Abstract
The present research analyzes chemical solvents based on the use of diamines (Ethylenediamine-EDA, 1,2-Dimethylethylenediamine-DMEDA and Tetramethylethylenediamine-TMEDA) for carbon dioxide absorption, taking into account the type of amino centers in the molecules. The presence and type of radicals can affect amine solubility in water, reaction mechanism, reaction kinetics, etc. Diamines have been considered interesting candidates for carbon dioxide chemical absorption, observing a high influence of the molecule structure. The present work analyzes a series of solvents based on diamines with the same chain length between amino centers, but different types of radicals. This study shows an important variability in the behavior of these solvents. EDA-based solvents have shown high absorption rates and stability, but carbamate hydrolysis is relatively low, avoiding an increase in carbon dioxide loading.
1. Introduction
The development of new solvents with better efficiencies to be used in gas separation and specifically, for carbon dioxide separation, continues to be a research field with a high interest. One of the main causes of this interest is the important energy penalty associated with the overall process of carbon dioxide chemical absorption, mainly due to solvent regeneration section by stripping [1,2,3]. For this reason, new generations of solvents have begun to be developed. The use of sterically hindered amines was the main proposal in the second generation of solvents, which allow an important improvement in the value of carbon dioxide loading, avoiding negative characteristics in absorption and reaction rates [4,5]. In the last few years, new strategies in the development of chemical solvents are centered on the use of organic compounds [6,7] and ionic liquids with suitable characteristics [8,9]. Additionally, a research line with interesting results is focused on the use of di- or polyamines. One of the most important molecules in this research line is piperazine, which has been used in an important number of research studies and papers, from basic studies to industrial applications [10,11]. The use of diamines allows the increase of carbon dioxide loading without increasing amine concentration. In addition, it allows one to avoid the use of a high concentration of amine that can imply an undesirable increase in solvent viscosity.
Other diamines such as TMDAP—tetramethyl-1,3-diaminopropane, 3DMAPA—(dimethylamino)propylamine, DAP—1,3-diaminopropane and HMD—hexamethylenediamine) [12], have been previously analyzed; they showed suitable values of carbon dioxide loading, but a more complete analysis allows us to conclude that the energy needed for solvent regeneration is high. Only TMDAP has a suitable overall behavior that allows it to reduce the energy penalty regarding the best available technique (aqueous solution of monoethanolamine). Though this amine is a tertiary amine in both centers, it shows an acceptable reaction rate with carbon dioxide, with higher values than aqueous solutions of methyldiethanolamine (MDEA), which is used at the industrial level, for instance, in hydrogen purification.
In a previous work of our research team [13], the behavior of aqueous solutions of dimethylethylenediamine (DMEDA) for carbon dioxide chemical absorption was studied. The aim of this work was to take advantage of the presence of primary and tertiary amino centers on the basis of previous studies using amine blends [14,15]. The present research is focused on the evaluation of diamines with the same chain length but with different natures in the amino groups, in order to analyze the interactions between amino groups and how they can influence the behavior of this type of compound in order to be used as chemical solvents for carbon dioxide separation. One of these amines is ethylenediamine (EDA), which is considered a promising carbon dioxide-capturing solvent [16].
2. Materials and Methods
2.1. Materials
Ethylenediamine-EDA, 1,2-Dimethylethylenediamine-DMEDA and Tetramethylethylenediamine-TMEDA were supplied by Alfa Aesar with purities ≥ 98%. Different amounts of amines were dissolved in bidistilled water for solvent preparation (concentration (CB) range: 0.1–0.6 mol·L−1).
Carbon dioxide was purchased by Nippon Gases with a purity of 99.998% and used as a feed stream for absorption experiments. The total pressure of experiments was 1 atm and the gas flow-rate (QG) range was 0.1–0.3 L·min−1.
2.2. Absorption Studies
Carbon dioxide absorption studies were carried out in semicontinuous regime (continuous for gas stream and batch for chemical solvent) in a square bubble column contactor (4 cm in diameter and 65 cm in height) with a liquid phase volume of 0.9 L. The absorption rate was determined as the difference between inlet and outlet gas streams. Mass flow controllers (M-Series Alicat Scientific) were used to maintain a constant inlet gas flow-rate and to determine and record the outlet gas flow-rate. This type of measurement has an associated uncertainty in the range of 10%. Figure 1 shows a scheme of the experimental set-up employed to evaluate the behavior of these solvents for carbon dioxide absorption.
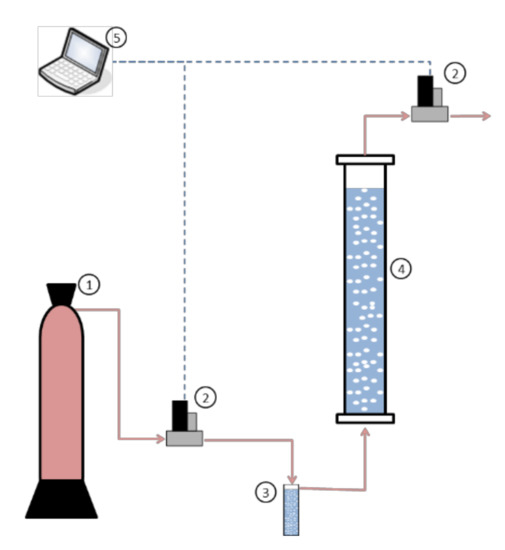
Figure 1.
Experimental set-up for carbon dioxide absorption experiments. ① Gas cylinder, ② mass flow controllers, ③ gas saturator, ④ bubble column, ⑤ computer.
2.3. Regeneration Studies
The experimental work carried out to evaluate solvent regeneration by stripping was performed using a three-necked flask with (0.5 L), and a heating mantle (Selecta Fibroman, Barcelona, Spain) with a temperature sensor (Selecta Sensoterm, Barcelona, Spain). After each carbon dioxide absorption experiment, the chemical solvent was introduced into the flask to carry out the gas desorption and then the solvent regeneration. A thermometer was used for monitoring temperature and two condensers were installed to prevent solvent evaporation. After cooling the solvent was used again in absorption experiments. Eight absorption/regeneration cycles were carried out in order to analyze the influence of aqueous phase regeneration upon absorption rate and carbon dioxide loading.
3. Results
The present research evaluates the use of different diamines with the same chain length between nitrogen atoms changing the type of active centers for the chemical reaction with carbon dioxide. Figure 2 and Figure 3 show the behavior of these chemical solvents based on aqueous solutions of EDA (two primary amino centers) and TMEDA (two tertiary amino centers) during the chemical absorption of carbon dioxide using several amine concentrations in the liquid phase.
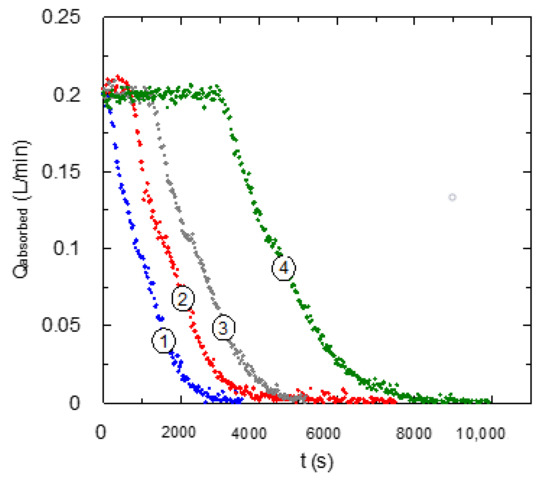
Figure 2.
Influence of EDA concentration upon carbon dioxide absorption curve. QG = 0.2 L·min−1. ① CB = 0.1 mol·L−1; ② CB = 0.2 mol·L−1; ③ CB = 0.3 mol·L−1; ④ CB = 0.6 mol·L−1.
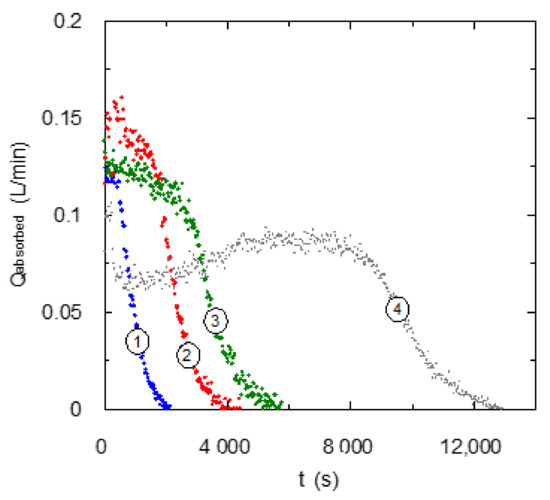
Figure 3.
Influence of TMEDA concentration upon carbon dioxide absorption curve. QG = 0.2 L·min−1. ① CB = 0.1 mol·L−1; ② CB = 0.2 mol·L−1; ③ CB = 0.3 mol·L−1; ④ CB = 0.6 mol·L−1.
The experimental data corresponding to the carbon dioxide absorption flow-rate using EDA-based solvents (Figure 2) shows absorption curves with a similar behavior to the expected one for primary amines in aqueous solution. In the first part of the experiments, complete carbon dioxide absorption is produced due to the fast chemical reaction corresponding to this type of amines (primary) [17].
An increase of amine (EDA) concentration in the solvent causes a displacement in the absorption curve to the right side, increasing the area under the curve (large amount of carbon dioxide absorbed) in agreement with the higher quantity of amine molecules in the liquid phase that are available to react with the absorbed gas.
In relation with the shape of the absorption curve, after the initial period with a complete carbon dioxide absorption, a monotonic decrease in the absorption rate is observed. Previous studies [18] concluded that the shape of the absorption curve could have an important relation with the reaction mechanism, and specifically with the weight of each reaction involved in the overall mechanism of carbon dioxide absorption in amine-based solvents. On the basis of this previous experience, the observed shapes in Figure 2 could show a low importance of carbamate hydrolysis upon the overall behavior, because it has not shown changes in the trend of absorption rate values [19]. Carbamate hydrolysis causes deprotonation of amines, and it increases the concentration of this reagent in the liquid phase. This fact allows it to maintain a higher absorption and reaction rate in comparison with a solvent that avoids this type of reaction.
The other diamine used in the present work is TMEDA, with the presence of two tertiary amino centers. The experimental data corresponding to the absorption curve using this amine with different concentrations in the solvent are presented in Figure 3. They show a very different behavior than EDA-based solvents (Figure 2), probably due to the reaction mechanism.
Solvents using TMEDA do not reach absorption values corresponding to a complete absorption of carbon dioxide fed to the gas-liquid contactor at the beginning of the experiments. This behavior is assigned to a lower reaction rate of carbon dioxide with tertiary centers in comparison with primary and secondary amines. Experimental results included in Figure 2 also show more complex absorption curves when TMEDA concentration increases in the chemical solvent. When tertiary amines are used for carbon dioxide chemical absorption, kinetics of the main reaction (between carbon dioxide and water in the presence of amine) can be the limiting step of the overall process. This fact indicates that the absorption curve could show a slight decrease while a chemical reaction is produced. A change in the trend (a decrease in the slope) is observed when amine is consumed, and physical saturation of liquid phase is produced. This type of behavior is clearly observed when solvents with a concentration of up to 0.3 M is used, but solvents with higher concentrations show a very different behaviors. In the TMEDA high concentration range, the behavior is complex and consists of a first period with a decrease in absorption rate, and after it an increase in absorption rate is observed.
The behavior shown by aqueous solutions of TMEDA is not a commonly observed characteristic, because an increase in concentration generally causes an increase in mass transfer rate, mainly at the beginning of experiments. It is produced by an increase in reaction rate due to the high amine concentration. This fact is clearer for tertiary amines because the reaction rate is generally low and an enhancement in this parameter causes a higher effect upon the overall mass transfer process. Taking into account this previous discussion, the behavior shown in Figure 3 for experiments with high TMEDA concentrations (that show lower absorption rates in the first part of the experiments) can be due to mass transfer steps. Specifically, an increase in amine concentration in the aqueous solution causes a significant increase in viscosity [20], and this fact can produce several negative influences upon gas-liquid mass transfer processes:
- (i)
- An increase in the rigidity of the gas–liquid interface is produced by an increase in viscosity [21], but also the presence of molecules of amine are higher in the gas–liquid interface due to the accumulation observed with surface tension data. Thus, local viscosity can be higher than in the liquid bulk, which enhances the rigidity of interface.
- (ii)
- A higher viscosity in the liquid solvent causes other undesirable effects in bubbling reactors. It generally increases the size of bubbles produced in the sparger. Additionally, it enhances bubble coalescence and reduces breakage. Both effects cause a reduction in the value of the gas–liquid interfacial area [22,23] and then, a decrease in the mass transfer rate.
The previously described effects can explain the reduction in carbon dioxide mass transfer rates at the beginning of experiments with the increase of TMEDA concentration. This chemical solvent based on TMEDA also shows an increase in absorption rate after the first decrease. This behavior is similar to the previously observed [18], for primary amines influenced by the presence of the carbamate hydrolysis reaction; for an aqueous solution of TMEDA it cannot be responsible for a change in the reaction mechanism, because carbamate is not present in this type of solvent [24]. In this case, the reaction products generated during the reaction between carbon dioxide and TMEDA (carbonate/bicarbonate and protonated amine) can modify the physical properties of the solvent. The reaction products have an ionic character, and it can cause an important influence upon the hydrodynamic of bubbling reactors. Previous works have concluded that the presence of this type of substance can inhibit bubble coalescence [25,26], and it can maintain an interfacial area, increasing the mass transfer rate. During absorption experiments it is possible to observe a decrease in bubble size, mainly at the beginning of experiments due to the enhancement of mass transfer caused by the chemical reaction. This is not the commonly observed behavior considering the overall chemical absorption process in a semicontinuous regime, due to the concentration of amine decreasing, which maintains a monotonic increase in bubble size [27]. In general, changes in the gas–liquid interface due to liquid phase composition modifications during carbon dioxide chemical absorption are not observed because the weight of the chemical reaction is higher than the hydrodynamic issues. For TMEDA aqueous solutions, the low reaction rate allows one to observe this type of influence.
A comparison between behaviors of these two solvents analyzed in the present work (aqueous solutions of EDA and TMEDA) with experimental values corresponding to aqueous solutions of DMEDA from a previous study [13] has been carried out and it is included in Figure 4. The experimental values corresponding to the use of EDA and DMEDA show similar behaviors, with a complete absorption of carbon dioxide fed to the bubble column reactor, and after that a monotonic decrease in the absorbed flow-rate is produced. The main difference is that the drop of carbon dioxide absorption rate is produced in a shorter time for DMEDA. This fact is related with the previously reached conclusions [13] for DMEDA solvents based on an enhancement in the carbamate stabilization. It causes a low utilization of amino groups through interactions between the primary and tertiary centers of the amine. It allows us to confirm that solvents based on EDA maintain a slightly higher carbamate hydrolysis that improves solvent characteristics for this application: high reaction rate, high carbon dioxide loading and a low regeneration energy, because the main reaction product is bicarbonate [23,28]. These results are in agreement with a previous work that suggested a decrease in solvent basicity with an increase in steric hindrance in amino centers [29].
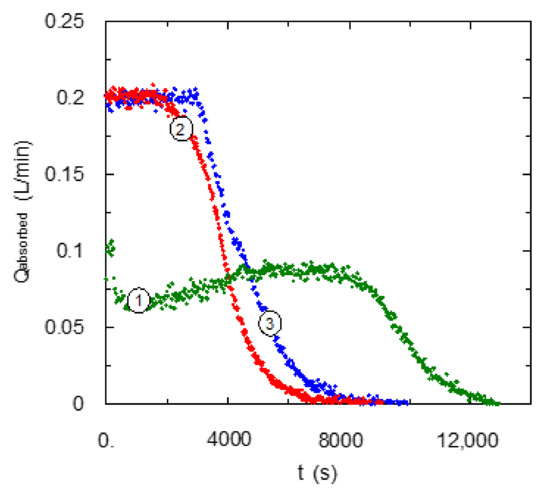
Figure 4.
Effect of type of center of amino centers upon carbon dioxide absorption curve. CB = 0.6 mol·L−1. QG = 0.2 L·min−1. ① TMEDA; ② DMEDA [13]; ③ EDA.
In relation to the behavior of the absorption rate for TMEDA in comparison with EDA and DMEDA solvents, important differences are present mainly due to the absence of primary centers. The main difference is the lower value of absorption rate from the beginning of the experiments. This fact is due to the low reaction rate for carbon dioxide with tertiary centers, but as previously commented, a special behavior is observed in the middle of the experiment with a slight increase in carbon dioxide absorption rate.
These three solvents based on EDA, DMEDA and TMEDA have been compared in relation to absorption rate in the first part of experiments (as the slope of carbon dioxide concentration vs. time) using different initial amine concentrations in the chemical solvent (see Figure 5). The experimental results show different behaviors when the chemical solvents include primary amino centers (EDA and DMEDA) regarding TMEDA-based solvent. In relation to the solvents based on EDA and DMEDA, they reached similar values for absorption rate because these solvents were allowed to absorb the majority amount of carbon dioxide fed to the reactor (in this case a mass transfer rate limitation is observed). A different trend is observed for aqueous solutions of TMEDA with an increase in absorption rate with low amine concentrations (caused by an increase in reaction rate), but an important decrease in absorption rate is observed at higher amine concentration. This behavior agrees with the previous explanation developed for Figure 3, and is based on the influence of physical properties upon mass transfer process, mainly the effect caused by a high viscosity of solvent that reduces carbon dioxide physical absorption.
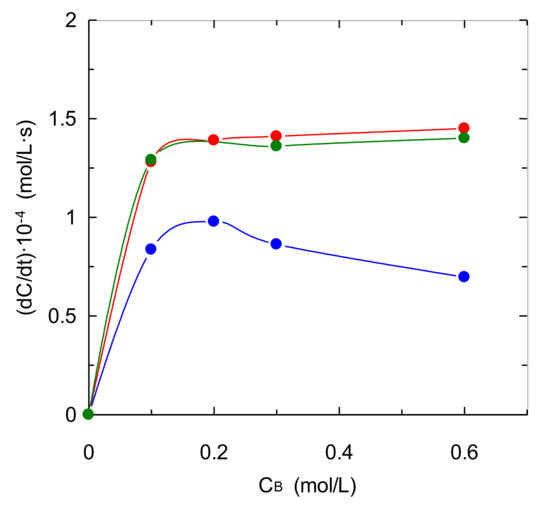
Figure 5.
Effect of type of center of amino centers upon absorption rate. CB = 0.6 mol·L−1. QG = 0.2 L·min−1.  TMEDA;
TMEDA;  DMEDA [13];
DMEDA [13];  EDA.
EDA.
 TMEDA;
TMEDA;  DMEDA [13];
DMEDA [13];  EDA.
EDA.
In order to develop a deeper analysis about the behavior of these chemical solvents for carbon dioxide separation, the value corresponding to carbon dioxide loading was calculated during absorption experiments. Figure 6 includes this information that complements the experimental data previously shown Figure 4. The calculated values for carbon dioxide loading confirm the lower absorption rate for the TMEDA solvent in comparison with systems with the presence of primary centers. The carbon dioxide loading data show that DMEDA-based solvent reaches values lower than the other amines employed in this work in agreement with the area under the curves in Figure 4.
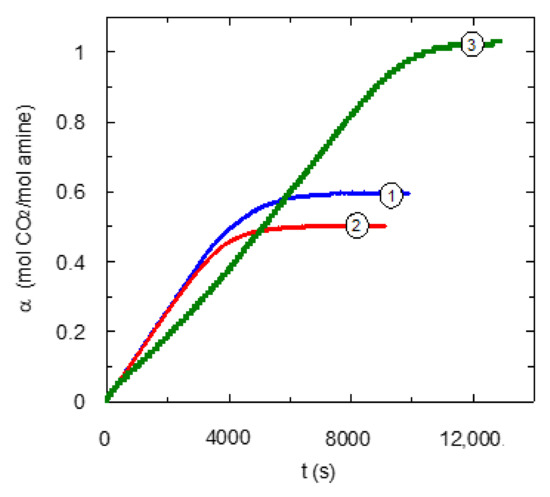
Figure 6.
Effect of type of center of amino centers upon carbon dioxide loading. CB = 0.6 mol·L−1. QG = 0.2 L·min−1. ① EDA; ② DMEDA [13]; ③ TMEDA. The carbon dioxide loading has been calculated per mol of amino centers.
The expected behavior corresponding to the analysis of carbon dioxide loading for these three diamines could be an increase in this parameter with the trend EDA < DMEDA < TMEDA. This hypothesis is based on the presence of tertiary centers, because this type of amine center increases carbon dioxide loading due to a more favorable stoichiometry [30].
Experimental data corresponding to the TMEDA solvent is in agreement with the hypothesis, reaching the highest carbon dioxide loading, and close to 1 mol CO2·mol amino centers−1. This value implies the complete reaction between carbon dioxide and TMEDA.
Alternatively, experimental data corresponding to EDA and DMEDA is not supported by the hypothesis because EDA-based solvents reached values of carbon dioxide loading higher than DMEDA solvents. This behavior was observed though EDA does not have a tertiary center, and thus, it is not possible to increase carbon dioxide loading by the direct reaction of tertiary amines with carbon dioxide to produce bicarbonate. EDA solvent has shown values included in the range of 0.6–0.8 mol CO2·mol amino centers−1 (1.2–1.6 mol CO2·mol amine−1). These results for carbon dioxide loading agree with previously published ones included in the range 0.7–1.7 mol CO2·mol amine−1 [31,32].
The behavior corresponding to EDA can be considered similar to monoethanolamine-based solvent [33], because both solvents reach close values for carbon dioxide loading. This behavior is compatible with a low degree of carbamate hydrolysis.
The DMEDA solvent shows a different behavior because of the presence of the same amount of primary and tertiary amino centers must allow it to reach carbon dioxide loading values between 0.75 and 1 mol CO2·mol amino centers−1. The fact that the DMEDA solvent reaches lower values of carbon dioxide loading can be explained on the basis of studies carried out in a previous work [13], based on the existence of inhibitions during carbon dioxide chemical absorption with the tertiary center of a DMEDA molecule. A similar conclusion was reached [34] using diamines with small alkyl chain length.
On the basis of the previous discussion, the reaction mechanism proposed for the chemical absorption of carbon dioxide in aqueous solutions of EDA and TMEDA are shown in Figure 7.
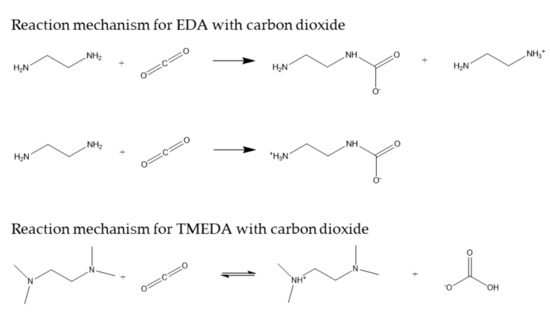
Figure 7.
Reaction mechanisms for EDA- and TMEDA-based solvents for carbon dioxide chemical absorption.
A design parameter with high importance in gas-liquid absorbers, and specifically in bubbling equipment, is the gas flow-rate fed to the reactor. Figure 8 shows the influence of this operation variable upon carbon dioxide absorption curves using the different solvents employed in this work. An increase in the amount of gas fed to the gas-liquid contactor increases the absorbed flow-rate that is related with an increase in gas-liquid interfacial area. This enhancement is mainly caused by an increase in gas hold-up (due to an increase in the number of bubbles dispersed in the liquid phase). An increase in gas flow-rate can also increase the bubbles diameter that tend to reduce gas-liquid interfacial area, but generally the parameter with the highest effect in this type of contactor is the gas hold-up [35,36]. A comparison between the behaviors of both solvents shows that the most favorable kinetic for EDA solvent causes a higher degree of carbon dioxide absorption reaching the 100% at the beginning of experiments (all the fed gas was absorbed). TMEDA solvent does not reach these high values of carbon dioxide removal and specifically the removal degree tends to reduce with carbon dioxide flow-rate fed to reactor showing this trend: 85–61–58%. The lower reaction rate for tertiary amines plays an important role causing a decrease in mass transfer rate.
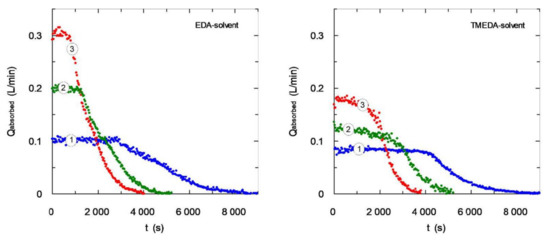
Figure 8.
Influence of gas flow-rate fed to bubble column using EDA- and TMEDA-based solvents. CB = 0.3 mol·L−1 (0.6 mol·L−1 of amine centers) (①) QG = 0.1 L·min−1; (②) QG = 0.2 L·min−1; (③) QG = 0.3 L·min−1.
Though the presence of tertiary amino centers tends to increase carbon dioxide loading, the EDA solvent has shown better results than DMEDA aqueous solutions, taking into account absorption rate and carbon dioxide loading. The TMEDA solvent allows large values of carbon dioxide loading due the different mechanism, but the low reaction rate reduces its interest to be applied in large scale applications.
For these reasons, additional work centered on chemical solvent regeneration has been carried out. The solvent regeneration section in this type of process has become increasing in importance due to the high energy penalty and costs associated to solvent regeneration and make-up flow. As previously discussed, solvents based on the use of EDA reach values of carbon dioxide loading higher than 0.5 mol CO2·mol amino centers−1, caused by partial carbamate hydrolysis. This characteristic has some positive issues, one of them related with a decrease in the amount of energy in solvent regeneration due to the presence of bicarbonate as reaction product instead of carbamate.
In carbon dioxide separation by chemical absorption the analysis of solvent stability during absorption/regeneration cycles is important. For this reason, several absorption/desorption cycles have been carried out in order to detect a lost in absorption capacity that could indicate the existence of solvent degradation. The experimental data corresponding to the carbon dioxide absorbed flow-rate are shown in plot A of Figure 9. It is possible to observe a comparison with the experiment using fresh solvent. A decrease in absorption rate and also in the value of the area under the curve is detected. This type of behavior is commonly observed for chemical solvents in carbon dioxide absorption processes [27,28]. This decrease in carbon dioxide loading is produced due to limitations in the acidic constant that does not allow the complete reversibility of the chemical reaction. After the first regeneration, the absorption curves for other cycles show a very similar behavior and it indicates that this type of solvent has a suitable stability.
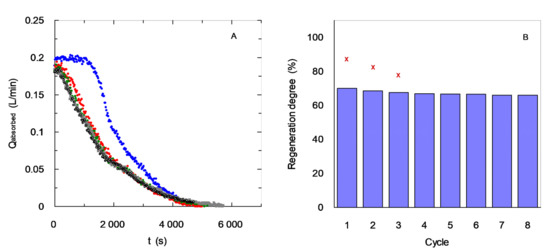
Figure 9.
Influence of absorption/regeneration cycles upon absorption curve (A) and regeneration degree (B) using EDA-based chemical solvent. CB = 0.6 mol·L−1. QG = 0.2 L·min−1.  Fresh solvent;
Fresh solvent;  1st cycle;
1st cycle;  3rd cycle;
3rd cycle;  5th cycle;
5th cycle;  7th cycle. (x) MEA solvent [37] CB = 0.3 mol·L−1. QG = 0.3 L·min−1.
7th cycle. (x) MEA solvent [37] CB = 0.3 mol·L−1. QG = 0.3 L·min−1.
 Fresh solvent;
Fresh solvent;  1st cycle;
1st cycle;  3rd cycle;
3rd cycle;  5th cycle;
5th cycle;  7th cycle. (x) MEA solvent [37] CB = 0.3 mol·L−1. QG = 0.3 L·min−1.
7th cycle. (x) MEA solvent [37] CB = 0.3 mol·L−1. QG = 0.3 L·min−1.
Plot B in Figure 9 show a comparison between the different absorption/regeneration cycles using the experimental values for carbon dioxide loading. It allows us to confirm a loss in this value after the first regeneration (about 30% is observed caused by the equilibrium previously commented). Additional absorption/regeneration cycles for the EDA-based solvent do not cause important decreases reaching a consistent behavior with a value of carbon dioxide loading close to 65% of the value corresponding to the use of fresh solvent. This behavior indicates that undesirable effects are not observed, such as amine degradation in the chemical solvent during stripping regeneration.
Figure 9B also shows a comparison between experimental data corresponding to the behavior in absorption–regeneration cycles of Monoethanolamine (MEA)-based solvent for carbon dioxide separation. For both solvents, a decrease in the degree of regeneration is observed with the use in the initial cycles, which is a commonly observed behavior [38].
Firstly, higher values in relation to the degree of regeneration for the MEA-based solvent are observed at low number of cycles. However, the decrease rate in the degree of regeneration appears with a higher slope than the EDA solvent. The values corresponding to the EDA-based solvent show a slight decrease during the three initial cycles, but it reaches a constant value in degree of regeneration.
Several reasons can explain the decrease in carbon dioxide loading after several cycles. One of them is related with the equilibrium constant (acidity constant) related with the reversible reaction that could have a strong influence upon the absorption behavior. Alternatively, previous studies have shown an important decrease in the amount of MEA available to react with carbon dioxide after regeneration caused by the high degradation of this molecule at high temperatures [39]. This fact is considered a very negative issue in the use of this solvent; the decrease caused by the amount of carbon dioxide absorbed, and the by-products generated during stripping can be related to corrosion issues. Solvents based on the use of EDA have shown a not very high degree of regeneration, but there have been suitable results regarding solvent chemical stability in comparison with benchmark solvents without the addition of other chemicals.
4. Conclusions
Different behaviors regarding the shape of the CO2 absorption curves were observed when the active centers of the diamine employed in present work were different (primary or tertiary). The diamine with primary centers (EDA) obtains absorption curves with the same shape for different initial concentrations. In the case of the presence of tertiary amines (TMEDA), the form of absorption curves is different due to the higher weight of hydrodynamic and mass transfer aspects, because the reaction rate in tertiary amines is lower. EDA-based solvents showed higher absorption rates than the rest of the solvents.
The EDA-based solvents have shown higher CO2 loading values than the corresponding diamine with primary and tertiary groups in the molecule (DMEDA). The CO2 loading data in an EDA-based solvent indicate that the presence of carbamate is still observed in the reaction products, but it still achieves better results than the use of DMEDA.
The low reaction rate between carbon dioxide and TMEDA-based solvents causes important limitations in the overall carbon dioxide absorption process, considerably reducing the effect of the increase in the gas flow-rate fed to the absorber.
EDA-based solvents have shown adequate chemical stability and/or chemical equilibrium and they maintain a similar value of CO2 loading after several absorption/regeneration cycles, which is not the case for a significant number of amines used in this type of solvent, in which a constant decrease is observed due to thermal or chemical degradation processes of the amines.
Author Contributions
Conceptualization, D.G.-D., J.M.N. and A.R.; methodology, D.G.-D. and A.R.; writing—original draft preparation, D.G.-D.; writing—review and editing, D.G.-D., J.M.N. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia, Innovación y Universidades, grant number PGC2018-101047-B-I00 and Consellería de Educación, Universidade e Formación Profesional, grant number ED431B 2020/39.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, T.; Keener, T.C. A review: Desorption of CO2 from rich solutions in chemical absorption processes. Int. J. Greenh. Gas Control. 2016, 51, 290–304. [Google Scholar] [CrossRef]
- Oyenekan, B.A.; Rochelle, G.T. Alternative stripper configurations for CO2 capture by aqueous amines. AIChE J. 2007, 53, 3144–3154. [Google Scholar] [CrossRef]
- Nwaoha, C.; Saiwan, C.; Supap, T.; Idema, R.; Tontiwachwuthikul, P.; Rongwong, W.; Al-Marri, M.J.; Benamor, A. Carbon dioxide (CO2) capture performance of aqueous tri-solvent blends containing 2-amino-2-methyl-1-propanol (AMP) and methyldiethanolamine (MDEA) promoted by diethylenetriamine (DETA). Int. J. Greenh. Gas Control. 2016, 53, 292–304. [Google Scholar] [CrossRef]
- Bougie, F.; Iliuta, M.C. Analysis of regeneration of sterically hindered alkanolamines aqueous solutions with and without activator. Chem. Eng. Sci. 2010, 65, 4746–4750. [Google Scholar] [CrossRef]
- Pei, Z.; Yao, S.; Jianwen, W.; Wei, Z.; Qing, Y. Regeneration of 2-amino-2-methyl-1-propanol used for carbon dioxide absorption. J. Environ. Sci. 2008, 20, 39–44. [Google Scholar]
- Ozturk, M.C.; Yuksel-Orhan, O.; Alper, E. Kinetics of carbon dioxide binding by 1,1,3,3-tetramethylguanidine in 1-hexanol. Int. J. Greenh. Gas Control. 2014, 26, 76–82. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Yonker, C.R.; Jessop, P.G.; Phan, L. CO2-binding organic liquids (CO2BOLs) for post-combustion CO2 capture. Energy Procedia 2009, 1, 1187–1195. [Google Scholar] [CrossRef]
- Zhang, X.M.; Huang, K.; Xia, S.; Chen, Y.L.; Wu, Y.T.; Hu, X.B. Low-viscous fluorine-substituted phenolic ionic liquids with high performance for capture of CO2. Chem. Eng. J. 2015, 274, 30–33. [Google Scholar] [CrossRef]
- Lu, J.G.; Lu, Z.Y.; Chen, Y.; Wang, J.T.; Gao, L.; Gao, X.; Tang, Y.Q.; Liu, D.G. CO2 absorption into aqueous blends of ionic liquid and amine in a membrane contactor. Sep. Purif. Technol. 2015, 150, 278–285. [Google Scholar] [CrossRef]
- Gao, T.; Selinger, J.L.; Rochelle, G.T. Demonstration of 99% CO2 removal from coal flue gas by amine scrubbing. Int. J. Greenh. Gas Control. 2019, 83, 236–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Sachde, D.; Chen, E.; Rochelle, G. Modeling of absorber pilot plant performance for CO2 capture with aqueous piperazine. Int. J. Greenh. Gas Control. 2017, 64, 300–313. [Google Scholar] [CrossRef]
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.V.; Abu Zahra, M.R.M. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy 2017, 185, 1433–1449. [Google Scholar] [CrossRef]
- Gómez-Díaz, D.; Muñiz-Mouro, A.; Navaza, J.M.; Rumbo, A. Diamine versus amines blend for CO2 chemical absorption. AIChE J. 2021, 67, e17071. [Google Scholar] [CrossRef]
- Horng, S.Y.; Li, M.H. Kinetics of absorption of carbon dioxide into aqueous solutions of monoethanolamine + triethanolamine. Ind. Eng. Chem. Res. 2002, 41, 257–266. [Google Scholar] [CrossRef]
- Liao, C.H.; Li, M.H. Kinetics of absorption of carbon dioxide into aqueous solutions of monoethanolamine + N-methyldiethanolamine. Chem. Eng. Sci. 2002, 57, 4569–4582. [Google Scholar] [CrossRef]
- Salvi, A.P.; Vaidya, P.D.; Kenig, E.Y. Kinetics of carbon dioxide removal by ethylenediamine and diethylenetriamine in aqueous solutions. Can. J. Chem. Eng. 2014, 92, 2021–2028. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Kenig, E.Y. CO2-alkanolamine reaction kinetics: A review of recent studies. Chem. Eng. Technol. 2007, 30, 1467–1474. [Google Scholar] [CrossRef]
- García-Abuín, A.; Gómez-Díaz, D.; Muñiz-Mouro, A.; Navaza, J.M.; Rumbo, A. CO2 capture by pyrrolidine: Reaction mechanism and mass transfer. AIChE J. 2014, 60, 1098–1106. [Google Scholar] [CrossRef]
- García-Abuín, A.; Gómez-Díaz, D.; López, A.B.; Navaza, J.M.; Rumbo, A. NMR characterization of carbon dioxide chemical absorption with monoethanolamine, diethanolamine, and triethanolamine. Ind. Eng. Chem. Res. 2013, 52, 13432–13438. [Google Scholar] [CrossRef]
- Gómez-Díaz, D.; Navaza, J.M. Density, speed of sound, viscosity, and surface tension of tetramethylethylenediamine aqueous solutions from T = 293.15 to 323.15 K. J. Chem. Eng. Data 2020, 65, 1565–1570. [Google Scholar] [CrossRef]
- Li, J.; You, C.; Chen, L.; Ye, Y.; Qi, Z.; Sundmacher, K. Dynamics of CO2 absorption and desorption processes in alkanolamine with cosolvent polyethylene glycol. Ind. Eng. Chem. Res. 2012, 51, 12081–12088. [Google Scholar] [CrossRef]
- McCann, N.; Phan, D.; Wang, X.; Conway, W.; Burns, R.; Attalla, M.; Puxty, G.; Maeder, M. Kinetics and mechanism of carbamate formation from CO2(aq), carbonate species, and monoethanolamine in aqueous solution. J. Phys. Chem. A 2009, 113, 5022–5029. [Google Scholar] [CrossRef] [PubMed]
- Muraleedharan, R.; Mondal, A.; Mandal, B. Absorption of carbon dioxide into aqueous blends of 2-amino-2-hydroxymethyl-1,3- propanediol and monoethanolamine. Sep. Purif. Technol. 2012, 94, 92–96. [Google Scholar] [CrossRef]
- Tong, D.; Trusler, J.P.M.; Maitland, G.C.; Gibbins, J.; Fennell, P.S. Solubility of carbon dioxide in aqueous solution of monoethanolamine or 2-amino-2methyl-1-propanol: Experimental measurements and modelling. Int. J. Greenh. Gas Control. 2012, 6, 37–47. [Google Scholar] [CrossRef]
- Chilekar, V.P.; Van der Schaaf, J.; Kuster, B.F.M.; Tinge, J.T.; Schouten, J.C. Influence of elevated pressure and particle lyophobicity on hydrodynamics and gas-liquid mass transfer in slurry bubble columns. AIChE J. 2010, 56, 584–596. [Google Scholar] [CrossRef]
- Orvalho, S.; Ruzicka, M.C.; Drahos, J. Bubble column with electrolytes: Gas holdup and flow regimes. Ind. Eng. Chem. Res. 2009, 48, 8237–8243. [Google Scholar] [CrossRef]
- López, A.B.; La Rubia, M.D.; Navaza, J.M.; Pacheco, R.; Gómez-Díaz, D. 1-amine-2-propanol + triethanolamine aqueous blends for carbon dioxide absorption in a bubble reactor. Energy Fuels 2015, 29, 5237–5244. [Google Scholar] [CrossRef]
- Mac Dowell, N.; Shah, N. Identification of the cost-optimal degree of CO2 capture: An optimisation study using dynamic process models. Int. J. Greenh. Gas Control. 2013, 13, 44–58. [Google Scholar] [CrossRef]
- Panda, D.; Kumar, E.A.; Singh, S.K. Amine modification of binder-containing zeolite 4A bodies for post-combustion CO2 capture. Ind. Eng. Chem. Res. 2019, 58, 5301–5313. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, M.; Pan, Y.; Yan, S.; Luo, Z. Amine-based absorbents selection for CO2 membrane vacuum regeneration technology by combined absorption-desorption analysis. Chem. Eng. Sci. 2013, 93, 238–249. [Google Scholar] [CrossRef]
- Muchan, P.; Narku-Tetteh, J.; Saiwan, C.; Idem, R.; Supap, T. Effect of number of amine groups in aqueous polyamine solution on carbon dioxide (CO2) capture activities. Sep. Purif. Technol. 2017, 184, 128–134. [Google Scholar] [CrossRef]
- Villarroel, J.A.; Palma-Cando, A.; Viloria, A.; Ricaurte, M. Kinetic and thermodynamic analysis of high-pressure CO2 capture using ethylenediamine: Experimental study and modeling. Energies 2021, 14, 6822. [Google Scholar] [CrossRef]
- Aghela, B.; Sahraiea, S.; Heidaryan, E. Carbon dioxide desorption from aqueous solutions of monoethanolamine and diethanolamine in a microchannel reactor. Sep. Purif. Technol. 2020, 237, 116390. [Google Scholar] [CrossRef]
- Yu, B.; Yu, H.; Yang, Q.; Li, K.; Ji, L.; Zhang, R.; Megharaj, M.; Chen, Z. Postcombustion capture of CO2 by diamines containing one primary and one tertiary amino group: Reaction rate and mechanism. Energy Fuels 2019, 33, 7500–7508. [Google Scholar] [CrossRef]
- Gómez-Díaz, D.; Gomes, N.; Teixeira, J.A.; Belo, I. Oxygen mass transfer to emulsions in a bubble column contactor. Chem. Eng. J. 2009, 152, 354–360. [Google Scholar] [CrossRef]
- Cerri, M.O.; Baldacin, J.C.; Cruz, A.J.G.; Hokka, C.O.; Badino, A.C. Prediction of mean bubble size in pneumatic reactors. Biochem. Eng. J. 2010, 53, 12–17. [Google Scholar] [CrossRef]
- Gómez-Díaz, D.; Navaza, J.M.; Rodríguez, P.; Vega, R. CO2 absorption and regeneration using amines with different degrees of steric hindrance. Chem. Eng. Technol. 2017, 40, 1767–1773. [Google Scholar] [CrossRef]
- Singh, P.; Versteeg, G.F. Structure and activity relationships for CO2 regeneration from aqueous amine-based absorbents. Process Saf. Environ. Prot. 2008, 86, 347–359. [Google Scholar] [CrossRef]
- Gao, H.; Liang, Z.; Liao, H.; Idem, R.O. Thermal degradation of aqueous DEEA solution at stripper conditions for post-combustion CO2 capture. Chem. Eng. Sci. 2015, 135, 330–342. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).