Development, Validation and Application of a Novel UHPLC-UV Method for the Simultaneous Determination of Valsartan and Nifedipine in the New Formulation of Self-Nanoemulsifying Drug Delivery Systems
Abstract
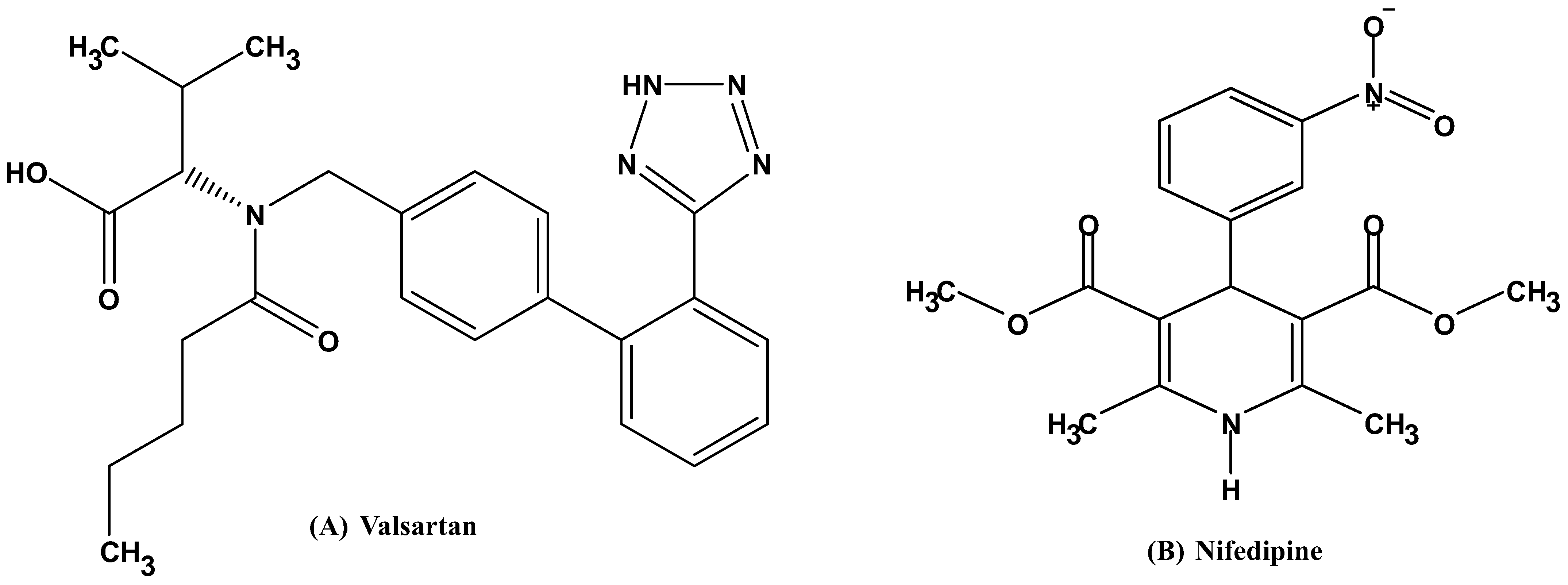
1. Introduction
2. Experimental
2.1. Materials and Equipment
2.2. Preparation of Self-Nanoemulsifying Lipid-Based Formulations
2.3. Characterization of Self-Nanoemulsifying Lipid-Based Formulation
2.4. SNEDDS Drug Loading
2.5. Instrumentation
2.6. Preparation of Mobile Phase, Standard Solutions and Quality Control Sample
3. Method Validation
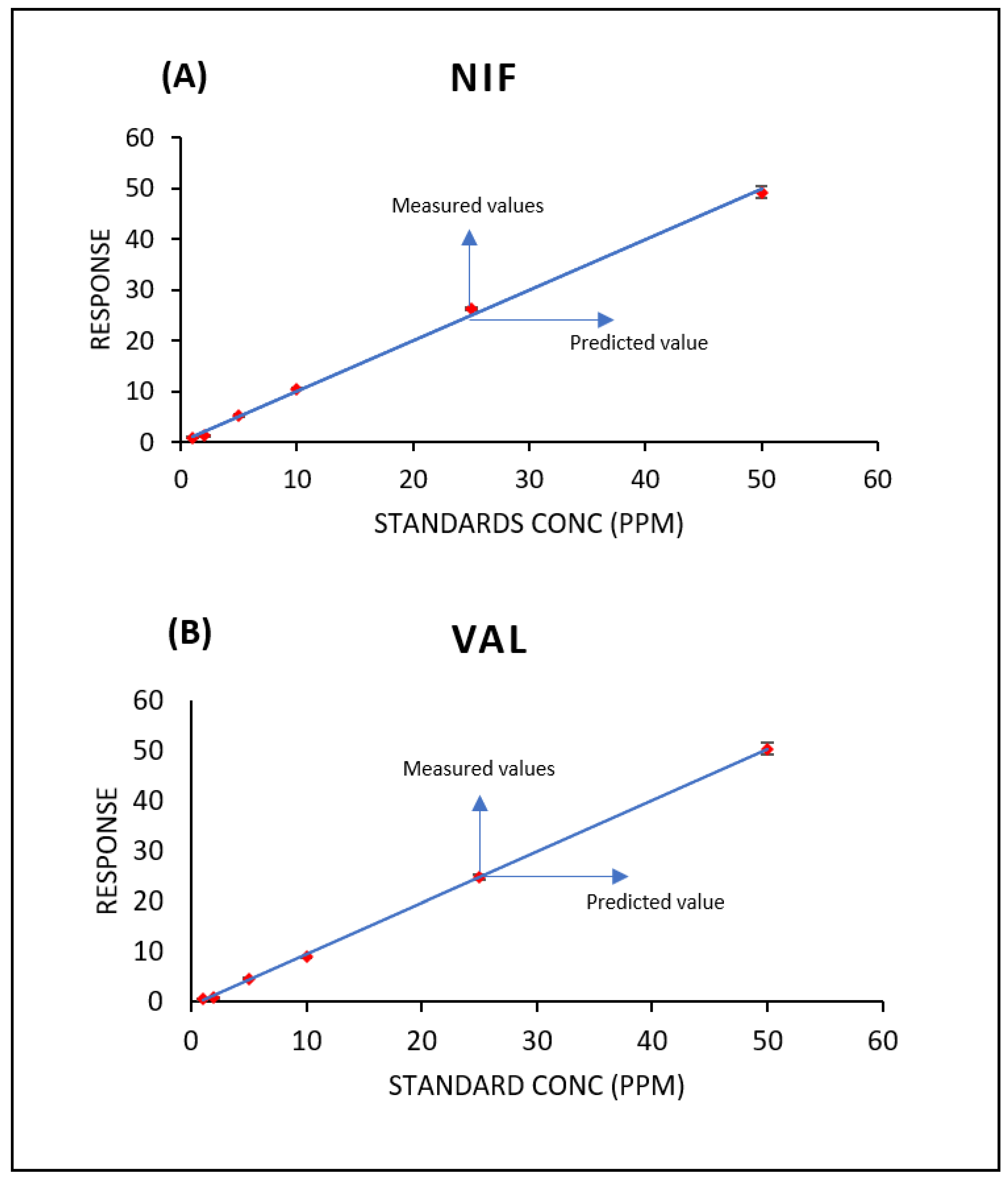
3.1. Linearity and Calibration
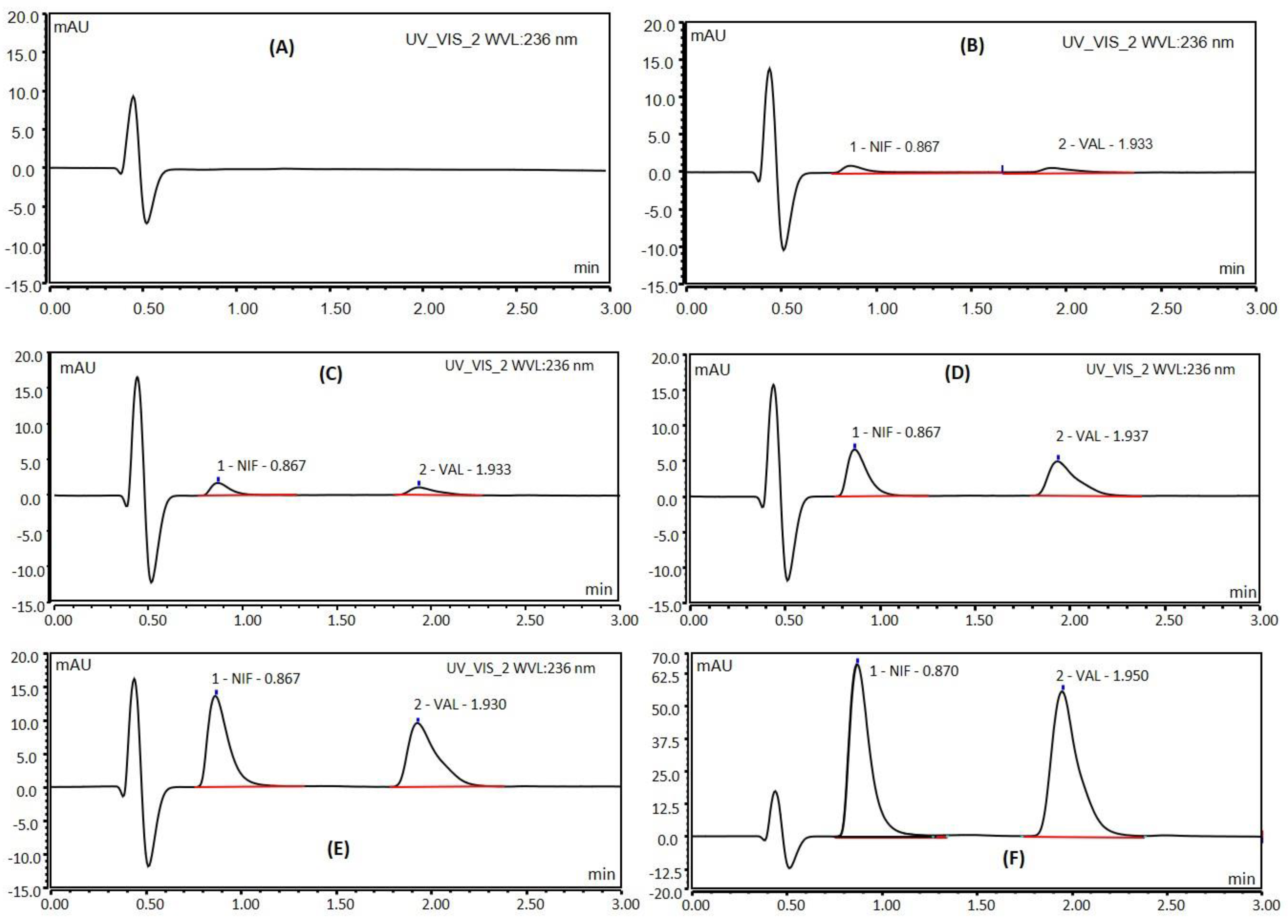
3.2. Specificity
3.3. Accuracy and Precision
3.4. Limit of Detection (LOD) and Lower Limit of Quantification (LLOQ)
3.5. Statistical Analysis
4. Results and Discussion
4.1. System Suitability Studies
4.2. Linearity and Calibration Curves
4.3. Accuracy
4.4. LOD and LOQ
4.5. Applicability of the Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danaei, G.; Ding, E.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.L.; Ezzati, M. The Preventable Causes of Death in the United States: Comparative Risk Assessment of Dietary, Lifestyle, and Metabolic Risk Factors. PLoS Med. 2009, 6, e1000058. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: A systematic analysis for the Global Burden of Disease Study. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Abstract 16828: Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-based Studies From 90 Countries. Circulation 2015, 132, A16828. [Google Scholar] [CrossRef]
- Murray, C.J.; Abraham, J.; Ali, M.K.; Alvarado, M.; Atkinson, C.; Baddour, L.M.; Bartels, D.H.; Benjamin, E.J.; Bhalla, K.; Birbeck, G.; et al. The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. Jama 2013, 310, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990-2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Dorans, K.S.; Mills, K.T.; Liu, Y.; He, J. Trends in Prevalence and Control of Hypertension According to the 2017 American College of Cardiol-ogy/American Heart Association (ACC/AHA) Guideline. J. Am. Heart Assoc. 2018, 7, e008888. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Executive summary: Heart disease and stroke statistics-2013 update: A report from the American Heart Asso-ciation. Circulation 2013, 127, 143–152. [Google Scholar] [CrossRef] [PubMed]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). Jama 2014, 311, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Law, M.; Morris, J.K.; Bestwick, J.P.; Wald, N.J. Combination Therapy Versus Monotherapy in Reducing Blood Pressure: Meta-analysis on 11,000 Participants from 42 Trials. Am. J. Med. 2009, 122, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Gradman, A.H.; Basile, J.N.; Carter, B.L.; Bakris, G.L.; American Society of Hypertension Writing Group. Combination therapy in hypertension. J. Am. Soc. Hypertens 2010, 4, 42–50. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 60846; National Center for Biotechnology Information: Bethesda, MD, USA, 2021.
- National Center for Biotechnology Information. PubChem Database. Nifedipine, C; National Center for Biotechnology Information: Bethesda, MD, USA, 2021.
- Nerenberg, K.A.; Zarnke, K.B.; Leung, A.A.; Dasgupta, K.; Butalia, S.; McBrien, K.; Harris, K.C.; Nakhla, M.; Cloutier, L.; Gelfer, M.; et al. Hypertension Canada’s 2018 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults and Children. Can. J. Cardiol. 2018, 34, 506–525. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Fu, J. Clinical effect and safety of nifedipine controlled-release tablets combined with valsartan in the treatment of primary hypertension. Pak. J. Pharm. Sci. 2019, 32, 2419–2422. [Google Scholar] [PubMed]
- Almasi, M.; Matiasova, A.; Sulecova, M.; Benova, E.; Sevc, J.; Vahovska, L.; Lisnichuk, M.; Girman, V.; Zelenakova, A.; Hudak, A.; et al. In vivo study of light-driven naproxen release from gated mesoporous silica drug delivery system. Sci. Rep. 2021, 11, 20191. [Google Scholar] [CrossRef] [PubMed]
- Sood, J.; Sapra, B.; Tiwary, A.K. Microemulsion Transdermal Formulation for Simultaneous Delivery of Valsartan and Nifedipine: Formulation by Design. AAPS Pharm. Sci. Tech. 2016, 18, 1901–1916. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- ICH Steering Committee. ICH Harmonized Tripartite Guidlines, Q1C, Stability Testing for New Dosage Forms; ICH Steering Committee: Geneva, Switzerland, 1996. [Google Scholar]
- Kazi, M.; Nasr, F.A.; Noman, O.; Alharbi, A.; Alqahtani, M.S.; Alanazi, F.K. Development, Characterization Optimization, and Assessment of Curcumin-Loaded Bioactive Self-Nanoemulsifying Formulations and Their Inhibitory Effects on Human Breast Cancer MCF-7 Cells. Pharmaceutics 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Alhajri, A.; AlShehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, In Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Alqahtani, A.A.; Alsaadi, B.S.; Alkholief, M.; Alanazi, F.K. UHPLC Method Development for Determining Sitagliptin and Dapagliflozin in Lipid-Based Self-Nanoemulsifying Systems as Combined Dose in Commercial Products and its Application to Pharmacokinetic Study of Dapagliflozin in Rats. Pharm. Chem. J. 2019, 53, 79–87. [Google Scholar] [CrossRef]
| F. No. | Components (% W/W) | Solubility (mg/g) | ||||
|---|---|---|---|---|---|---|
| OS | Maisene 35 | TOP-OV | CrEL | NIF | VAL | |
| F1 | 35 | 15 | 50 | 30.89 ± 0.55 | 12.6 ± 1.12 | |
| F2 | 15 | 35 | 50 | 29.13 ± 0.82 | 10.76 ± 0.97 | |
| Formulation No. | Formulation Composition | Particle Size (d.nm) | PDI | Zeta Potential |
|---|---|---|---|---|
| F1 | ** OS: Maisene-35/TOP-OV [35/15/50] | 483.26 | 0.492 | −48.8 |
| F2 | ** OS: Maisene-35/CrEL [15/35/50] | 198.4333 | 0.725 | −14.6 |
| Std1 | Std2 | Std3 | Std4 | Std5 | Std6 | |
|---|---|---|---|---|---|---|
| Methanol volume (µL) | 950 | 500 | 600 | 500 | 600 | 500 |
| Addition | Stock | Std1 | Std2 | Std3 | Std4 | Std5 |
| Volume of addition (µL) | 50 | 500 | 400 | 500 | 400 | 500 |
| Final conc. (µg/mL) | 50 | 25 | 10 | 5 | 2 | 1 |
| System Suitability Parameter | NIF a | VAL b | Status |
|---|---|---|---|
| Retention Time (Min) | 0.867 | 1.933 | Passed |
| % RSD | 0.045 | 0.067 | Passed |
| Peak Tailing | 1.08 | 1.34 | Passed |
| Theoretical Plate number | 2563 | 2345 | Passed |
| Conc (ppm) | NIF a | VAL b | |
|---|---|---|---|
| Std1 | 1 | 14.75 | 7.85 |
| Std2 | 2 | 10.25 | 1.83 |
| Std3 | 5 | 6.68 | 3.07 |
| Std4 | 10 | 2.36 | 1.74 |
| Std5 | 25 | 1.95 | 3.60 |
| Std6 | 50 | 4.40 | 4.56 |
| Amount of Drug in SNEDDS | NIF a | VAL b |
|---|---|---|
| % Recovery | % Recovery | |
| 2.5 mg/g | 99.09 ± 0.35 | 98.99 ± 0.66 |
| 5 mg/g | 99.67 ± 0.15 | 99.10 ± 0.45 |
| 10 mg/g | 99.91 ± 0.09 | 99.76 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.A.; Bilani, M.; Shazly, G.; Kazi, M. Development, Validation and Application of a Novel UHPLC-UV Method for the Simultaneous Determination of Valsartan and Nifedipine in the New Formulation of Self-Nanoemulsifying Drug Delivery Systems. Separations 2022, 9, 325. https://doi.org/10.3390/separations9110325
Rashid MA, Bilani M, Shazly G, Kazi M. Development, Validation and Application of a Novel UHPLC-UV Method for the Simultaneous Determination of Valsartan and Nifedipine in the New Formulation of Self-Nanoemulsifying Drug Delivery Systems. Separations. 2022; 9(11):325. https://doi.org/10.3390/separations9110325
Chicago/Turabian StyleRashid, Md Abdur, Mohammad Bilani, Gamal Shazly, and Mohsin Kazi. 2022. "Development, Validation and Application of a Novel UHPLC-UV Method for the Simultaneous Determination of Valsartan and Nifedipine in the New Formulation of Self-Nanoemulsifying Drug Delivery Systems" Separations 9, no. 11: 325. https://doi.org/10.3390/separations9110325
APA StyleRashid, M. A., Bilani, M., Shazly, G., & Kazi, M. (2022). Development, Validation and Application of a Novel UHPLC-UV Method for the Simultaneous Determination of Valsartan and Nifedipine in the New Formulation of Self-Nanoemulsifying Drug Delivery Systems. Separations, 9(11), 325. https://doi.org/10.3390/separations9110325








