Modeling the Adaptive Immune Response in an HBV Infection Model with Virus to Cell Transmission in Both Liver with CTL Immune Response and the Extrahepatic Tissue
Abstract
:1. Introduction
2. Preliminary Results
- and are continuous on .
- for all , with and are two positive constants.
3. Positivity and Boundedness of Solutions
4. Local Stability of Equilibrium
5. Global Stability of the Equilibrium
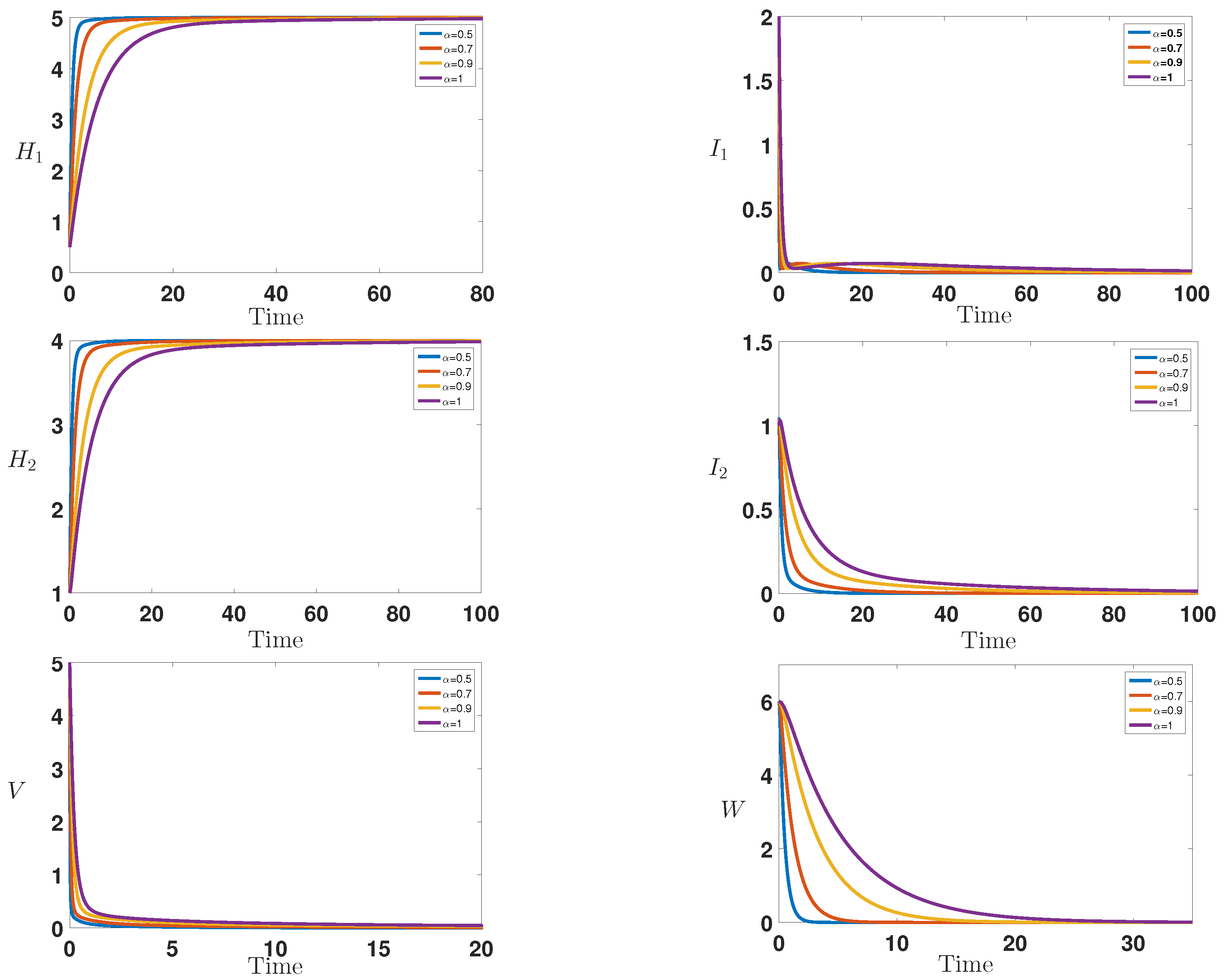
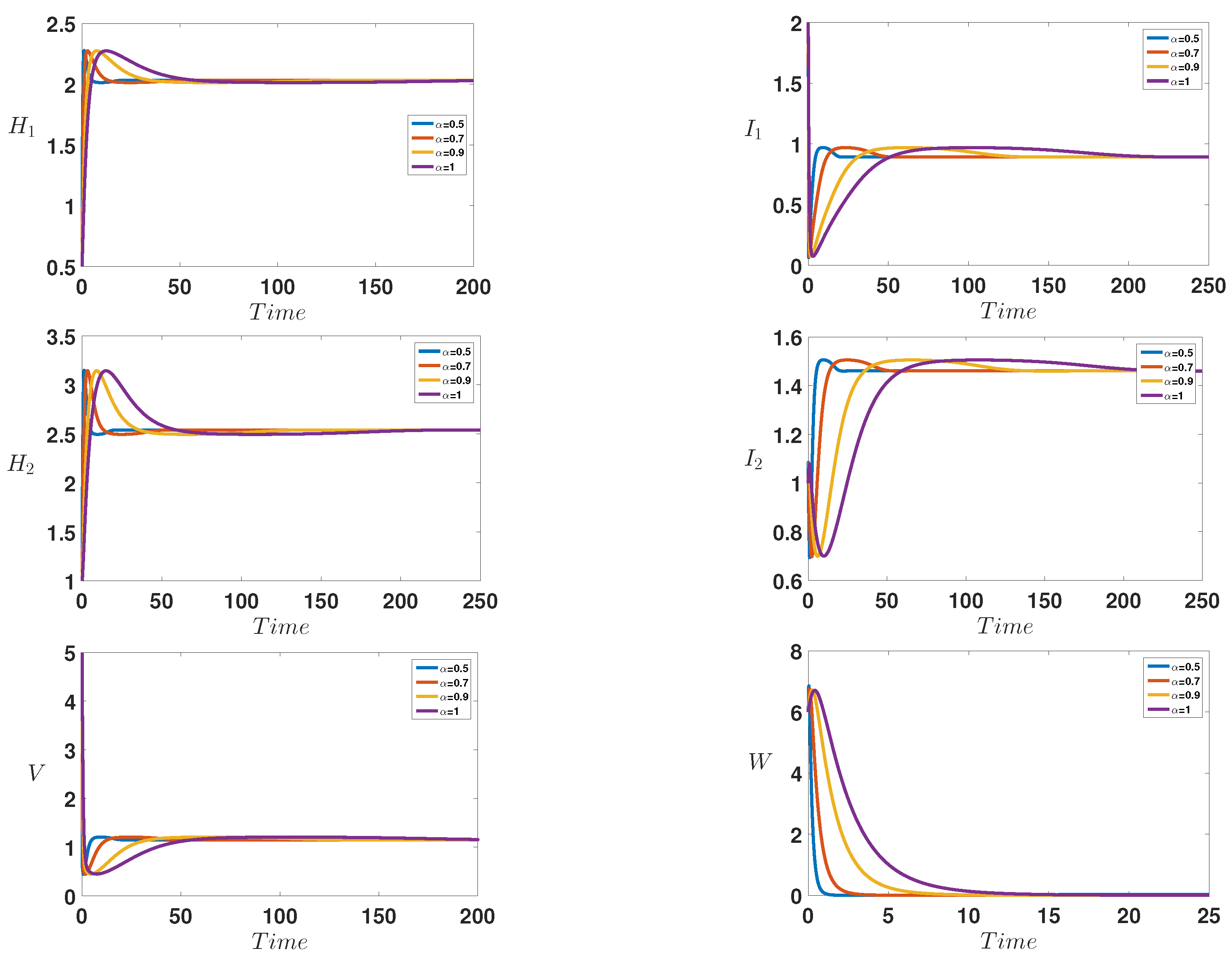
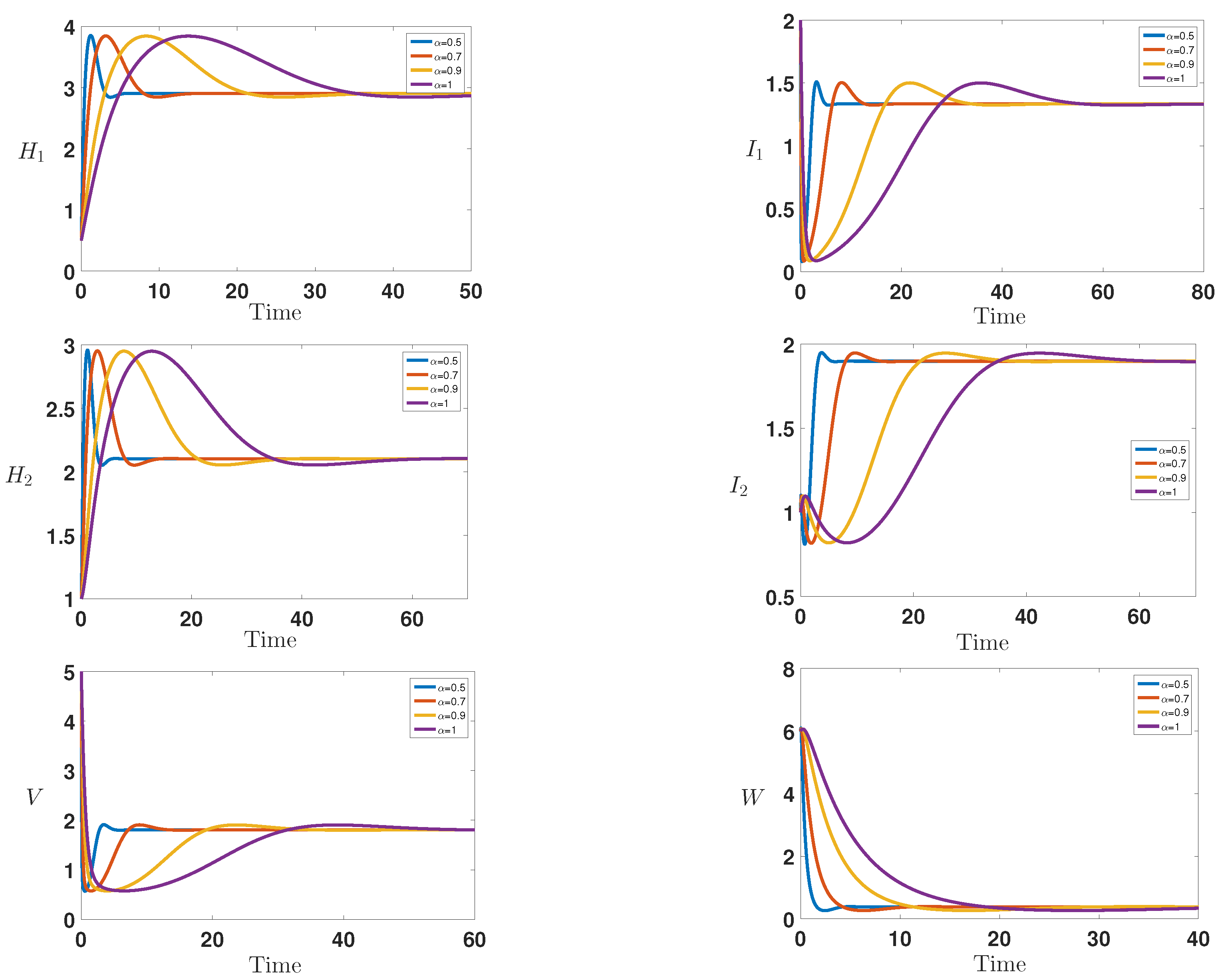
6. Numerical Simulations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beasley, R.P.; Lin, C.C.; Wang, K.Y.; Hsieh, F.J.; Hwang, L.Y.; Stevens, C.E.; Sun, T.S.; Szmuness, W. Hepatocellular carcinoma and hepatitis B virus. Lancet 1981, 2, 1129–1133. [Google Scholar] [CrossRef]
- World Health Organization Media Centre. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs204/en/ (accessed on 24 June 2022).
- Alter, M.J.; Margolis, H.S.; Krawczynski, K.; Judson, F.N.; Mares, A.; Alexander, W.J.; Hu, P.Y.; Miller, J.K.; Gerber, M.A.; Sampliner, R.E.; et al. The natural history of community-acquired hepatitis C in the United States. N. Engl. J. Med. 1992, 327, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- LaSalle, J.P. The Stability of Dynamical Systems; SIAM: Philadelphia, PA, USA, 1976. [Google Scholar]
- WHO. Global Hepatitis Report; WHO: Paris, France, 2017. [Google Scholar]
- Zhang, S.; Zhou, Y. The analysis and application of an HBV model. Appl. Math. Model. 2012, 36, 1302–1312. [Google Scholar] [CrossRef]
- Thornley, S.; Bullen, C.; Roberts, M. Hepatitis B in a high prevalence New Zealand population: A mathematical model applied to infection control policy. Nat. Med. 2008, 254, 599–603. [Google Scholar] [CrossRef]
- Pang, J.; Cui, J.; Zhou, X. Dynamical behavior of a hepatitis B virus transmission model with vaccination. Nat. Med. 2010, 265, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhang, W.; Ruan, S. Modeling the transmission dynamics and control of hepatitis B. Nat. Med. 2010, 262, 330–338. [Google Scholar]
- Hu, X.; Li, J.; Feng, X. Threshold dynamics of a HCV model with virus to cell transmission in both liver with CTL immune response and the extrahepatic tissue. J. Biol. Dyn. 2021, 15, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wang, Z.; Hu, H. Measuring memory with the order of fractional derivative. Sci. Rep. 2013, 3, 3431. [Google Scholar] [CrossRef]
- Danane, J.; Allali, K.; Hammouch, Z. Mathematical analysis of a fractional differential model of HBV infection with antibody immune response. Chaos Solitons Fractals 2020, 136, 109787. [Google Scholar] [CrossRef]
- Bhatter, S.; Mathur, A.; Kumar, D.; Singh, J. A new analysis of fractional Drinfeld–Sokolov–Wilson model with exponential memory. Physica A 2020, 537, 122578. [Google Scholar] [CrossRef]
- Bhatter, S.; Mathur, A.; Kumar, D.; Nisar, K.S.; Singh, J. Fractional modified Kawahara equation with Mittag–Leffler law. Chaos Solitons Fractals 2020, 131, 109508. [Google Scholar] [CrossRef]
- Veeresha, P.; Prakasha, D.G.; Singh, J. Solution for fractional forced KdV equation using fractional natural decomposition method. AIMS Math. 2020, 5, 798–810. [Google Scholar] [CrossRef]
- Boukhouima, A.; Hattaf, K.; Yousfi, N. Dynamics of a fractional order HIV infection model with specific functional response and cure rate. Int. J. Differ. Equ. 2017, 2017, 8372140. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.; El-Saka, H.A. On fractional-order models for hepatitis C. Nonlinear Biomed. Phys. 2010, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Rihan, F.A.; Sheek-Hussein, M.; Tridane, A.; Yafia, R. Dynamics of hepatitis C virus infection: Mathematical modeling and parameter estimation. Math. Model. Nat. Phenom. 2017, 12, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Sun, Q. Stability analysis of a fractional-order HBV infection model. Int. J. Adv. Appl. Math. Mech. 2014, 2, 1–6. [Google Scholar]
- Salman, S.M.; Yousef, A.M. On a fractional-order model for HBV infection with cure of infected cells. J. Egypt. Math. Soc. 2017, 25, 445–451. [Google Scholar]
- Yildiz, T.A.; Jajarmi, A.; Yildiz, B.; Baleanu, D. New aspects of time fractional optimal control problems within operators with nonsingular kernel. Discret. Contin. Dyn. Syst. Ser. S 2020, 13, 407. [Google Scholar]
- Baleanu, D.; Jajarmi, A.; Sajjadi, S.S.; Mozyrska, D. A new fractional model and optimal control of a tumor-immune surveillance with non-singular derivative operator. Chaos 2019, 29, 083127. [Google Scholar] [CrossRef] [PubMed]
- Rihan, F.A.; Lakshmanan, S.; Hashish, A.H.; Rakkiyappan, R.; Ahmed, E. Fractional-order delayed predator–prey systems with Holling type-II functional response. Nonlinear Dyn. 2015, 80, 777–789. [Google Scholar] [CrossRef]
- Rihan, F.A.; Arafa, A.A.; Rakkiyappan, R.; Rajivganthi, C.; Xu, Y. Fractional-order delay differential equations for the dynamics of hepatitis C virus infection with IFN-α treatment. Alex. Eng. J. 2021, 60, 4761–4774. [Google Scholar] [CrossRef]
- Rihan, F.A. Numerical modeling of fractional-order biological systems. Abstr. Appl. Anal. 2013, 2013, 816803. [Google Scholar] [CrossRef] [Green Version]
- Rihan, F.A.; Baleanu, D.; Lakshmanan, S.; Rakkiyappan, R. On fractional SIRC model with Salmonella bacterial infection. Abstr. Appl. Anal. 2014, 2014, 136263. [Google Scholar] [CrossRef] [Green Version]
- Delavari, H.; Baleanu, D.; Sadati, J. Stability analysis of Caputo fractional-order nonlinear systems revisited. Nonlinear Dyn. 2012, 67, 2433–2439. [Google Scholar] [CrossRef]
- Magin, R.L. Fractional calculus models of complex dynamics in biological tissues. Comput. Math. Appl. 2010, 59, 1586–1593. [Google Scholar] [CrossRef] [Green Version]
- Podlubny, I. Fractional Differential Equations; Academic Press: San Diego, CA, USA, 1999; p. 198. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fikri, F.E.; Allali, K. Modeling the Adaptive Immune Response in an HBV Infection Model with Virus to Cell Transmission in Both Liver with CTL Immune Response and the Extrahepatic Tissue. Math. Comput. Appl. 2022, 27, 65. https://doi.org/10.3390/mca27040065
Fikri FE, Allali K. Modeling the Adaptive Immune Response in an HBV Infection Model with Virus to Cell Transmission in Both Liver with CTL Immune Response and the Extrahepatic Tissue. Mathematical and Computational Applications. 2022; 27(4):65. https://doi.org/10.3390/mca27040065
Chicago/Turabian StyleFikri, Fatima Ezzahra, and Karam Allali. 2022. "Modeling the Adaptive Immune Response in an HBV Infection Model with Virus to Cell Transmission in Both Liver with CTL Immune Response and the Extrahepatic Tissue" Mathematical and Computational Applications 27, no. 4: 65. https://doi.org/10.3390/mca27040065
APA StyleFikri, F. E., & Allali, K. (2022). Modeling the Adaptive Immune Response in an HBV Infection Model with Virus to Cell Transmission in Both Liver with CTL Immune Response and the Extrahepatic Tissue. Mathematical and Computational Applications, 27(4), 65. https://doi.org/10.3390/mca27040065






