidazoles: Solvent-Dependent Fluorescence Response of Hoechst 33258 to Radiation Exposure" Photonics 10, no. 6: 671. https://doi.org/10.3390/photonics10060671
APA StyleKolyvanova, M. A., Klimovich, M. A., Koshevaya, E. D., Nikitin, E. A., Lifanovsky, N. S., Tyurin, V. Y., Belousov, A. V., Trofimov, A. V., Kuzmin, V. A., & Morozov, V. N. (2023). Chemical Dosimetry Using Bisbenzimidazoles: Solvent-Dependent Fluorescence Response of Hoechst 33258 to Radiation Exposure. Photonics, 10(6), 671. https://doi.org/10.3390/photonics10060671





