Quantitative Analysis of Anthocyanins in Grapes by UPLC-Q-TOF MS Combined with QAMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. The Instrument Conditions
2.4. QuantitativeAnalysis of Anthocyanins
2.5. Data Analysis
3. Resultsand Discussion
3.1. Optimization of the Instrumental Conditions
3.2. Validation of the Method
3.3. QAMS Method Development
3.4. Application of Proposed Method to Grape Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhao, X.; Zhang, S.-S.; Zhang, X.-K.; He, F.; Duan, C.-Q. An effective method for the semi-preparative isolation of high-purity anthocyanin monomers from grape pomace. Food Chem. 2020, 310, 125830. [Google Scholar] [CrossRef] [PubMed]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Crupi, P.; Palattella, D.; Corbo, F.; Clodoveo, M.L.; Masi, G.; Caputo, A.R.; Battista, F.; Tarricone, L. Effect of pre-harvest inactivated yeast treatment on the anthocyanin content and quality of table grapes. Food Chem. 2021, 337, 128006. [Google Scholar] [CrossRef]
- Lima, Á.S.; de Oliveira, B.S.; Shabudin, S.V.; Almeida, M.; Freire, M.G.; Bica, K. Purification of anthocyanins from grape pomace by centrifugal partition chromatography. J. Mol. Liq. 2021, 326, 115324. [Google Scholar] [CrossRef]
- Kharadze, M.; Japaridze, I.; Kalandia, A.; Vanidze, M. Anthocyanins and antioxidant activity of red wines made from endemic grape varieties. Ann. Agrar. Sci. 2018, 16, 181–184. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Yi, J.; Yang, B.; Li, M.; He, D.; Yang, W.; Zhang, Y.; Ni, H. Anti-tumor properties of anthocyanins from Lonicera caerulea ‘Beilei’ fruit on human hepatocellular carcinoma: In vitro and in vivo study. Biomed. Pharmacother. 2018, 104, 520–529. [Google Scholar] [CrossRef]
- Decendit, A.; Mamani-Matsuda, M.; Aumont, V.; Waffo-Teguo, P.; Moynet, D.; Boniface, K.; Richard, E.; Krisa, S.; Rambert, J.; Mérillon, J.-M.; et al. Malvidin-3-O-β glucoside, major grape anthocyanin, inhibits human macrophage-derived inflammatory mediators and decreases clinical scores in arthritic rats. Biochem. Pharmacol. 2013, 86, 1461–1467. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes—A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Xie, L.; Su, H.; Sun, C.; Zheng, X.; Chen, W. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci. Technol. 2018, 72, 13–24. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S. Molecular mechanisms involved in the cardiovascular and neuroprotective effects of anthocyanins. Arch. Biochem. Biophys. 2014, 559, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh, S.; Begum, R.F.; Singh, A.S.; Chitra, V. Anthocyanin as a therapeutic in Alzheimer’s disease: A systematic review of preclinical evidences. Ageing Res. Rev. 2022, 76, 101595. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, J.G.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The potential of anthocyanins in smart, active, and bioactive eco-friendly polymer-based films: A review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Y.; Chen, H.; Zhang, Z.; Ge, M. Anthocyanin degradation and the underlying molecular mechanism in a red-fleshed grape variety. LWT 2021, 151, 112198. [Google Scholar] [CrossRef]
- Ju, Y.; Yang, L.; Yue, X.; Li, Y.; He, R.; Deng, S.; Yang, X.; Fang, Y. Anthocyanin profiles and color properties of red wines made from Vitisdavidii and Vitis vinifera grapes. Food Sci. Hum. Wellness 2021, 10, 335–344. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, F.; Ning, J.; Liu, X.; Zhang, Z.; Yang, S. Predicting the anthocyanin content of wine grapes by NIR hyperspectral imaging. Food Chem. 2015, 172, 788–793. [Google Scholar] [CrossRef]
- Pinelli, P.; Romani, A.; Fierini, E.; Agati, G. Prediction models for assessing anthocyanins in grape berries by fluorescence sensors: Dependence on cultivar, site and growing season. Food Chem. 2018, 244, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.J. Application situation of multi-components quantitation by one marker new method for quality evaluation and control of Chinese herbal medicine. Pharm. Clin. Chin. Mater. Med. 2013, 4, 18–20. [Google Scholar]
- Ning, Z.; Liu, Z.; Song, Z.; Zhao, S.; Dong, Y.; Zeng, H.; Shu, Y.; Lu, C.; Liu, Y.; Lu, A. A single marker choice strategy in simultaneous characterization and quantification of multiple components by rapid resolution liquid chromatography coupled with triple quadrupole tandem mass spectrometry (RRLC-QqQ-MS). J. Pharm. Biomed. Anal. 2016, 124, 174–188. [Google Scholar] [CrossRef]
- Zhu, C.; Li, X.; Zhang, B.; Lin, Z. Quantitative analysis of multi-components by single marker—a rational method for the internal quality of Chinese herbal medicine. Integr. Med. Res. 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Jia, X.-H.; Zhu, S.; Komatsu, K.; Wang, X.; Cai, S.-Q. A systematic study on the influencing parameters and improvement of quantitative analysis of multi-component with single marker method using notoginseng as research subject. Talanta 2015, 134, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Y.; Qi, X.; Zhao, T.; Wang, X.; Ma, F.; Zhang, L.; Zhang, Q.; Li, P. Quantitative analysis of metabolites in the aflatoxin biosynthesis pathway for early warning of aflatoxin contamination by UHPLC-HRMS combined with QAMS. J. Hazard. Mater. 2022, 431, 128531. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, C.; Sun, K.; Li, W.; Liu, R. Quantitative analysis of bioactive components in walnut leaves by UHPLC-Q-Orbitrap HRMS combined with QAMS. Food Chem. 2020, 331, 127180. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-W.; Zhu, M.; Shao, Y.-D.; Shen, Z.; Weng, C.-C.; Yan, W.-D. Determination and quality evaluation of green tea extracts through qualitative and quantitative analysis of multi-components by single marker (QAMS). Food Chem. 2016, 197, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Stekolshchikova, E.; Turova, P.; Shpigun, O.; Rodin, I.; Stavrianidi, A. Application of quantitative analysis of multi-component system approach for determination of ginsenosides in different mass-spectrometric conditions. J. Chromatogr. A 2018, 1574, 82–90. [Google Scholar] [CrossRef]
- He, J.J.; Liu, Y.X.; Pan, Q.H.; Cui, X.Y.; Duan, C.Q. Different Anthocyanin Profiles of the Skin and the Pulp of Yan73 (Muscat Hamburg × Alicante Bouschet) Grape Berries. Molecules 2010, 15, 1141–1153. [Google Scholar] [CrossRef]
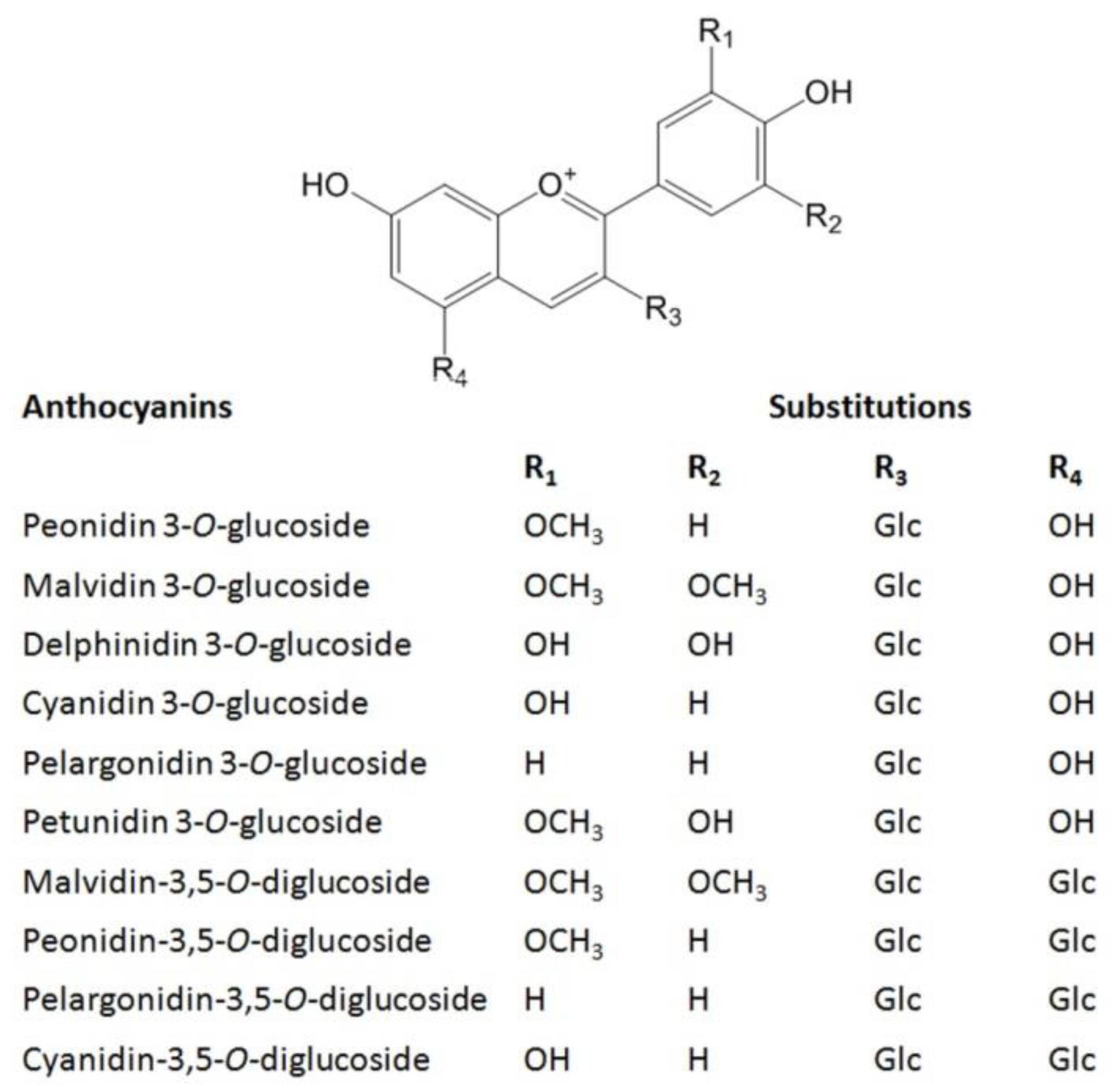

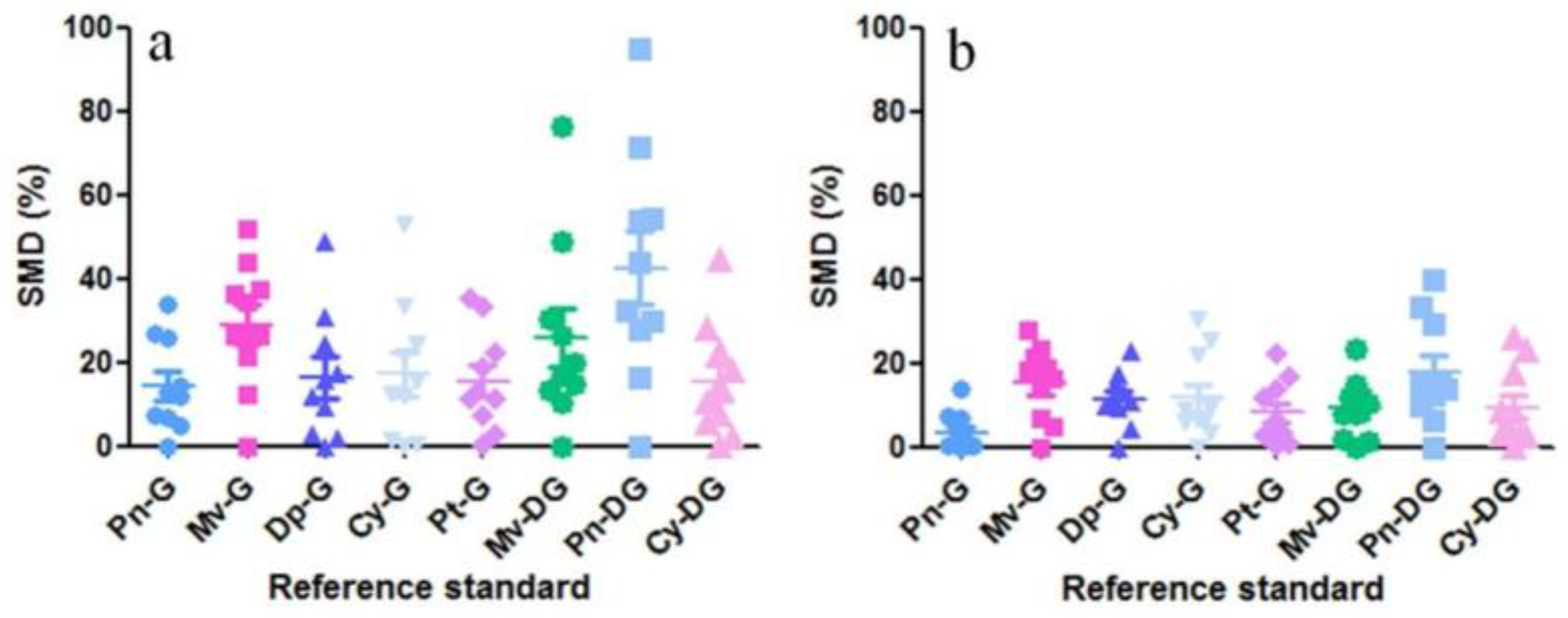
| No. | Compound | Regression Equation | Linearity Range (μg/mL) | Correlation Coefficient (R2) | LOD (μg/mL) | LOQ (μg/mL) | Precision | Repeatability | |
|---|---|---|---|---|---|---|---|---|---|
| Intra-Day RSD (%) | Inter-Day RSD (%) | RSD (%) | |||||||
| 1 | Pn-G | Y = 9558X + 1598.3 | 0.25–5.00 | 0.9992 | 0.05 | 0.20 | 1.33 | 0.79 | 1.22 |
| 2 | Mv-G | Y = 7824.3X + 740.48 | 0.24–4.88 | 0.9999 | 0.05 | 0.20 | 2.62 | 1.65 | 1.76 |
| 3 | Dp-G | Y = 2387X + 2139.5 | 0.50–9.90 | 0.9943 | 0.10 | 0.40 | 3.08 | 1.71 | 2.16 |
| 4 | Cy-G | Y = 5336.4X + 3894.4 | 0.75–15.00 | 0.9988 | 0.15 | 0.50 | 3.99 | 0.61 | 2.22 |
| 5 | Pe-G | Y = 8655.4X + 1850.4 | 0.25–4.99 | 0.9963 | 0.05 | 0.20 | 3.45 | 2.35 | 2.43 |
| 6 | Pt-G | Y = 5021X + 2382.5 | 0.50–9.90 | 0.9949 | 0.10 | 0.40 | 4.00 | 2.28 | 1.59 |
| 7 | Mv-DG | Y = 547.08X + 1619.8 | 1.75–34.95 | 0.9958 | 0.50 | 1.50 | 3.31 | 1.87 | 1.95 |
| 8 | Pn-DG | Y = 3951.6X + 2697.6 | 0.37–7.42 | 0.9932 | 0.10 | 0.30 | 0.58 | 0.80 | 2.01 |
| 9 | Pe-DG | Y = 9817X + 6488.5 | 0.75–7.5 | 0.9951 | 0.15 | 0.50 | 4.45 | 4.23 | 2.15 |
| 10 | Cy-DG | Y = 2130.2X + 3240.5 | 1.25–12.5 | 0.9922 | 0.30 | 1.00 | 4.09 | 3.72 | 2.05 |
| No. | Compound | PrecursorIon (m/z) | Fragment Ion (m/z) | Rt (min) |
|---|---|---|---|---|
| 1 | Pn-G | 463.2524 | 301 | 5.76 |
| 2 | Mv-G | 493.2640 | 331 | 6.82 |
| 3 | Dp-G | 465.1743 | 303 | 2.44 |
| 4 | Cy-G | 449.2361 | 287 | 3.26 |
| 5 | Pe-G | 433.2406 | 271 | 4.56 |
| 6 | Pt-G | 479.2479 | 317 | 3.84 |
| 7 | Mv-DG | 655.2778 | 331 | 2.87 |
| 8 | Pn-DG | 625.2689 | 301 | 2.74 |
| 9 | Pe-DG | 595.2590 | 271 | 2.48 |
| 10 | Cy-DG | 611.2512 | 287 | 2.08 |
| Compounds | Quantitative Method | Mean | RSD% | p |
|---|---|---|---|---|
| Pn-G | ESM | 2.07 | 2.50 | / |
| Mv-G | ESM | 1.96 | 1.79 | 0.63 |
| QAMS | 1.98 | 3.77 | ||
| Dp-G | ESM | 4.94 | 0.54 | 0.63 |
| QAMS | 4.96 | 1.04 | ||
| Cy-G | ESM | 6.39 | 1.18 | 0.56 |
| QAMS | 6.35 | 1.40 | ||
| Pe-G | ESM | 1.92 | 4.90 | 0.56 |
| QAMS | 1.96 | 4.15 | ||
| Pt-G | ESM | 4.19 | 3.41 | 0.42 |
| QAMS | 4.09 | 3.07 | ||
| Mv-DG | ESM | 18.64 | 1.31 | 0.08 |
| QAMS | 18.28 | 0.66 | ||
| Pn-DG | ESM | 4.07 | 0.74 | 0.06 |
| QAMS | 3.86 | 3.43 | ||
| Pe-DG | ESM | 5.60 | 1.19 | 0.78 |
| QAMS | 5.62 | 1.07 | ||
| Cy-DG | ESM | 10.44 | 0.97 | 0.15 |
| QAMS | 10.20 | 2.14 |
| Dp-G | Cy-G | Pt-G | Mv-G | |
|---|---|---|---|---|
| R | 1.000 | 1.000 | 1.000 | 1.000 |
| R2 | 1.000 | 0.999 | 0.999 | 0.999 |
| F | 7326.6 | 2435.9 | 2165.0 | 2085.4 |
| Sig. | 0.000 | 0.000 | 0.000 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, W.; Sun, S.; Wang, J.; Zhu, J.; Liang, F.; Zhang, Y.; Hu, G. Quantitative Analysis of Anthocyanins in Grapes by UPLC-Q-TOF MS Combined with QAMS. Separations 2022, 9, 140. https://doi.org/10.3390/separations9060140
Li X, Wang W, Sun S, Wang J, Zhu J, Liang F, Zhang Y, Hu G. Quantitative Analysis of Anthocyanins in Grapes by UPLC-Q-TOF MS Combined with QAMS. Separations. 2022; 9(6):140. https://doi.org/10.3390/separations9060140
Chicago/Turabian StyleLi, Xue, Wei Wang, Suling Sun, Junhong Wang, Jiahong Zhu, Feng Liang, Yu Zhang, and Guixian Hu. 2022. "Quantitative Analysis of Anthocyanins in Grapes by UPLC-Q-TOF MS Combined with QAMS" Separations 9, no. 6: 140. https://doi.org/10.3390/separations9060140
APA StyleLi, X., Wang, W., Sun, S., Wang, J., Zhu, J., Liang, F., Zhang, Y., & Hu, G. (2022). Quantitative Analysis of Anthocyanins in Grapes by UPLC-Q-TOF MS Combined with QAMS. Separations, 9(6), 140. https://doi.org/10.3390/separations9060140






