Silver Nanoparticles Functionalized with Sodium Mercaptoethane Sulfonate to Remove Copper from Water by the Formation of a Micellar Phase
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instrumentation
2.2. Preparation of Silver Nanoparticles Functionalized with Sodium 2-Mercaptoethanesulfonate (AgNPs@MESNa)
2.3. Proposed Procedure for the Removal of Cu2+ from Water
3. Results and Discussion
3.1. Selection of the Functionalizing Reagent
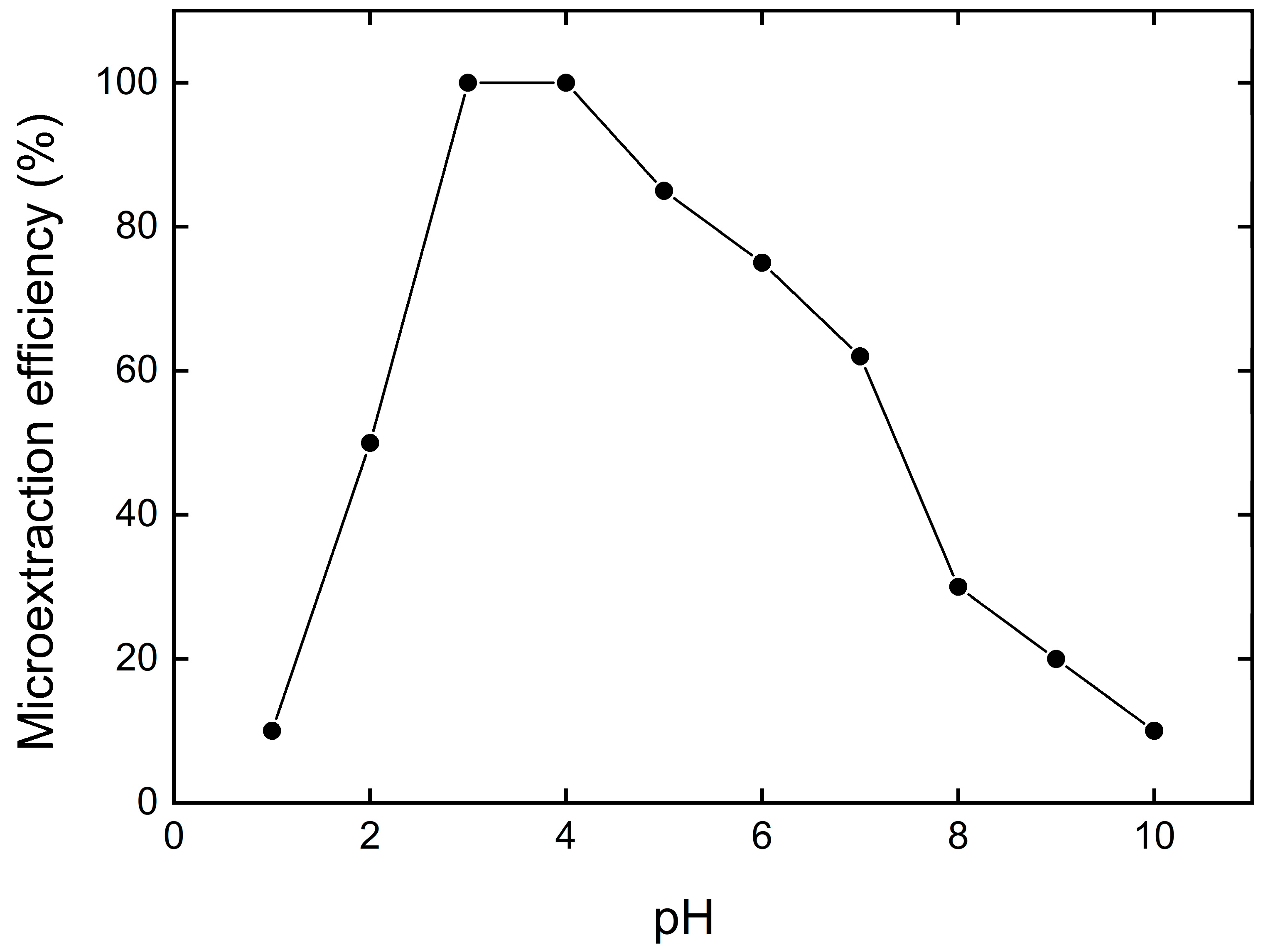
3.2. Effect of pH on Cu2+ Removal
3.3. Effect of Triton X-114 Concentration
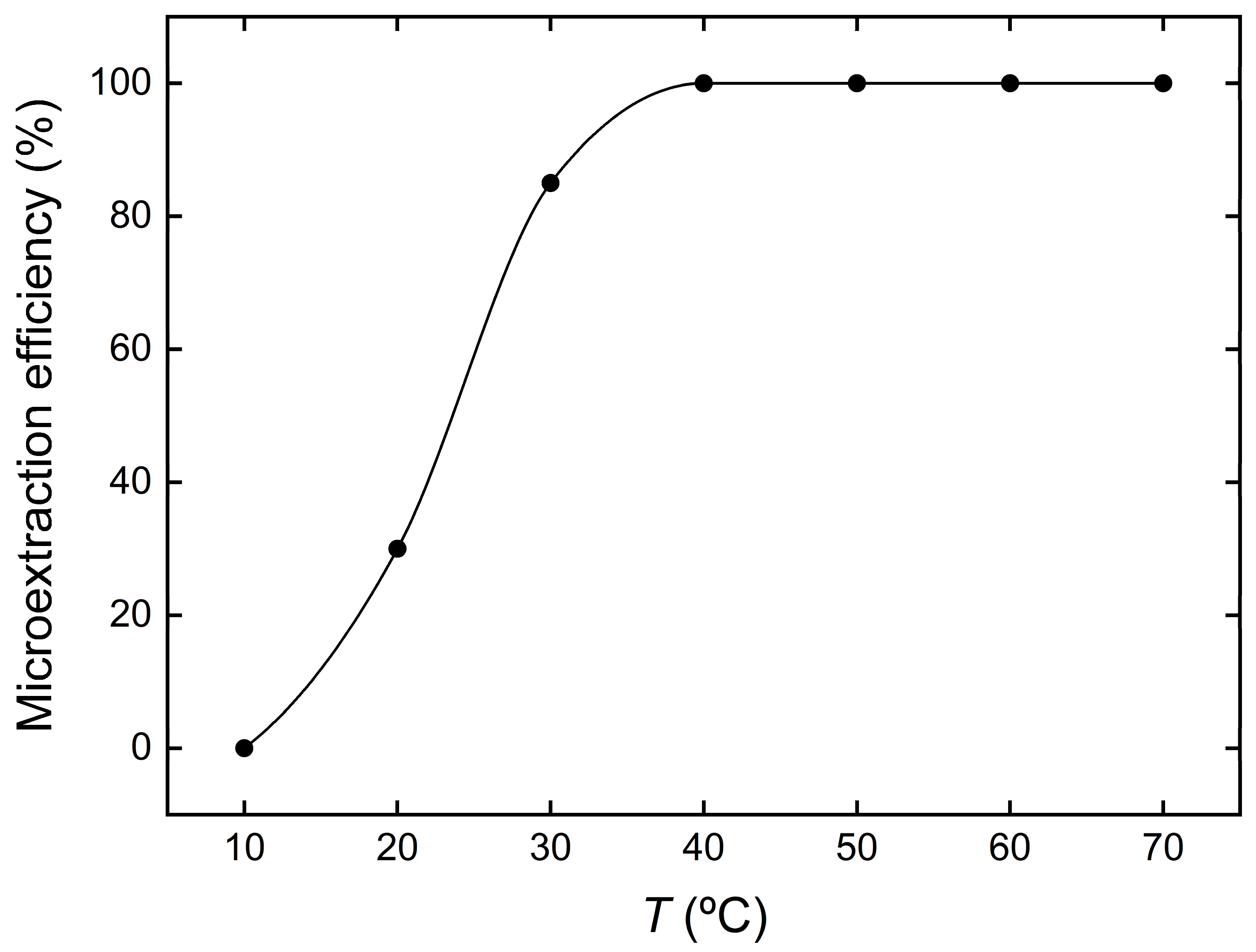
3.4. Effect of Temperature on the Microextraction Efficiency of Cu2+
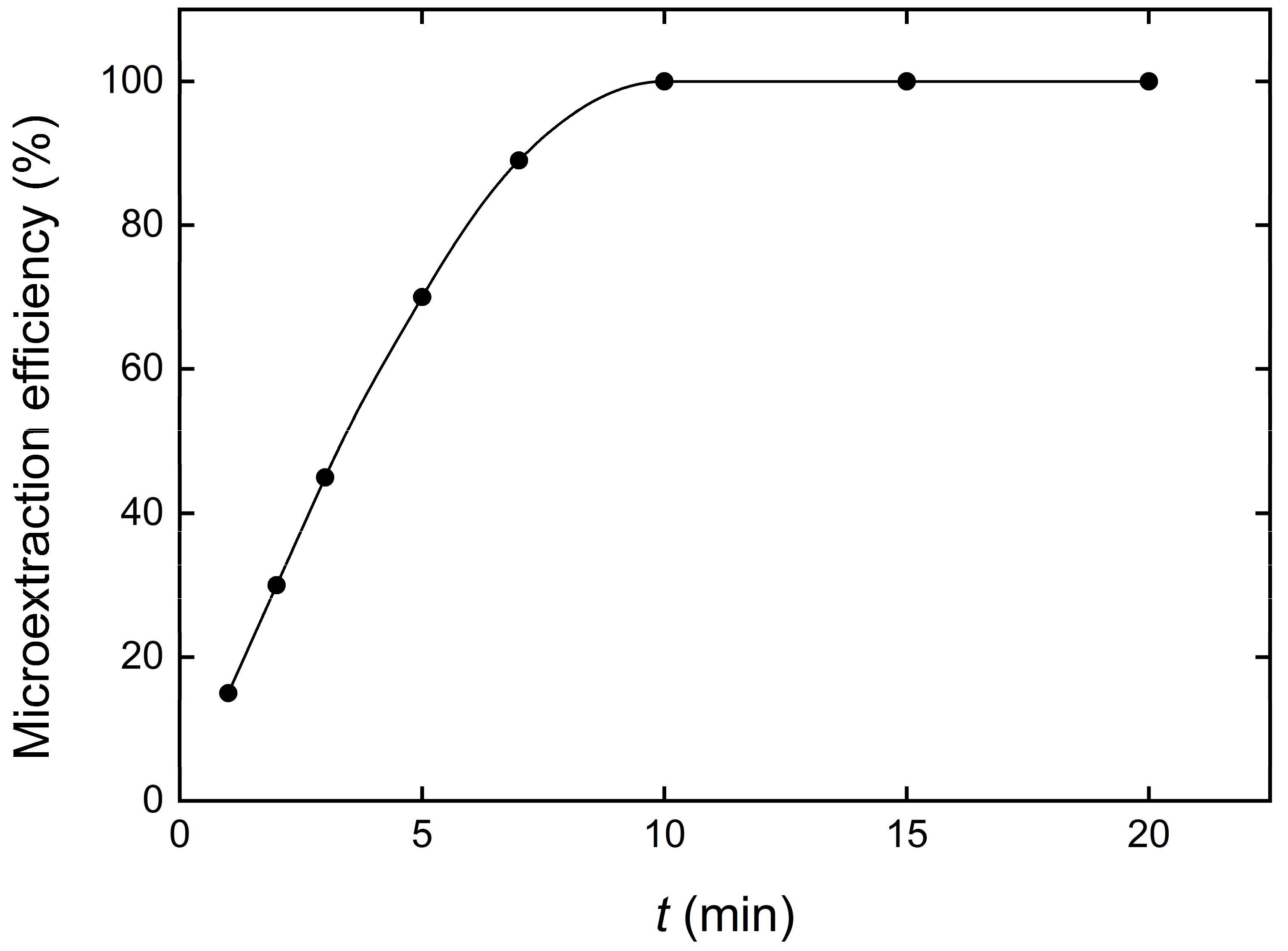
3.5. Contact Time Effect
3.6. Effect of Other Ions Present in the Medium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Beavington, F. Trace-Elements in Rainwater and Dry Deposition around a Smelting Complex. Environ. Pollut. 1977, 13, 127–131. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Frag, E.Y.; El Brawy, M.H. Rapid potentiometric sensor for determination of Cu(II) ions in food samples. Microchem. J. 2021, 164, 7. [Google Scholar] [CrossRef]
- Zhu, F.K.; Wang, X.J.; Fan, W.X.; Qu, L.; Qiao, M.Y.; Yao, S.W. Assessment of potential health risk for arsenic and heavy metals in some herbal flowers and their infusions consumed in China. Environ. Monit. Assess. 2013, 185, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Karadjova, I.; Izgi, B.; Gucer, S. Fractionation and speciation of Cu, Zn and Fe in wine samples by atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2002, 57, 581–590. [Google Scholar] [CrossRef]
- Ramlan; Basir-Cyio, M.; Napitupulu, M.; Inoue, T.; Anshary, A.; Mahfudz; Isrun; Rusydi, M.; Golar; Sulbadana; et al. Pollution and contamination level of Cu, Cd, and Hg heavy metals in soil and food crop. Int. J. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Sinaei, M.; Zare, R.; Matin, M.T.; Ghasemzadeh, J. Marine Debris and Trace Metal (Cu, Cd, Pb, and Zn) Pollution in the Stranded Green Sea Turtles (Chelonia mydas). Arch. Environ. Contam. Toxicol. 2021, 80, 634–644. [Google Scholar] [CrossRef]
- Armid, A.; Shinjo, R.; Takwir, A.; Ruslan, R.; Wijaya, A.R. Spatial Distribution and Pollution Assessment of Trace Elements Pb, Cu, Ni, Fe and As in the Surficial Water of Staring Bay, Indonesia. J. Braz. Chem. Soc. 2021, 32, 299–310. [Google Scholar] [CrossRef]
- Lopez-Garcia, I.; Vicente-Martinez, Y.; Hernandez-Cordoba, M. Determination of very low amounts of free copper and nickel ions in beverages and water samples using cloud point extraction assisted by silver nanoparticles. Anal. Methods 2015, 7, 3786–3792. [Google Scholar] [CrossRef]
- Yan, C.Y.; Zhuang, T.; Bai, J.H.; Wen, X.J.; Lu, Q.Q.; Zhang, L. Assessment of As, Cd, Zn, Cu and Pb Pollution and Toxicity in River Wetland Sediments and Artificial Wetland Soils Affected by Urbanization in a Chinese Delta. Wetlands 2020, 40, 2799–2809. [Google Scholar] [CrossRef]
- Kamnoet, P.; Aeungmaitrepirom, W.; Menger, R.F.; Henry, C.S. Highly selective simultaneous determination of Cu(ii), Co(ii), Ni(ii), Hg(ii), and Mn(ii) in water samples using microfluidic paper-based analytical devices. Analyst 2021, 146, 2229–2239. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Tran, V.S.; Pham, T.T.; Pham, H.G.; Hoang, B.L.; Nguyen, T.H.; Tran, T.H.; Ngo, H.H. Fenton/ozone-based oxidation and coagulation processes for removing metals (Cu, Ni)-EDTA from plating wastewater. J. Water Process Eng. 2021, 39. [Google Scholar] [CrossRef]
- Honarmandrad, Z.; Javid, N.; Malakootian, M. Efficiency of ozonation process with calcium peroxide in removing heavy metals (Pb, Cu, Zn, Ni, Cd) from aqueous solutions. SN Appl. Sci. 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.P.; Cao, Y.J.; Peng, W.J.; Miao, Y.H.; Fan, G.X.; Li, C.; Huang, Y.K.; Song, X.Y. Enhancing the ion flotation removal of Cu(II) via regulating the oxidation degree of nano collector-graphene oxide. J. Clean. Prod. 2021, 295. [Google Scholar] [CrossRef]
- Grudzien, K.; Nogas, W.; Szczepaniak, G.; Grela, K. Larger scale Stahl oxidation with instant Cu removal in convenient synthesis of chiral bidentate N-heterocyclic carbene precursor. Polyhedron 2021, 199. [Google Scholar] [CrossRef]
- Wu, K.; Wang, M.; Li, A.Z.; Zhao, Z.X.; Liu, T.; Hao, X.D.; Yang, S.J.; Jin, P.K. The enhanced As(III) removal by Fe-Mn-Cu ternary oxide via synergistic oxidation: Performances and mechanisms. Chem. Eng. J. 2021, 406. [Google Scholar] [CrossRef]
- Wu, L.Y.; Peng, B.; Li, Q.Z.; Wang, Q.W.; Yan, X.; Lin, Q.H.; Ji, C.L. Formation of high crystalline LDH sludge for removing Cu and Zn from wastewater by controlled double-jet precipitation. Environ. Sci. Pollut. Res. 2019, 26, 19665–19675. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.F.; Xue, M.M.; Song, R.Y.; Zhang, Y.; Qu, G.Z.; Wang, T.C. High-efficient decomplexation of Cu-EDTA and Cu removal by high-frequency non-thermal plasma oxidation/alkaline precipitation. Sep. Purif. Technol. 2021, 257. [Google Scholar] [CrossRef]
- Guo, P.P.; Kong, L.H.; Hu, X.Y.; Peng, X.J.; Wang, X.L. Removal of Cl(-I) from strongly acidic wastewater containing Cu(II) by complexation-precipitation using thiourea: Efficiency enhancement by ascorbic acid. J. Hazard. Mater. 2021, 402. [Google Scholar] [CrossRef]
- Xiong, B.W.; Zhang, T.T.; Zhao, Y.L.; Wen, T.; Zhang, Q.W.; Bao, S.X.; Song, S.X. Removal of Cu(II) from wastewater by using mechanochemically activated carbonate-based tailings through chemical precipitation. Environ. Sci. Pollut. Res. 2019, 26, 35198–35207. [Google Scholar] [CrossRef]
- Shan, C.; Xu, Z.; Zhang, X.L.; Xu, Y.; Gao, G.D.; Pan, B.C. Efficient removal of EDTA-complexed Cu(II) by a combined Fe(III)/UV/alkaline precipitation process: Performance and role of Fe(II). Chemosphere 2018, 193, 1235–1242. [Google Scholar] [CrossRef]
- Pavithra, S.; Thandapani, G.; Sugashini, S.; Sudha, P.N.; Alkhamis, H.H.; Alrefaei, A.F.; Almutairi, M.H. Batch adsorption studies on surface tailored chitosan/orange peel hydrogel composite for the removal of Cr(VI) and Cu(II) ions from synthetic wastewater. Chemosphere 2021, 271. [Google Scholar] [CrossRef]
- Luo, H.Y.; Liu, Y.; Lu, H.X.; Fang, Q.; Rong, H.W. Efficient Adsorption of Tetracycline from Aqueous Solutions by Modified Alginate Beads after the Removal of Cu(II) Ions. ACS Omega 2021, 6, 6240–6251. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, R.R.; Li, X.G.; Yan, L.G.; Ma, Z.M.; Jia, R.B.; Sun, S.H. Effective removal of Cu(II), Pb(II) and Cd(II) by sodium alginate intercalated MgAl-layered double hydroxide: Adsorption properties and mechanistic studies. Water Sci. Technol. 2021, 83, 975–984. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.J.; Chelme-Ayala, P.; Wei, J.; Zhou, X.T.; Min, Y.H.; El-Din, M.G.; Wu, Z.R. The removal of Cu(II)-EDTA chelates using green rust adsorption combined with ferrite formation process. J. Environ. Manag. 2021, 279. [Google Scholar] [CrossRef]
- Abou-Zeid, R.E.; Ali, K.A.; Gawad, R.M.A.; Kamal, K.H.; Kamel, S.; Khiari, R. Removal of Cu(II), Pb(II), Mg(II), and Fe(II) by Adsorption onto Alginate/Nanocellulose Beads as Bio-Sorbent. J. Renew. Mater. 2021, 9, 601–613. [Google Scholar] [CrossRef]
- Yang, L.W.; Peng, Y.Q.; Qian, C.F.; Xing, G.H.; He, J.J.; Zhao, C.L.; Lai, B. Enhanced adsorption/photocatalytic removal of Cu(II) from wastewater by a novel magnetic chitosan@ bismuth tungstate coated by silver (MCTS-Ag/Bi2WO6) composite. Chemosphere 2021, 263. [Google Scholar] [CrossRef] [PubMed]
- Bozejewicz, D.; Osmialowski, B.; Kaczorowska, M.A.; Witt, K. 2,6-Bis((benzoyl-R)amino)pyridine (R = H, 4-Me, and 4-NMe2) Derivatives for the Removal of Cu(II), Ni(II), Co(II), and Zn(II) Ions from Aqueous Solutions in Classic Solvent Extraction and a Membrane Extraction. Membranes 2021, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Isosaari, P.; Marjavaara, P.; Lehmus, E. Sequential electrokinetic treatment and oxalic acid extraction for the removal of Cu, Cr and As from wood. J. Hazard. Mater. 2010, 182, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Chague-Goff, C. Assessing the removal efficiency of Zn, Cu, Fe and Pb in a treatment wetland using selective sequential extraction: A case study. Water Air Soil Pollut. 2005, 160, 161–179. [Google Scholar] [CrossRef]
- Lin, S.H.; Juang, R.S. Removal of free and chelated Cu(II) ions from water by a nondispersive solvent extraction process. Water Res. 2002, 36, 3611–3619. [Google Scholar] [CrossRef]
- Wen, J.; Hu, X.H. Metal selectivity and effects of co-existing ions on the removal of Cd, Cu, Ni, and Cr by ZIF-8-EGCG nanoparticles. J. Colloid Interface Sci. 2021, 589, 578–586. [Google Scholar] [CrossRef]
- Chang, L.L.; Pu, Y.P.; Jing, P.P.; Cui, Y.F.; Zhang, G.C.; Xu, S.; Cao, B.Y.; Guo, J.Y.; Chen, F.Y.; Qiao, C.F. Magnetic core-shell MnFe2O4@TiO2 nanoparticles decorated on reduced graphene oxide as a novel adsorbent for the removal of ciprofloxacin and Cu(II) from water. Appl. Surf. Sci. 2021, 541. [Google Scholar] [CrossRef]
- Hamdy, A. Experimental Study of the Relationship Between Dissolved Iron, Turbidity, and Removal of Cu(II) Ion From Aqueous Solutions Using Zero-Valent Iron Nanoparticles. Arab. J. Sci. Eng. 2021, 46, 5543–5565. [Google Scholar] [CrossRef]
- Hosain, A.N.A.; El Nemr, A.; El Sikaily, A.; Mahmoud, M.E.; Amira, M.F. Surface modifications of nanochitosan coated magnetic nanoparticles and their applications in Pb(II), Cu(II) and Cd(II) removal. J. Environ. Chem. Eng. 2020, 8. [Google Scholar] [CrossRef]
- Wu, X.Y.; Xu, Y.B.; Dong, Y.J.; Jiang, X.; Zhu, N.N. Colorimetric determination of hexavalent chromium with ascorbic acid capped silver nanoparticles. Anal. Methods 2013, 5, 560–565. [Google Scholar] [CrossRef]
- Catarino, S.; Trancoso, I.M.; de Sousa, R.B.; Curvelo-Garcia, A.S. Grape must mineralization by high pressure microwave digestion for trace element analysis: Development of a procedure. Cienc. E Tec. Vitivinic. 2010, 25, 87–93. [Google Scholar]
- Niemeyer, C.M. Nanoparticles, proteins, and nucleic acids: Biotechnology meets materials science. Angew. Chem. Int. Edit. 2001, 40, 4128–4158. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Gittins, D.I.; Caruso, F. Tailoring the polyelectrolyte coating of metal nanoparticles. J. Phys. Chem. B 2001, 105, 6846–6852. [Google Scholar] [CrossRef]
- Sommer, W.J.; Weck, M. Facile functionalization of gold nanoparticles via microwave-assisted 1,3 dipolar cycloaddition. Langmuir 2007, 23, 11991–11995. [Google Scholar] [CrossRef]
- Wilton-Ely, J. The surface functionalisation of gold nanoparticles with metal complexes. Dalton Trans. 2008, 25–29. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.H.; Chen, Y.H.; Bi, N.; Zheng, X.; Qi, H.B.; Qin, M.H.; Liao, X.; Zhang, H.Q.; Tian, Y. Determination of mercury(II) by surface-enhanced Raman scattering spectroscopy based on thiol-functionalized silver nanoparticles. Microchim. Acta 2012, 177, 341–348. [Google Scholar] [CrossRef]
- Rivas, B.L.; Schiappacasse, L.N.; Pereira, U.E.; Moreno-Villoslada, I. Interactions of polyelectrolytes bearing carboxylate and/or sulfonate groups with Cu(II) and Ni(II). Polymer 2004, 45, 1771–1775. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, H.; Li, C.; He, P.; Peng, W.B.; Yuan, L.F.; Zeng, L.X.; He, Y.J. Colorimetric detection of Mn2+ using silver nanoparticles cofunctionalized with 4-mercaptobenzoic acid and melamine as a probe. Talanta 2012, 97, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.; Mani, V.; Chandrasekaran, N.; Mukherjee, A. Selective colorimetric sensing of cysteine in aqueous solutions using silver nanoparticles in the presence of Cr3+. Talanta 2011, 85, 533–540. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, H.; He, Y.J.; Ding, N.; Cao, Q. Colorimetric detection of Cu2+ using 4-mercaptobenzoic acid modified silver nanoparticles. Colloid Surf. A-Physicochem. Eng. Asp. 2011, 391, 179–183. [Google Scholar] [CrossRef]
- Liu, J.F.; Chao, J.B.; Liu, R.; Tan, Z.Q.; Yin, Y.G.; Wu, Y.; Jiang, G.B. Cloud Point Extraction as an Advantageous Preconcentration Approach for Analysis of Trace Silver Nanoparticles in Environmental Waters. Anal. Chem. 2009, 81, 6496–6502. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Arceo, E.; Goulet, P.J.G.; Garrido, J.J.; Aroca, R.F. Role of nanoparticle surface charge in surface-enhanced Raman scattering. J. Phys. Chem. B 2005, 109, 3787–3792. [Google Scholar] [CrossRef] [PubMed]
- Quina, F.H.; Hinze, W.L. Surfactant-mediated cloud point extractions: An environmentally benign alternative separation approach. Ind. Eng. Chem. Res. 1999, 38, 4150–4168. [Google Scholar] [CrossRef]
- Meeravali, N.N.; Kumar, S.J. Determination of Cd, Pb, Cu, Ni and Mn in effluents and natural waters by a novel salt induced mixed-micelle cloud point extraction using ETAAS. Anal. Methods 2012, 4, 2435–2440. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Martínez, Y.; Caravaca Garratón, M.; García-Onsurbe, M.d.C.; Soto-Meca, A. Silver Nanoparticles Functionalized with Sodium Mercaptoethane Sulfonate to Remove Copper from Water by the Formation of a Micellar Phase. Separations 2021, 8, 108. https://doi.org/10.3390/separations8080108
Vicente-Martínez Y, Caravaca Garratón M, García-Onsurbe MdC, Soto-Meca A. Silver Nanoparticles Functionalized with Sodium Mercaptoethane Sulfonate to Remove Copper from Water by the Formation of a Micellar Phase. Separations. 2021; 8(8):108. https://doi.org/10.3390/separations8080108
Chicago/Turabian StyleVicente-Martínez, Yesica, Manuel Caravaca Garratón, María del Carmen García-Onsurbe, and Antonio Soto-Meca. 2021. "Silver Nanoparticles Functionalized with Sodium Mercaptoethane Sulfonate to Remove Copper from Water by the Formation of a Micellar Phase" Separations 8, no. 8: 108. https://doi.org/10.3390/separations8080108





