Capturing Dioclea Reflexa Seed Bioactives on Halloysite Nanotubes and pH Dependent Release of Cargo against Breast (MCF-7) Cancers In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Loading and pH-Dependent Release of DR Metabolites
2.2.2. Characterization of HNTs and DR Loaded HNTs
2.2.3. Culturing and Cyclic Voltammetry Analysis of MCF-7 Breast Cancer Cell Lines
3. Results
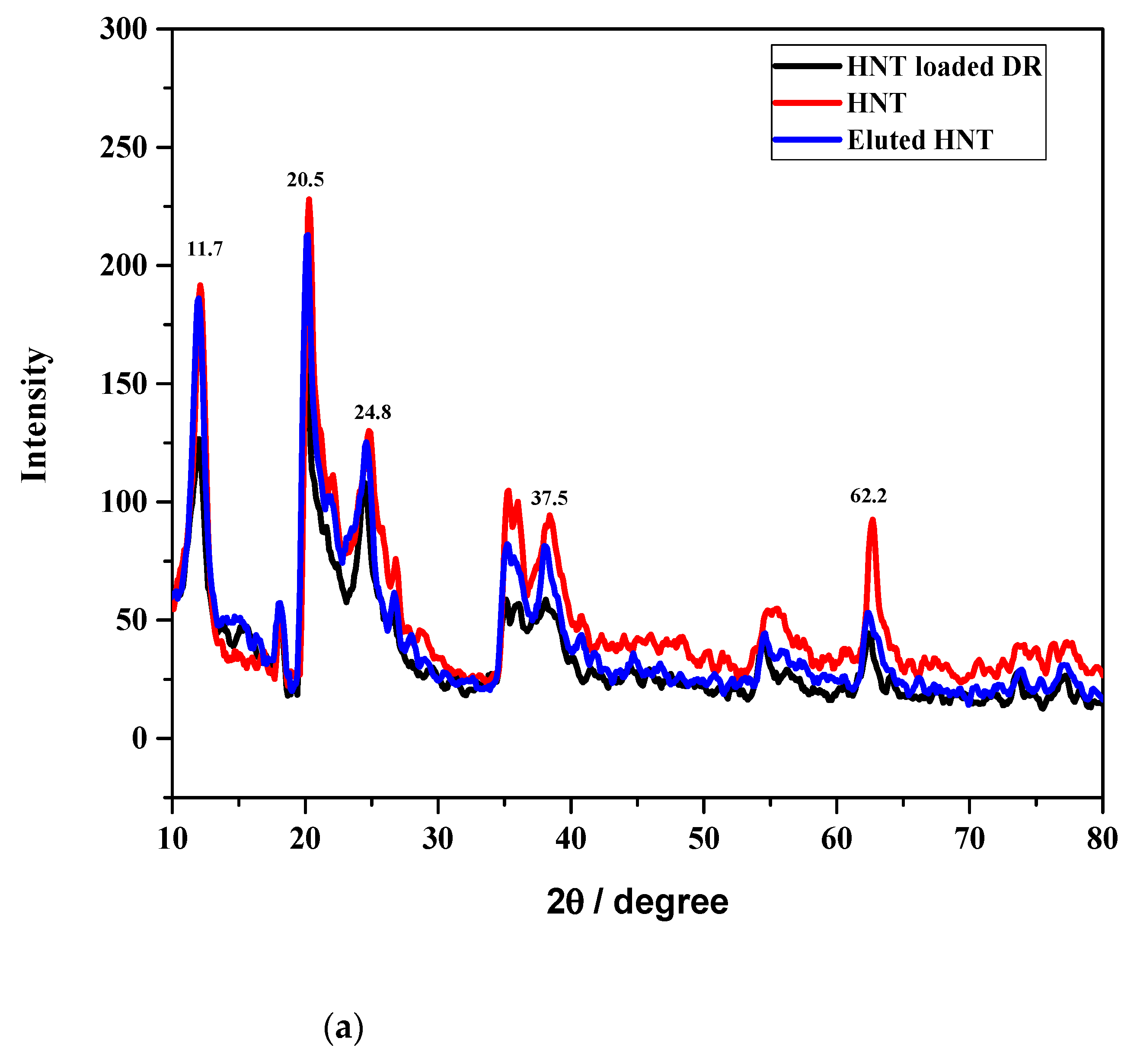
3.1. Characterization of HNT and loaded HNT using XRD, TGA, and FTIR
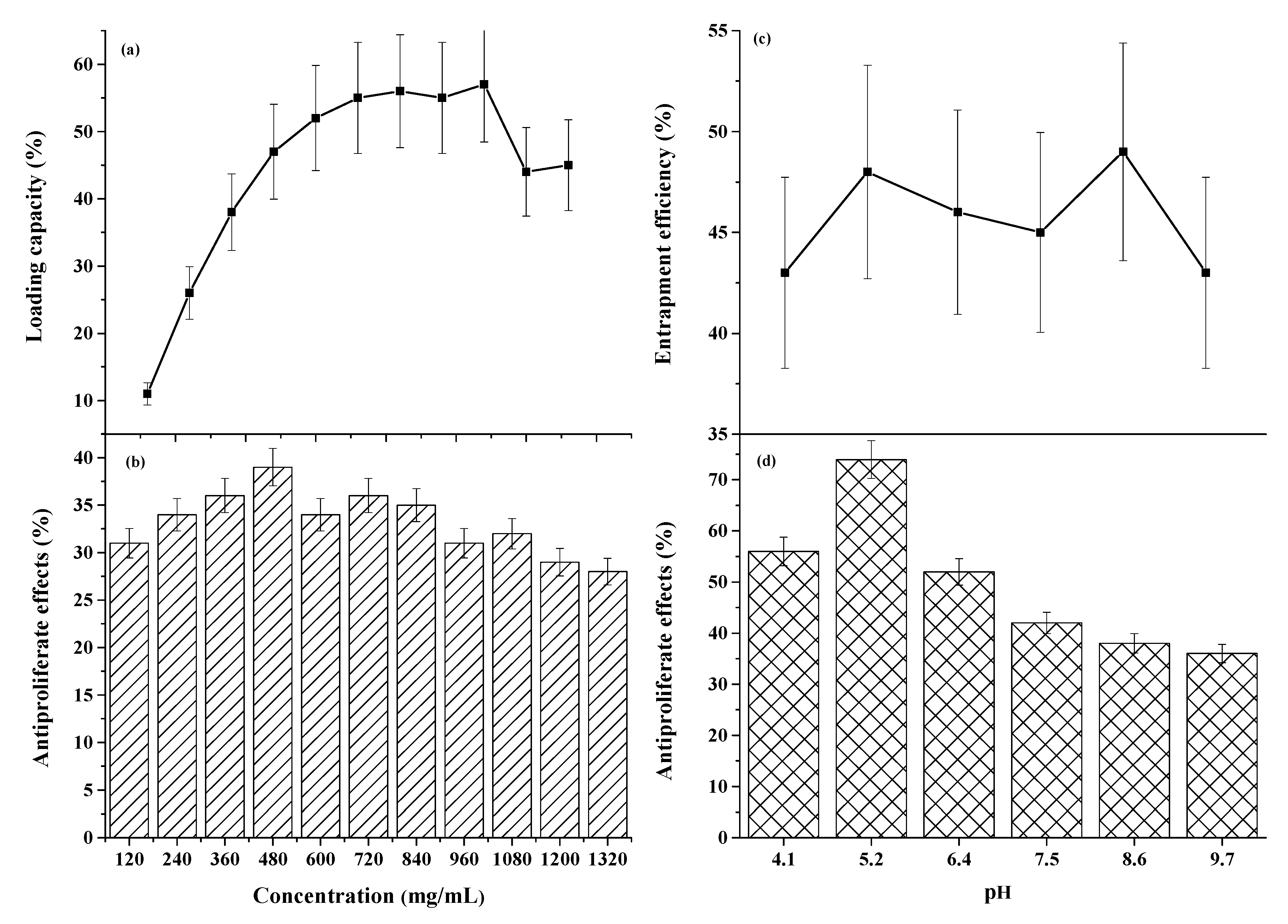
3.1.1. Optimization of Parameters to Increase Metabolites Immobilization onto HNTs
3.1.2. PH-Dependent Elution of the Metabolites from HNTs
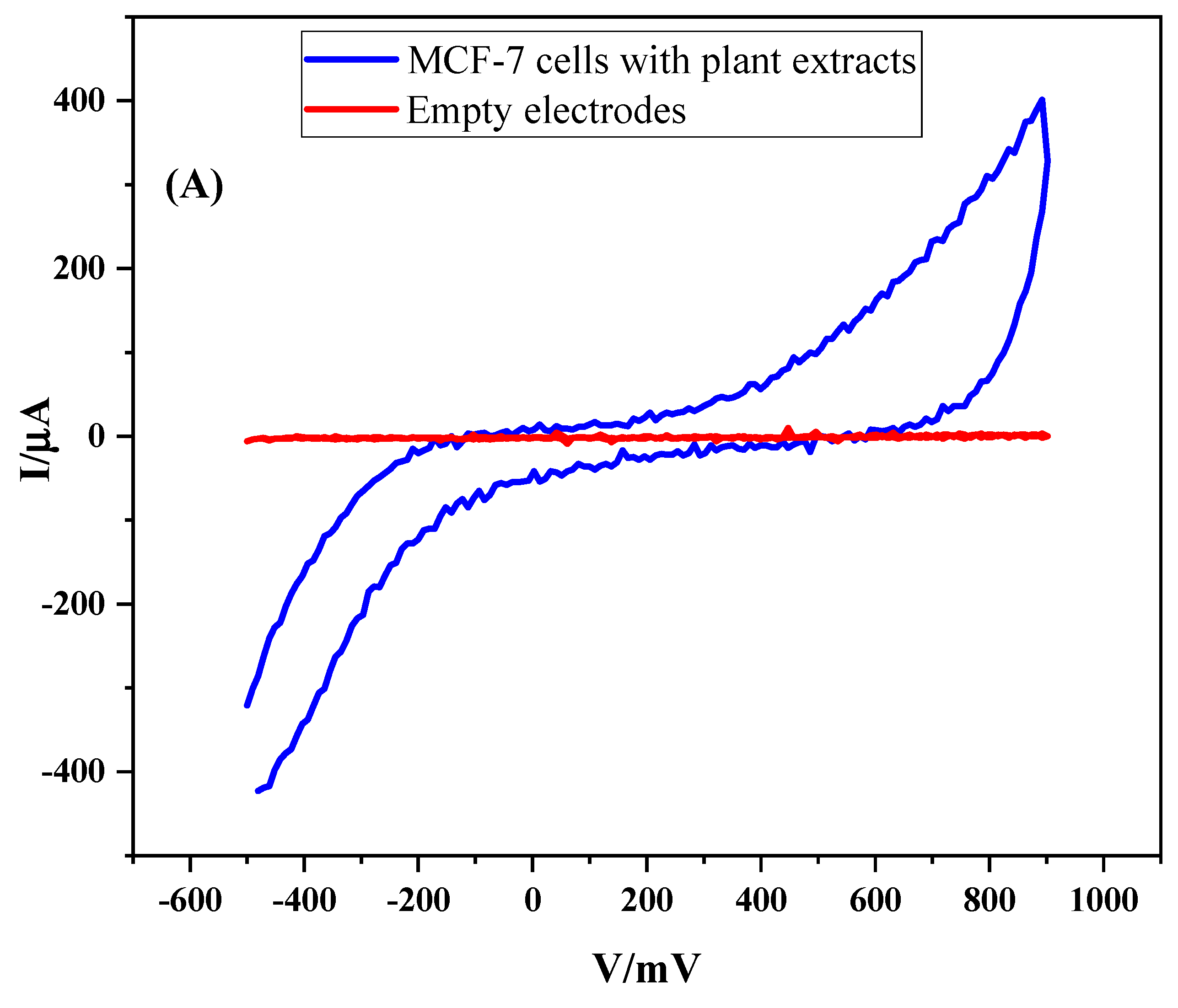
3.1.3. CV Response of the MC-7 Cells in the Presence of the Metabolites
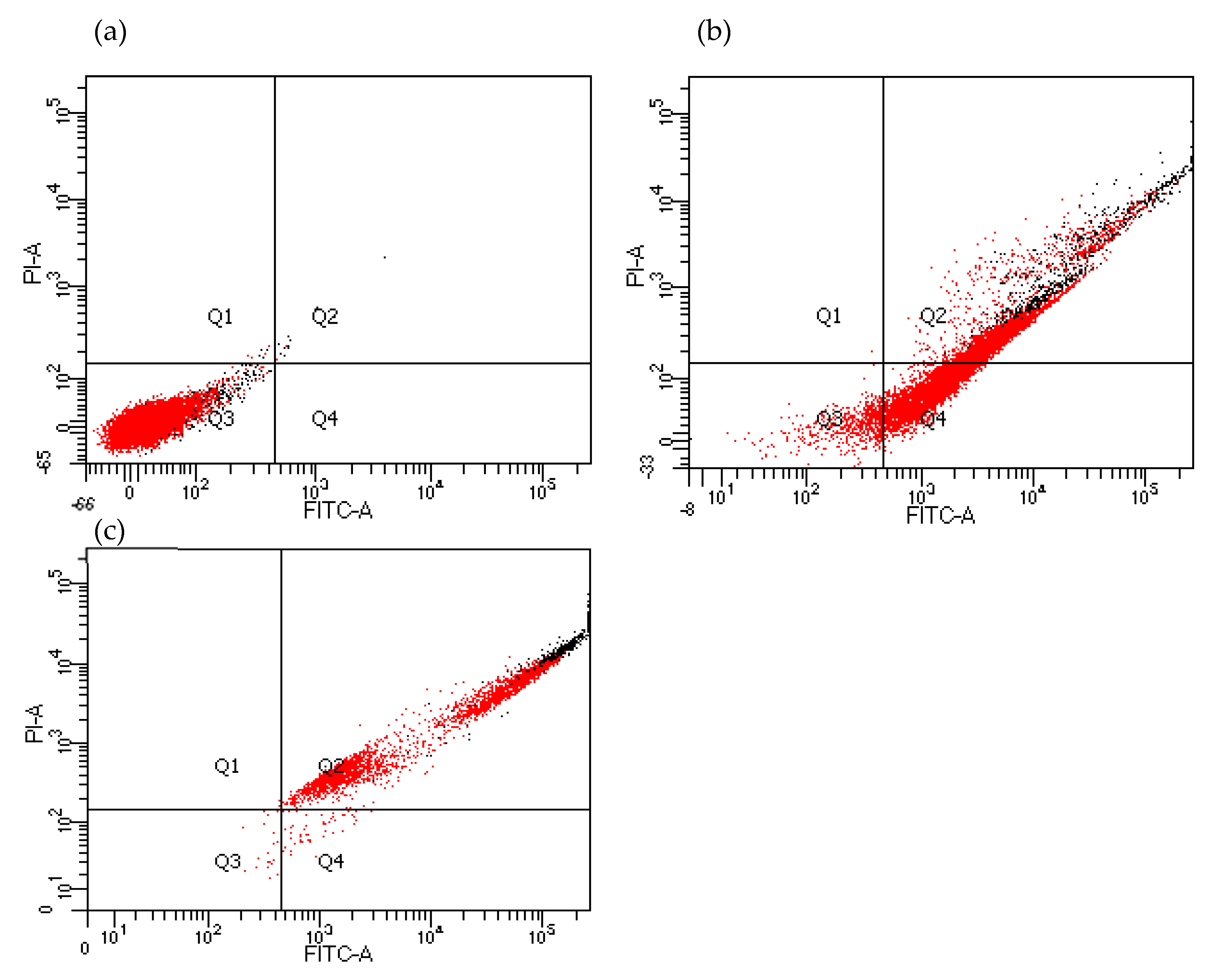
3.1.4. Flow Cytometry Analysis of the Active Metabolites on Cell Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saqib, Z.; Mahmood, A.; Malik, R.N.; Syed, J.H.; Ahmad, T. Indigenous knowledge of medicinal plants in Kotli Sattian, Rawalpindi district, Pakistan. J. Ethnopharmacol. 2014, 151, 820–828. [Google Scholar] [CrossRef]
- Pal, D.; Mandal, M.; Senthilkumar, G.; Padhiari, A. Antibacterial activity of Cuscuta reflexa stem and Corchorus olitorius seed. Fitoterapia 2006, 77, 589–591. [Google Scholar] [CrossRef]
- Bhandari, P.; Sendri, N.; Devidas, S.B. Dammarane triterpenoid glycosides in Bacopa monnieri: A review on chemical diversity and bioactivity. Phytochemistry 2020, 172, 112276. [Google Scholar] [CrossRef]
- Ge, Y.-W.; Wang, S.-M.; Zhang, L.-Y. Label-free small molecule probe and target discovery of traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi 2019, 44, 4152–4157. [Google Scholar] [PubMed]
- Benattia, F.K.; Arrar, Z.; Dergal, F.; Khabbal, Y. Pharmaco-Analytical Study and Phytochemical Profile of Hydroethanolic Extract of Algerian Prickly Pear (Opuntia ficus-indica.L). Curr. Pharm. Biotechnol. 2019, 20, 696–706. [Google Scholar] [CrossRef]
- Arthur, P.K.; Yeboah, A.B.; Issah, I.; Balapangu, S.; Kwofie, S.K.; Asimeng, B.O.; Foster, E.J.; Tiburu, E.K. Electrochemical Response of Saccharomyces cerevisiae Corresponds to Cell Viability upon Exposure to Dioclea reflexa Seed Extracts and Antifungal Drugs. Biosensor 2019, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Bittner, M.; Schenk, R.; Springer, A.; Melzig, M.F. Economical, Plain, and Rapid Authentication of Actaea racemosa L. (syn. Cimicifuga racemosa, Black Cohosh) Herbal Raw Material by Resilient RP-PDA-HPLC and Chemometric Analysis. Phytochem. Anal. 2016, 27, 318–325. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Wang, S.; Tao, S.; Haibo, W.; Wang, Y. Fabrication of enzyme-immobilized halloysite nanotubes for affinity enrichment of lipase inhibitors from complex mixtures. J. Chromatogr. A 2015, 1392, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, D.; Ji, L.; Li, J.; Liu, S.; Liu, X.; Jiang, S. A novel TiO2 nanotube array/Ti wire incorporated solid-phase microextraction fiber with high strength, efficiency and selectivity. J. Chromatogr. A 2010, 1217, 1898–1903. [Google Scholar] [CrossRef]
- Gianni, E.; Avgoustakis, K.; Papoulis, D. Kaolinite group minerals: Applications in cancer diagnosis and treatment. Eur. J. Pharm. Biopharm. 2020, 154, 359–376. [Google Scholar] [CrossRef]
- Saif, M.J.; Asif, H.M.; Naveed, M. PROPERTIES AND MODIFICATION METHODS OF HALLOYSITE NANOTUBES: A STATE-OF-THE-ART REVIEW. J. Chil. Chem. Soc. 2018, 63, 4109–4125. [Google Scholar] [CrossRef]
- Park, K.; Lee, J.; Chang, J.H.; Hwang, K.H.; Lee, Y. Characterization of Surface-Modified Halloysite Nanotubes by Thermal Treatment Under Reducing Atmosphere. J. Nanosci. Nanotechnol. 2020, 20, 4221–4226. [Google Scholar] [CrossRef]
- Tas, C.E.; Ozbulut, E.B.S.; Ceven, O.F.; Tas, B.A.; Unal, S.; Unal, H. Purification and Sorting of Halloysite Nanotubes into Homogeneous, Agglomeration-Free Fractions by Polydopamine Functionalization. ACS Omega 2020, 5, 17962–17972. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Jang, S.R.; Lee, G.M.; Ryu, J.H.; Park, S.I.; Park, N.H. Halloysite Nanocapsules Containing Thyme Essential Oil: Preparation, Characterization, and Application in Packaging Materials. J. Food Sci. 2017, 82, 2113–2120. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Wicklein, B.; Lo Dico, G.; Lazzara, G.; Del Real, G.; Aranda, P.; Ruiz-Hitzky, E. Functional biohybrid materials based on halloysite, sepiolite and cellulose nanofibers for health applications. Dalton Trans. 2020, 49, 3830–3840. [Google Scholar] [CrossRef]
- Tully, J.; Yendluri, R.; Lvov, Y. Halloysite Clay Nanotubes for Enzyme Immobilization. Biomacromolecules 2016, 17, 615–621. [Google Scholar] [CrossRef]
- Barman, M.; Mahmood, S.; Augustine, R.; Hasan, A.; Thomas, S.; Ghosal, K. Natural halloysite nanotubes /chitosan based bio-nanocomposite for delivering norfloxacin, an anti-microbial agent in sustained release manner. Int. J. Biol. Macromol. 2020, 162, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, M.A.; Gentile, P.; Ferreira, A.M.; Cometa, S.; De Giglio, E. Insight into halloysite nanotubes-loaded gellan gum hydrogels for soft tissue engineering applications. Carbohydr. Polym. 2017, 163, 280–291. [Google Scholar] [CrossRef]
- Bottino, M.C.; Yassen, G.H.; Platt, J.A.; Labban, N.; Windsor, L.J.; Spolnik, K.J.; Bressiani, A.H.A. A novel three-dimensional scaffold for regenerative endodontics: materials and biological characterizations. J. Tissue Eng. Regen. Med. 2013, 9, E116–E123. [Google Scholar] [CrossRef]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Milbeau, C.L.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of diclofenac onto organoclays: Effects of surfactant and environmental (pH and temperature) conditions. J. Hazard. Mater. 2017, 323 Pt A, 558–566. [Google Scholar] [CrossRef]
- Li, W.; Liu, D.; Zhang, H.; Correia, A.; Mäkilä, E.; Salonen, J.; Hirvonen, J.; Santos, H.A. Microfluidic assembly of a nano-in-micro dual drug delivery platform composed of halloysite nanotubes and a pH-responsive polymer for colon cancer therapy. Acta Biomater. 2017, 48, 238–246. [Google Scholar] [CrossRef]
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Pasbakhsh, P.; Milioto, S.; Lazzara, G. Why does vacuum drive to the loading of halloysite nanotubes? The key role of water confinement. J. Colloid Interface Sci. 2019, 547, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Junior, V.R.; Osterne, V.J.S.; Santiago, M.Q.; Correia, J.L.A.; Pereira-Junior, F.N.; Leal, R.B.; Pereira, M.G.; Chicas, L.S.; Nagano, C.S.; Rocha, B.A.M.; et al. Structural studies of a vasorelaxant lectin from Dioclea reflexa Hook seeds: Crystal structure, molecular docking and dynamics. Int. J. Biol. Macromol. 2017, 98, 12–23. [Google Scholar] [CrossRef]
- Pinto-Junior, V.R.; Correia, J.L.; Pereira, R.I.; Pereira-Junior, F.N.; Santiago, M.Q.; Osterne, V.J.; Madeira, J.C.; Cajazeiras, J.B.; Nagano, C.S.; Delatorre, P.; et al. Purification and molecular characterization of a novel mannose-specific lectin from Dioclea reflexa hook seeds with inflammatory activity. J. Mol. Recognit. 2016, 29, 134–141. [Google Scholar] [CrossRef]
- Ajatta, M.A.; Akinola, S.A.; Otolowo, D.T.; Awolu, O.O.; Omoba, O.S.; Osundahunsi, O.F. Effect of Roasting on the Phytochemical Properties of Three Varieties of Marble Vine (Dioclea reflexa) Using Response Surface Methodology. Prev. Nutr. Food Sci. 2019, 24, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Kazenel, M.R.; Debban, C.L.; Ranelli, L.; Hendricks, W.Q.; Chung, Y.A.; Pendergast, T.H.; Charlton, N.D.; Young, C.A.; Rudgers, J.A. A mutualistic endophyte alters the niche dimensions of its host plant. AoB Plants 2015, 7, plv005. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mazumder, U.K.; Pal, D.K.; Bhattacharya, S. Anti-steroidogenic activity of methanolic extract of Cuscuta reflexa roxb. stem and Corchorus olitorius Linn. seed in mouse ovary. Indian J. Exp. Boil. 2003, 41, 641–644. [Google Scholar]
- Mazumder, U.K.; Gupta, M.; Pal, D.; Bhattacharya, S. Chemical and toxicological evaluation of methanol extract of Cuscuta reflexa Roxb. stem and Corchorus olitorius Linn. seed on hematological parameters and hepatorenal functions in mice. Acta Pol. Pharm. Drug Res. 2004, 60, 317–323. [Google Scholar]
- Gupta, M.; Mazumder, U.K.; Pal, D.; Bhattacharya, S.; Chakrabarty, S. Studies on brain biogenic amines in methanolic extract of Cuscuta reflexa Roxb. and Corchorus olitorius Linn. seed treated mice. Acta Pol. Pharm. Drug Res. 2003, 60, 207–210. [Google Scholar]
- Gupta, M.; Mazumder, U.; Pal, D.; Bhattacharya, S. Onset of puberty and ovarian steroidogenesis following adminstration of methanolic extract of Cuscuta reflexa Roxb. stem and Corchorus olitorius Linn. seed in mice. J. Ethnopharmacol. 2003, 89, 55–59. [Google Scholar] [CrossRef]
- Munge, B.S.; Stracensky, T.; Gamez, K.; DiBiase, D.; Rusling, J.F. Multiplex Immunosensor Arrays for Electrochemical Detection of Cancer Biomarker Proteins. Electroanalysis 2016, 28, 2644–2658. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, M.R.; Deepak, F.L.; Garcia, C.D. Adsorption of Glucose Oxidase to 3-D Scaffolds of Carbon Nanotubes: Analytical Applications. Electroanalysis 2011, 23, 1462–1469. [Google Scholar] [CrossRef]
- Szigeti, Z.; Vigassy, T.; Bakker, E.; Pretsch, E. Approaches to Improving the Lower Detection Limit of Polymeric Membrane Ion-Selective Electrodes. Electroanalysis 2006, 18, 1254–1265. [Google Scholar] [CrossRef]
- de Oliveira, L.A.; Soares, R.O.; Buzzi, M.; Mourão, C.F.A.B.; Kawase, T.; Kuckelhaus, S.A.S. Cell and platelet composition assays by flow cytometry: basis for new platelet-rich fibrin methodologies. J. Biol. Regul. Homeost. Agents 2020, 34, 1379–1390. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, X.; Zhang, J.; Sun, B.; Zhengfeng, Y.; Jinling, Z.; Liu, S.; Sui, G.; Yin, Z. Microfluidic chip for isolation of viable circulating tumor cells of hepatocellular carcinoma for their culture and drug sensitivity assay. Cancer Biol. Ther. 2016, 17, 1177–1187. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Y.; Wei, C.; Yang, X.; Cheng, J.; Yang, Z.; Chen, C.; Ji, Z. Anti-proliferative and pro-apoptotic effects of cinobufagin on human breast cancer MCF-7 cells and its molecular mechanism. Nat. Prod. Res. 2018, 32, 493–497. [Google Scholar] [CrossRef]
- Sargazi, S.; Kooshkaki, O.; Reza, J.Z.; Saravani, R.; Jaliani, H.Z.; Mirinejad, S.; Meshkini, F. Mild antagonistic effect of Valproic acid in combination with AZD2461 in MCF-7 breast cancer cells. Med J. Islam. Repub. Iran 2019, 33, 175–180. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and sur- vival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015, 111, A3. B.1–A3. B.3. [Google Scholar] [CrossRef]
- Owoseni, O.; Nyankson, E.; Zhang, Y.; Adams, S.J.; He, J.; McPherson, G.L.; Bose, A.; Gupta, R.B.; John, V.T. Surfactant-loaded halloysite clay nanotube dispersants for crude oil spill remediation. Ind. Eng. Chem. Res. 2015, 54, 9328–9341. [Google Scholar]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-Ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2014, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Oladele Oladimeji, A.; Adebayo Oladosu, I.; Shaiq Ali, M.; Lateef, M. Dioclins A and B, new antioxidant flavonoids from Dioclea reflexa. Nat. Prod. Res. 2018, 32, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, X.; Wang, C.; Chen, L.; Xiao, Y.; Pang, Y. Bipolar and fixable probe targeting mitochondria to trace local depolarization via two-photon fluorescence lifetime imaging. Analyst 2015, 140, 5488–5494. [Google Scholar] [CrossRef] [PubMed]
- Kuralay, F.; Dükar, N.; Bayramlı, Y. Poly-L-lysine Coated Surfaces for Ultrasensitive Nucleic Acid Detection. Electroanalytical 2018, 30, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, H.A.; van Griensven, L.J. Flow cytometric evaluation of the effects of 3-bromopyruvate (3BP) and dichloracetate (DCA) on THP-1 cells: a multiparameter analysis. J. Bioenergy Biomembr. 2012, 44, 91–99. [Google Scholar] [CrossRef]


| Treatment | Normalized | |
|---|---|---|
| Sample/Dry Extract | IC50 (mg) | R Squared Value |
| Water | 33.3 | 0.959 |
| Ethanol | 1.6 | 0.991 |
| pH 4.1 | 1.4 | 0.967 |
| pH 5.2 | 0.8 | 0.998 |
| pH 6.4 | 1.6 | 0.975 |
| pH7.4 | 1.9 | 0.983 |
| pH 8.1 | 2.3 | 0.948 |
| pH 9.6 | 3.1 | 0.994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balapangu, S.; Nyankson, E.; Asimeng, B.O.; Asiamah, R.; Arthur, P.K.; Tiburu, E.K. Capturing Dioclea Reflexa Seed Bioactives on Halloysite Nanotubes and pH Dependent Release of Cargo against Breast (MCF-7) Cancers In Vitro. Separations 2021, 8, 26. https://doi.org/10.3390/separations8030026
Balapangu S, Nyankson E, Asimeng BO, Asiamah R, Arthur PK, Tiburu EK. Capturing Dioclea Reflexa Seed Bioactives on Halloysite Nanotubes and pH Dependent Release of Cargo against Breast (MCF-7) Cancers In Vitro. Separations. 2021; 8(3):26. https://doi.org/10.3390/separations8030026
Chicago/Turabian StyleBalapangu, Srinivasan, Emmanuel Nyankson, Bernard O. Asimeng, Richard Asiamah, Patrick K. Arthur, and Elvis K. Tiburu. 2021. "Capturing Dioclea Reflexa Seed Bioactives on Halloysite Nanotubes and pH Dependent Release of Cargo against Breast (MCF-7) Cancers In Vitro" Separations 8, no. 3: 26. https://doi.org/10.3390/separations8030026
APA StyleBalapangu, S., Nyankson, E., Asimeng, B. O., Asiamah, R., Arthur, P. K., & Tiburu, E. K. (2021). Capturing Dioclea Reflexa Seed Bioactives on Halloysite Nanotubes and pH Dependent Release of Cargo against Breast (MCF-7) Cancers In Vitro. Separations, 8(3), 26. https://doi.org/10.3390/separations8030026







