Annual and Seasonal Trends in Density, Biomass, and Secondary Production of Chironomid Midges (Diptera: Chironomidae) in the Middle Reaches of the Shinano River, Japan, 2015–2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Collection of Adult Chironomid Midges
2.3. Estimation of Annual Secondary Production of Chironomidae
3. Results and Discussion
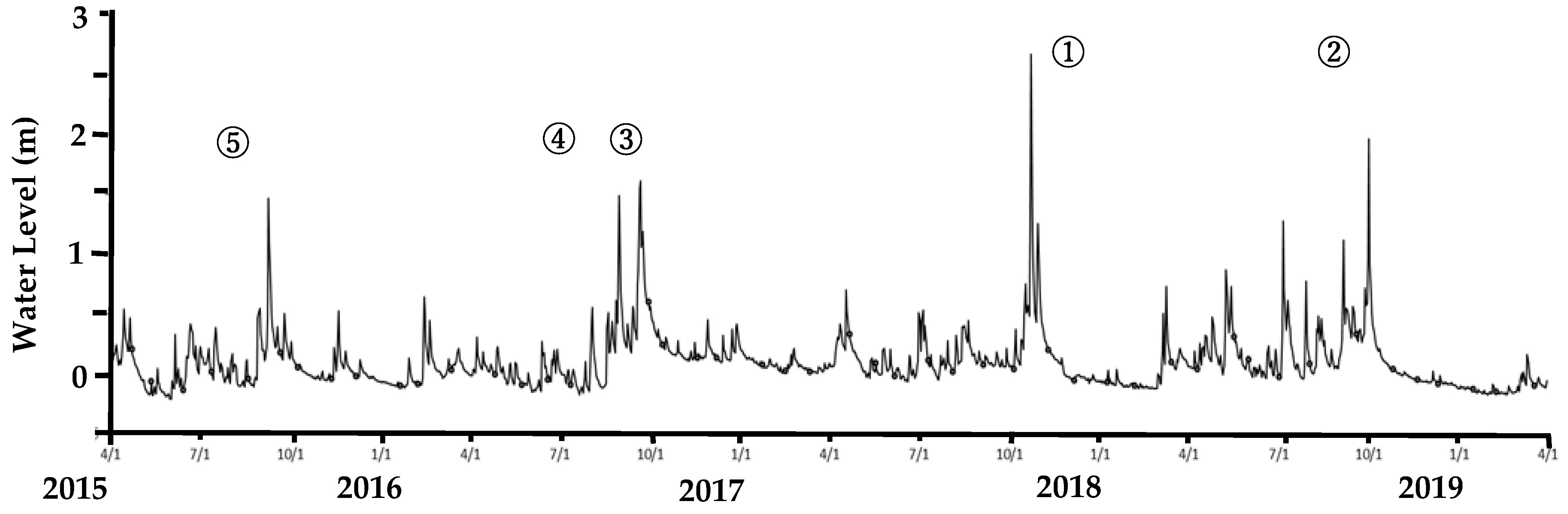
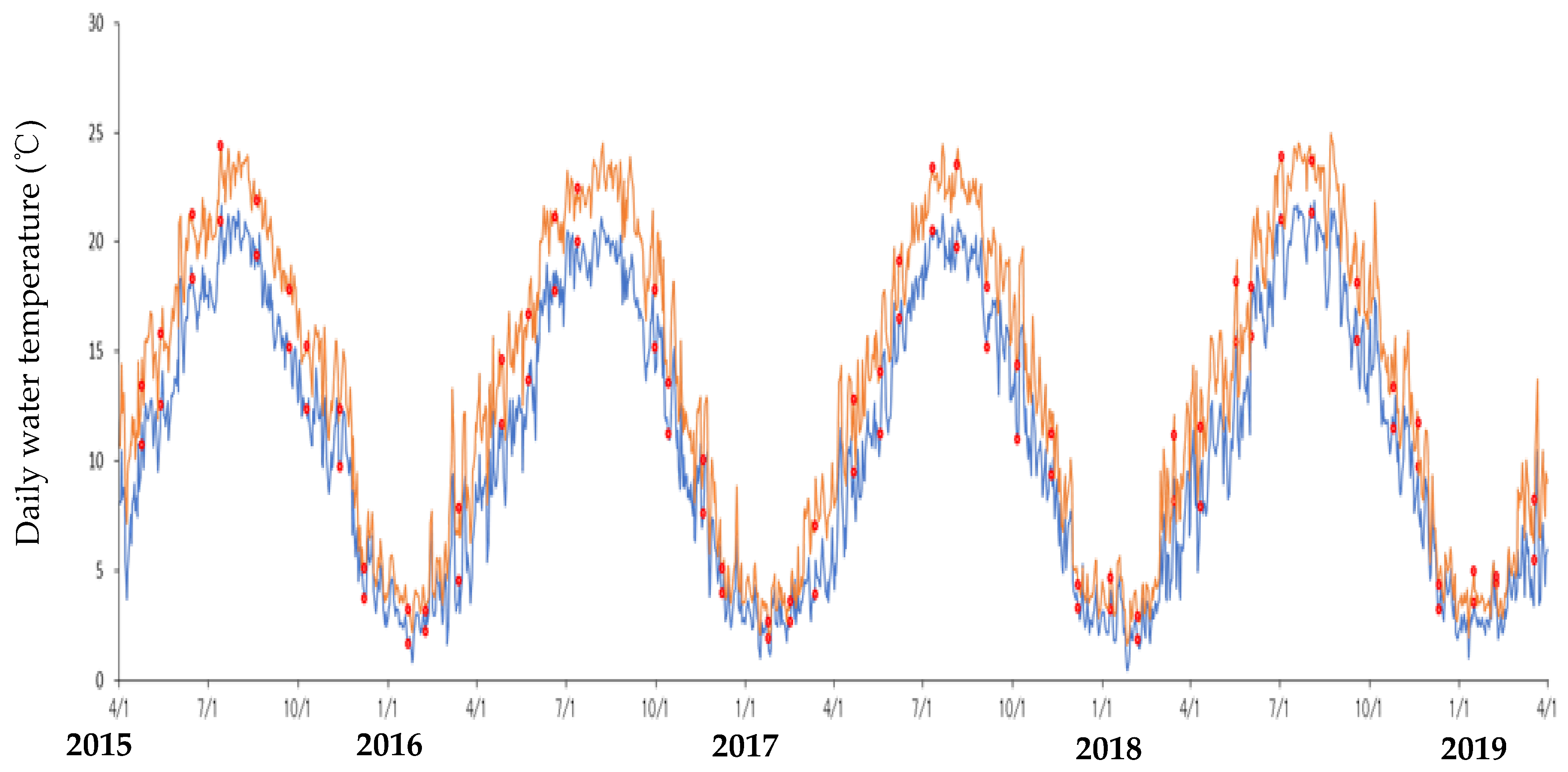
3.1. Environmental Conditions During the Investigation Periods
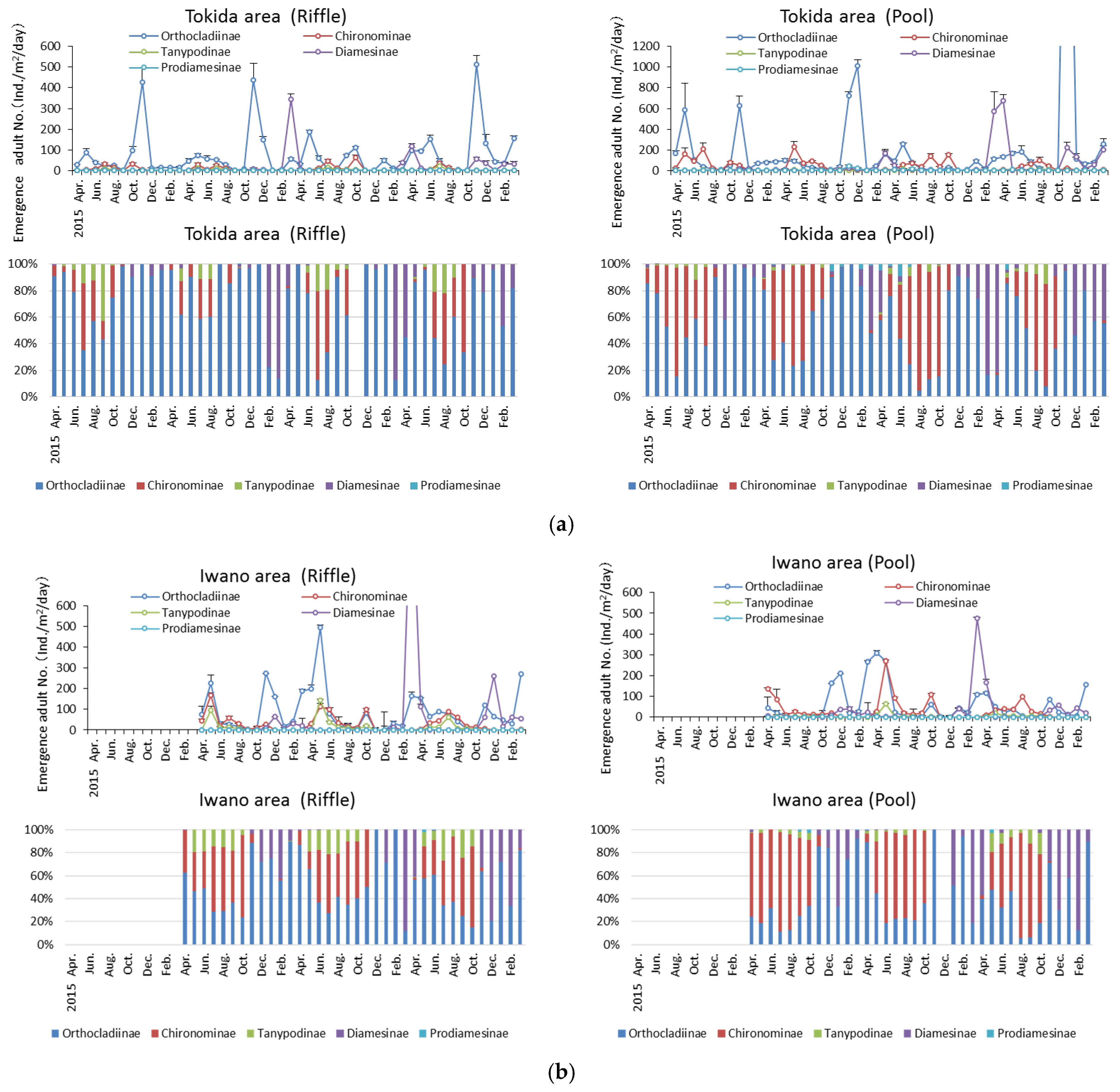
3.2. Seasonal Trends in Density and Biomass of Adult Chironomid Midges
3.3. Range of Annual Secondary Production of Chironomidae in Shinano River
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinder, L.C.V. The habitats of chironomid larvae. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman & Hall: London, UK, 1995; pp. 107–135. [Google Scholar]
- Lindegaard, C. Classification of water-bodies and pollution. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman & Hall: London, UK, 1995; pp. 385–404. [Google Scholar]
- Tokeshi, M. Life cycles and population dynamics. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman & Hall: London, UK, 1995; pp. 225–268. [Google Scholar]
- Tokeshi, M. Production ecology. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman & Hall: London, UK, 1995; pp. 269–296. [Google Scholar]
- Hirabayashi, K.; Wotton, R. Organic matter processing by chironomid larvae. Hydrobiologia 1998, 382, 151–159. [Google Scholar] [CrossRef]
- Wotton, R.; Hirabayashi, K. Midge larvae (Diptera: Chironomidae) as engineers in slow sand filter beds. Water Res. 1999, 33, 1509–1515. [Google Scholar] [CrossRef]
- Berg, M.B.; Hellenthal, R.A. Secondary production of Chironomidae (Diptera) in a north temperate stream. Freshw. Biol. 1991, 25, 497–505. [Google Scholar] [CrossRef]
- Grzybkowska, M.; Witczak, J. Distribution and production of Chironomidae (Diptera) in the lower course of the Grabia River (Central Poland). Freshw. Biol. 1990, 24, 519–531. [Google Scholar] [CrossRef]
- Ladle, M.; Cooling, D.A.; Welton, J.S.; Bass, A.B. Studies on Chironomidae in experimental recirculation stream system. II The growth, development and production of a spring generation of Orthocladius (Euothocladius) calvus Pinder. Freshw. Biol. 1985, 15, 243–255. [Google Scholar] [CrossRef]
- Iwakuma, T. Factors controlling the secondary productivity of benthic macroinvertebrates in freshwaters: A review. Jpn. J. Ecol. 1986, 36, 169–187. [Google Scholar]
- Kitagawa, N. Production of Chironomid larvae in the Takamigawa River. Investig. Product. Yoshino River 1971, 3, 14–15. [Google Scholar]
- Wiederholm, T. Chironomidae of Holarctic region. Keys and diagnoses. Part 1. larvae. Entmol. Scand. Suppl. 1983, 19, 1–457. [Google Scholar]
- Vallenduuk, H.J.; Moller-Pillot, H.K.M. Chironomidae Larvae. General Ecology and Tanypodinae; KNNV Publishing: Zeist, The Netherlands, 2007; pp. 1–144. [Google Scholar]
- Moller-Pillot, H.K.M. Chironomidae Larvae II. Biology and Ecology of the Chironomini; KNNV Publishing: Zeist, The Netherlands, 2009; pp. 1–144. [Google Scholar]
- Moller-Pillot, H.K.M. Chironomidae Larvae III. Biology and Ecology of the Aquatic Orthocladiinae; KNNV Publishing: Zeist, The Netherlands, 2013; pp. 1–314. [Google Scholar]
- Dolbeth, M.; Cusson, M.; Sousa, R.; Pardal, M.A. Secondary production as a tool for better understanding of aquatic ecosystems. Can. J. Fish Aqua. Sci. 2012, 69, 1230–1253. [Google Scholar] [CrossRef]
- Downing, J.A.; Rigler, F.H. A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters, 2nd ed.; Blackwell Scientific Publication: Oxford, UK, 1984; pp. 1–501. [Google Scholar]
- Speir, J.A.; Anderson, N.H. Use of emergence data for estimating annual production of aquatic insects. Limnol. Oceanogr. 1974, 19, 154–156. [Google Scholar] [CrossRef]
- Pinder, L.C.V. Biology of the eggs and first-instar larvae. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman & Hall: London, UK, 1995; pp. 87–106. [Google Scholar]
- Hirabayashi, K. Abundance and seasonal trend of dominant Chironomid midges in the middle reach of the Chikuma River. Jpn. J. Ecol. 2002, 52, 281–285. (In Japanese) [Google Scholar]
- Okada, S.; Hirabayashi, K. Effect of emergence trap size on catch in the riffles of the middle reaches of the Chikuma River. Jpn. J. Environ. Entomol. Zool. 2020, 31, 87–94. [Google Scholar]
- LeSage, L.; Harrison, A.D. Improved traps and techniques for the study of emerging aquatic insects. Entmol. News. 1979, 90, 65–78. [Google Scholar]
- Pinder, L.C.V. A key to adult males of British Chironomidae (Diptera) the non-biting midges. Freshw. Biol. Assoc. Sci. Publ. 1978, 37, 1–169. [Google Scholar]
- Ministry of Land, Infrastructure, Transport and Tourism, Japan. Water Data Base in Ikuta Station. Available online: http://www1.river.go.jp/cgi-bin/SrchWaterData.exe?ID=304031284416030&KIND=1&PAGE=0 (accessed on 10 March 2025).
- Meyer, E.I.; Poepperl, R. Secondary production of invertebrates in a Central European mountain stream (Steina, Black Forest, Germany). Arch. Hydrobiool. 2003, 158, 25–42. [Google Scholar] [CrossRef]
- Poff, N.L.; Huryn, A.D. Multi-scale determinants of secondary production in Atlantic salmon (Salmo salar) streams. Can. J. Fish Aqua. Sci. 1998, 55, 201–217. [Google Scholar] [CrossRef]
- Waters, T.F. Secondary production in inland waters. Adv. Ecol. Res. 1977, 10, 91–164. [Google Scholar]
- Benke, A.C. Secondary production of aquatic insects. In The Ecology of Aquatic Insects; Resh, V.H., Rosenberg, D.M., Eds.; Praeger Publish: New York, NY, USA, 1984; pp. 289–322. [Google Scholar]
- Benke, A.C.; Huryn, A.D.; Ward, G.M. Use of empirical models of stream invertebrate secondary production as applies to a functional feeding group. Verh. Internat. Verein. Limnol. 1998, 26, 2024–2029. [Google Scholar]
- Benke, A.C. Production dynamics of riverine chironomids: Extremely high biomass turnover rates of primary consumers. Ecology 1998, 79, 899–910. [Google Scholar] [CrossRef]
- Cummins, K.W. Ecology and distribution of aquatic insects. In An Introduction to the Aquatic Insects of North America, 3rd ed.; Merrit, R.W., Cummins, K.W., Eds.; Kendall Hunt: Dubuque, IA, USA, 1996; pp. 68–74. [Google Scholar]
- Berg, M.B. Larval food and feeding behavior. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman & Hall: London, UK, 1995; pp. 136–168. [Google Scholar]
- Iwakuma, T.; Yasuno, M.; Sugaya, Y. Chironomid production in relation to phytoplankton primary production in Lake Kasumigaura, Japan. Verh. Internat. Verein. Limnol. 1984, 22, 1150–1159. [Google Scholar] [CrossRef]
- Cranston, P.S. Biogeography. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman & Hall: London, UK, 1995; pp. 62–84. [Google Scholar]
- Okino, T. The Nature of Rivers Created by Floods; Shinano-Mainichi-Shinbunn Publishing: Nagano, Japan, 2006; pp. 1–253. [Google Scholar]
- United State Environmental Protection Agency. Climate Change Indicators: River Flooding. Available online: https://www.epa.gov/climate-indicators/climate-change-indicators-river-flooding (accessed on 20 May 2025).

| Orthocladiinae | Chironominae | Tanypodinae | Diamesinae | Prodiamesinae | Unknown | Total | ||
|---|---|---|---|---|---|---|---|---|
| Total individual number of chironomid midges/3 Emergence traps | ||||||||
| Total biomass (wet weight; mg) of chironomid midges/3 Emergence traps | ||||||||
| 2015 Tokida | Riffle (No.) | 1152 | 145 | 32 | 8 | 0 | 20 | 1357 |
| (wet-weight; mg) | 420.0 | 59.4 | 22.2 | 6.7 | 0.0 | 2.5 | 510.7 | |
| Pool (No.) | 2735 | 936 | 29 | 75 | 0 | 17 | 3792 | |
| (wet-weight; mg) | 1144.2 | 382.2 | 17.6 | 59.8 | 0.0 | 2.9 | 1606.6 | |
| sub-total | Riffle + Pool (No.) | 3887 | 1081 | 61 | 83 | 0 | 37 | 5149 |
| (wet-weight; mg) | 1564.2 | 441.5 | 39.8 | 66.5 | 0.0 | 5.4 | 2117.3 | |
| 2016 Tokida | Riffle (No.) | 1338 | 120 | 41 | 539 | 1 | 3 | 2042 |
| (wet-weight; mg) | 511.1 | 29.6 | 19.9 | 186.5 | 0.3 | 0.3 | 747.7 | |
| Pool (No.) | 3359 | 716 | 21 | 332 | 106 | 14 | 4548 | |
| (wet-weight; mg) | 1395.5 | 124.9 | 12.5 | 170.6 | 121.8 | 2.1 | 1827.3 | |
| sub-total | Riffle + Pool (No.) | 4697 | 836 | 62 | 871 | 107 | 17 | 6590 |
| (wet-weight; mg) | 1906.6 | 154.5 | 32.3 | 357.1 | 122.1 | 2.4 | 2574.9 | |
| 2016 Iwano | Riffle (No.) | 1566 | 531 | 196 | 194 | 0 | 17 | 2504 |
| (wet-weight; mg) | 370.5 | 93.5 | 95.5 | 84.3 | 0.0 | 1.3 | 645.0 | |
| Pool (No.) | 1137 | 457 | 11 | 178 | 4 | 8 | 1795 | |
| (wet-weight; mg) | 260.0 | 125.1 | 5.2 | 89.2 | 4.2 | 0.6 | 484.3 | |
| sub-total | Riffle + Pool (No.) | 2703 | 988 | 207 | 372 | 4 | 25 | 4299 |
| (wet-weight; mg) | 630.5 | 218.6 | 100.7 | 173.5 | 4.2 | 1.9 | 1129.3 | |
| 2017 Tokida | Riffle (No.) | 807 | 207 | 48 | 72 | 0 | 4 | 1138 |
| (wet-weight; mg) | 332.2 | 48.4 | 21.5 | 152.1 | 0.0 | 0.6 | 554.8 | |
| Pool (No.) | 1058 | 752 | 47 | 957 | 46 | 2 | 2862 | |
| (wet-weight; mg) | 147.4 | 157.4 | 22.3 | 50.2 | 34.3 | 0.5 | 412.1 | |
| sub-total | Riffle + Pool (No.) | 1865 | 959 | 95 | 1029 | 46 | 6 | 4000 |
| (wet-weight; mg) | 479.6 | 205.8 | 43.8 | 202.3 | 34.3 | 1.1 | 966.9 | |
| 2017 Iwano | Riffle (No.) | 1641 | 595 | 335 | 1842 | 0 | 2 | 4415 |
| (wet-weight; mg) | 229.4 | 96.4 | 139.9 | 3.4 | 0.0 | 0.1 | 469.2 | |
| Pool (No.) | 1246 | 805 | 110 | 769 | 1 | 0 | 2931 | |
| (wet-weight; mg) | 199.2 | 110.6 | 57.9 | 4.6 | 0.6 | 0.0 | 372.9 | |
| sub-total | Riffle + Pool (No.) | 2887 | 1400 | 445 | 2611 | 1 | 2 | 7346 |
| (wet-weight; mg) | 428.6 | 207.0 | 197.8 | 8.0 | 0.6 | 0.1 | 842.1 | |
| 2018 Tokida | Riffle (No.) | 1880 | 92 | 51 | 425 | 0 | 0 | 2448 |
| (wet-weight; mg) | 1294.5 | 40.7 | 43.8 | 503.8 | 0.0 | 0.0 | 1882.8 | |
| Pool (No.) | 8599 | 448 | 60 | 1938 | 12 | 0 | 11,057 | |
| (wet-weight; mg) | 5652.0 | 255.5 | 40.8 | 2511.5 | 14.9 | 0.0 | 8474.7 | |
| sub-total | Riffle + Pool (No.) | 10,479 | 540 | 111 | 2363 | 12 | 0 | 13,505 |
| (wet-weight; mg) | 6946.5 | 296.2 | 84.6 | 3015.3 | 14.9 | 0.0 | 10,357.5 | |
| 2018 Iwano | Riffle (No.) | 1438 | 399 | 157 | 839 | 5 | 0 | 2838 |
| (wet-weight; mg) | 794.9 | 166.0 | 97.2 | 983.1 | 6.2 | 0.0 | 2047.4 | |
| Pool (No.) | 767 | 389 | 64 | 482 | 4 | 0 | 1706 | |
| (wet-weight; mg) | 371.5 | 125.9 | 50.4 | 509.2 | 3.9 | 0.0 | 1060.9 | |
| sub-total | Riffle + Pool (No.) | 2205 | 788 | 221 | 1321 | 9 | 0 | 4544 |
| (wet-weight; mg) | 1166.4 | 291.9 | 147.6 | 1492.3 | 10.1 | 0.0 | 3108.3 | |
| Total Tokida | Riffle (No.) | 5177 | 564 | 172 | 1044 | 1 | 27 | 6985 |
| (4 years) | (wet-weight; mg) | 2557.8 | 178.1 | 107.4 | 849.1 | 0.3 | 3.4 | 3696.0 |
| Pool (No.) | 15,751 | 2852 | 157 | 3302 | 164 | 33 | 22,259 | |
| (wet-weight; mg) | 8339.1 | 919.9 | 93.2 | 2792.1 | 171.0 | 5.4 | 12,320.6 | |
| Sub-total | Riffle + Pool (No.) | 20,928 | 3416 | 329 | 4346 | 165 | 60 | 29,244 |
| (wet-weight; mg) | 10,896.9 | 1098.0 | 200.5 | 3641.2 | 171.3 | 8.8 | 16,016.6 | |
| Total Iwano | Riffle (No.) | 4645 | 1525 | 688 | 2875 | 5 | 19 | 9757 |
| (3 years) | (wet-weight; mg) | 1394.8 | 355.85 | 332.6 | 1070.8 | 6.2 | 1.35 | 3161.6 |
| Pool (No.) | 3150 | 1651 | 185 | 1429 | 9 | 8 | 6432 | |
| (wet-weight; mg) | 830.7 | 361.6 | 113.5 | 603.0 | 8.7 | 0.6 | 1918.1 | |
| Sub-total | Riffle + Pool (No.) | 7795 | 3176 | 873 | 4304 | 14 | 27 | 16,189 |
| (wet-weight; mg) | 2225.5 | 717.5 | 446.1 | 1673.8 | 14.9 | 2.0 | 5079.7 | |
| Tokida + Iwano | Tokida + Iwano (No.) | 28,723 | 6592 | 1202 | 8650 | 179 | 87 | 45,433 |
| % | 63.2 | 14.5 | 2.6 | 19.0 | 0.4 | 0.2 | 100.0 | |
| (wet-weight; mg) | 13,122.4 | 1815.4 | 646.6 | 5315.0 | 186.2 | 10.8 | 21,096.3 | |
| % | 62.2 | 8.6 | 3.1 | 25.2 | 0.9 | 0.1 | 100.0 | |
| 2015 | 2016 | 2017 | 2018 | Average | % | ||
|---|---|---|---|---|---|---|---|
| Tokida area | Orthocoladiinae | 24,692 | 29,627 | 17,580 | 101,469 | 43,342 | 63.0 |
| Chironominae | 6290 | 2005 | 3206 | 4206 | 3927 | 5.7 | |
| Tanypodinae | 791 | 569 | 826 | 1554 | 935 | 1.4 | |
| Diamesinae | 907 | 6925 | 28,646 | 43,464 | 19,986 | 29.1 | |
| Prodiamesinae | 0 | 1488 | 420 | 192 | 525 | 0.8 | |
| Unknown | 111 | 24 | 37 | 0 | 43 | 0.1 | |
| Sub-total | 32,766 | 40,675 | 50,703 | 150,885 | 68,757 | 100.0 | |
| Iwano area | Orthocoladiinae | 15,180 | 29,905 | 23,070 | 22,718 | 27.8 | |
| Chironominae | 6440 | 6195 | 7305 | 6647 | 8.1 | ||
| Tanypodinae | 710 | 3725 | 3105 | 2513 | 3.1 | ||
| Diamesinae | 4980 | 113,360 | 30,730 | 49,690 | 60.9 | ||
| Prodiamesinae | 150 | 50 | 2230 | 810 | 1.0 | ||
| Unknown | 55 | 0 | 0 | 18 | 0.0 | ||
| Sub-total | 27,460 | 152,825 | 64,440 | 81,575 | 100.0 | ||
| The middle reaches of the Shinano River (Average of Tokida and Iwano area) | Orthocoladiinae | 22,404 | 23,743 | 62,270 | 36,139 | 44.5 | |
| Chironominae | 4223 | 4701 | 5756 | 4893 | 6.0 | ||
| Tanypodinae | 640 | 2276 | 2330 | 1748 | 2.2 | ||
| Diamesinae | 5953 | 71,003 | 37,097 | 38,018 | 46.8 | ||
| Prodiamesinae | 819 | 235 | 1211 | 755 | 0.9 | ||
| Unknown | 40 | 19 | 0 | 19 | 0.0 | ||
| Sub-total | 34,068 | 101,764 | 107,663 | 81,165 | 100.0 |
| Year | Area | Orthocladiinae | Chironominae | Diamesinae | Prodiamesinae | Detritus feeder * | Tanypodinae | Unknown | Total |
|---|---|---|---|---|---|---|---|---|---|
| AFDW g/m2/year | |||||||||
| 2015 | Tokida Riffle | 4.50 ± 0.18 | 0.65 ± 0.28 | 0.07 ± 0.04 | 0.0 ± 0.0 | 5.22 | 0.20 ± 0.05 | 0.03 ± 0.02 | 5.44 ± 0.97 |
| Tokida Pool | 12.24 ± 1.61 | 4.15 ± 1.37 | 0.64 ± 0.22 | 0.0 ± 0.0 | 17.03 | 0.16 ± 0.08 | 0.03 ± 0.02 | 17.22 ± 2.47 | |
| Average of R & P | 8.37 ± 1.10 | 2.40 ± 0.81 | 0.36 ± 0.13 | 0.0 ± 0.0 | 11.13 | 0.18 ± 0.03 | 0.03 ± 0.01 | 11.33 ± 1.67 | |
| 2016 | Tokida Riffle | 5.28 ± 1.11 | 0.26 ± 0.07 | 2.03 ± 0.22 | 0.00 ± 0.01 | 7.57 | 0.15 ± 0.05 | 0.0 ± 0.0 | 7.73 ± 1.18 |
| Tokida Pool | 14.92 ± 1.77 | 1.21 ± 0.17 | 1.84 ± 0.46 | 1.30 ± 0.38 | 19.29 | 0.11 ± 0.10 | 0.02 ± 0.03 | 19.40 ± 1.90 | |
| Average of R & P | 10.10 ± 1.25 | 0.74 ± 0.10 | 1.93 ± 0.15 | 0.65 ± 0.19 | 13.43 | 0.13 ± 0.08 | 0.01 ± 0.02 | 13.56 ± 1.16 | |
| Iwano Riffle | 3.94 ± 0.33 | 0.92 ± 0.08 | 0.90 ± 0.19 | 0.0 ± 0.0 | 5.78 | 0.81 ± 0.25 | 0.01 ± 0.01 | 6.59 ± 0.19 | |
| Iwano Pool | 2.79 ± 0.84 | 1.24 ± 0.25 | 0.96 ± 0.19 | 0.04 ± 0.03 | 5.04 | 0.04 ± 0.04 | 0.01 ± 0.01 | 5.08 ± 0.63 | |
| Average | 3.37 ± 0.59 | 1.08 ± 0.12 | 0.93 ± 0.02 | 0.02 ± 0.02 | 5.41 | 0.42 ± 0.13 | 0.01 ± 0.0 | 5.83 ± 0.41 | |
| 2017 | Tokida Riffle | 3.78 ± 0.59 | 0.53 ± 0.05 | 1.66 ± 0.87 | 0.0 ± 0.0 | 5.97 | 0.20 ± 0.05 | 0.01 ± 0.01 | 6.17 ± 0.87 |
| Tokida Pool | 7.51 ± 0.72 | 1.71 ± 0.25 | 21.61 ± 12.81 | 0.37 ± 0.13 | 31.20 | 0.20 ± 0.10 | 0.01 ± 0.01 | 31.40 ± 13.42 | |
| Average of R & P | 5.64 ± 0.19 | 1.12 ± 0.14 | 11.63 ± 6.44 | 0.18 ± 0.07 | 18.59 | 0.20 ± 0.33 | 0.01 ± 0.01 | 18.79 ± 6.48 | |
| Iwano Riffle | 6.50 ± 0.93 | 1.04 ± 0.25 | 41.07 ± 6.70 | 0.0 ± 0.0 | 48.62 | 1.27 ± 0.23 | 0.0 ± 0.0 | 49.89 ± 7.13 | |
| Iwano Pool | 5.55 ± 0.60 | 1.20 ± 0.08 | 19.70 ± 3.92 | 0.01 ± 0.01 | 26.45 | 0.53 ± 0.22 | 0.0 ± 0.0 | 26.98 ± 3.43 | |
| Average of R & P | 6.03 ± 0.76 | 1.12 ± 0.09 | 30.39 ± 3.82 | 0.0 ± 0.01 | 37.53 | 0.90 ± 0.10 | 0.0 ± 0.0 | 38.43 ± 3.88 | |
| 2018 | Tokida Riffle | 13.83 ± 1.21 | 0.44 ± 0.32 | 5.41 ± 0.77 | 0.0 ± 0.0 | 19.69 | 0.37 ± 0.21 | 0.0 ± 0.0 | 20.06 ± 1.08 |
| Tokida Pool | 59.97 ± 25.78 | 2.77 ± 0.32 | 26.75 ± 1.72 | 0.16 ± 0.05 | 89.65 | 0.37 ± 0.09 | 0.0 ± 0.0 | 90.02 ± 26.97 | |
| Average of R & P | 36.90 ± 13.41 | 1.60 ± 0.19 | 16.08 ± 0.68 | 0.08 ± 0.03 | 54.67 | 0.37 ± 0.09 | 0.0 ± 0.0 | 55.04 ± 13.85 | |
| Iwano Riffle | 8.55 ± 2.26 | 1.80 ± 0.75 | 10.56 ± 4.52 | 0.07 ± 0.06 | 20.98 | 0.88 ± 0.65 | 0.0 ± 0.0 | 21.86 ± 8.18 | |
| Iwano Pool | 3.99 ± 0.18 | 1.35 ± 0.44 | 5.40 ± 0.77 | 0.04 ± 0.06 | 10.78 | 0.46 ± 0.10 | 0.0 ± 0.0 | 11.24 ± 1.10 | |
| Average of R & P | 6.27 ± 1.22 | 1.57 ± 0.37 | 7.98 ± 2.05 | 0.06 ± 0.02 | 15.88 | 0.67 ± 0.37 | 0.0 ± 0.0 | 16.55 ± 3.90 | |
| Average | Tokida area | 24.46 ± 20.38 | 0.22 ± 0.10 | ||||||
| Iwano area | 19.61 ± 16.38 | 0.66 ± 0.24 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirabayashi, K. Annual and Seasonal Trends in Density, Biomass, and Secondary Production of Chironomid Midges (Diptera: Chironomidae) in the Middle Reaches of the Shinano River, Japan, 2015–2019. Limnol. Rev. 2025, 25, 27. https://doi.org/10.3390/limnolrev25020027
Hirabayashi K. Annual and Seasonal Trends in Density, Biomass, and Secondary Production of Chironomid Midges (Diptera: Chironomidae) in the Middle Reaches of the Shinano River, Japan, 2015–2019. Limnological Review. 2025; 25(2):27. https://doi.org/10.3390/limnolrev25020027
Chicago/Turabian StyleHirabayashi, Kimio. 2025. "Annual and Seasonal Trends in Density, Biomass, and Secondary Production of Chironomid Midges (Diptera: Chironomidae) in the Middle Reaches of the Shinano River, Japan, 2015–2019" Limnological Review 25, no. 2: 27. https://doi.org/10.3390/limnolrev25020027
APA StyleHirabayashi, K. (2025). Annual and Seasonal Trends in Density, Biomass, and Secondary Production of Chironomid Midges (Diptera: Chironomidae) in the Middle Reaches of the Shinano River, Japan, 2015–2019. Limnological Review, 25(2), 27. https://doi.org/10.3390/limnolrev25020027






