Living with Breakthrough: Two-Dimensional Liquid-Chromatography Separations of a Water-Soluble Synthetically Grafted Bio-Polymer
Abstract
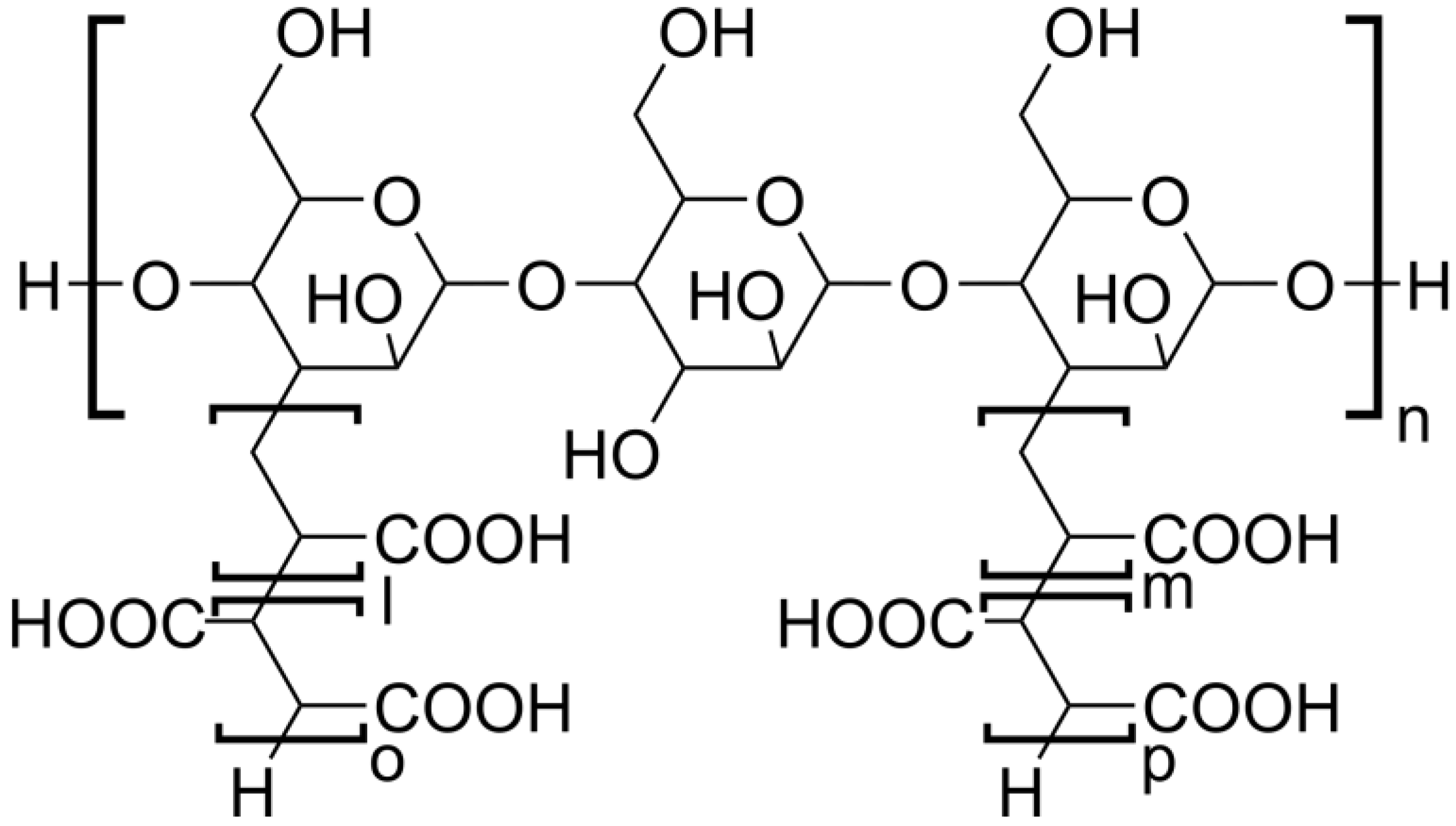
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumental
2.2.1. One-Dimensional Separations
Aqueous Size-Exclusion Chromatography
Hydrophilic-Interaction and Reversed-Phase Chromatography
Porous Graphitic Carbon Chromatography (PGC)
2.2.2. Two-Dimensional Separations
Aqueous Size-Exclusion Chromatography × Reversed Phase Chromatography and Porous Graphitic Carbon Chromatography × Reversed Phase Chromatography
Hydrophilic-Interaction × Reversed-Phase Chromatography
2.3. Identification
2.3.1. Mass Spectrometry Identification
2.3.2. Infrared-Spectroscopy Identification
2.4. Procedures
2.4.1. Sample Preparation
Preparation for Chromatography
Preparation for Off-Line Analysis
3. Results and Discussion
3.1. One-Dimensional Separations
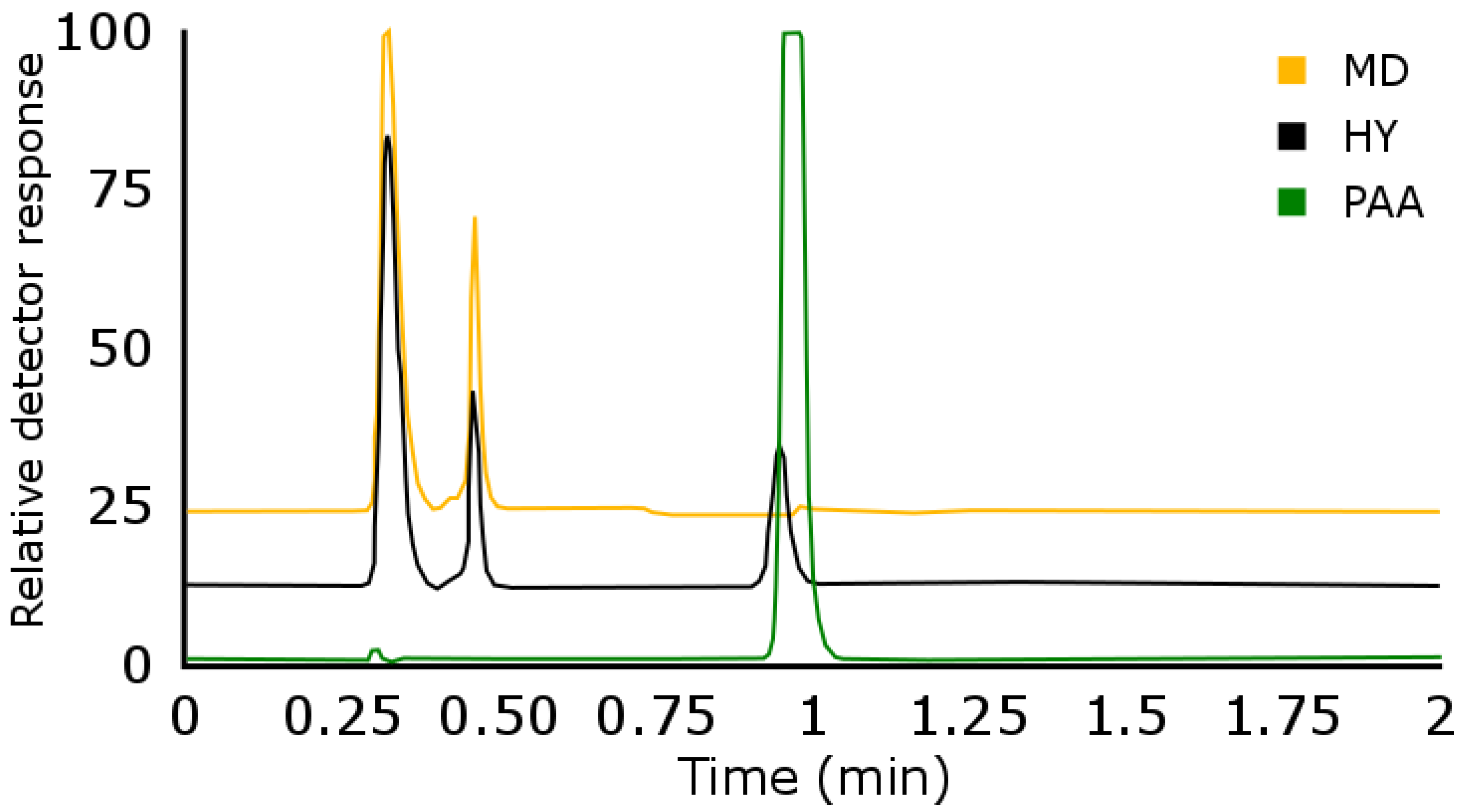
3.1.1. Aqueous Size-Exclusion Chromatography (aq-SEC)
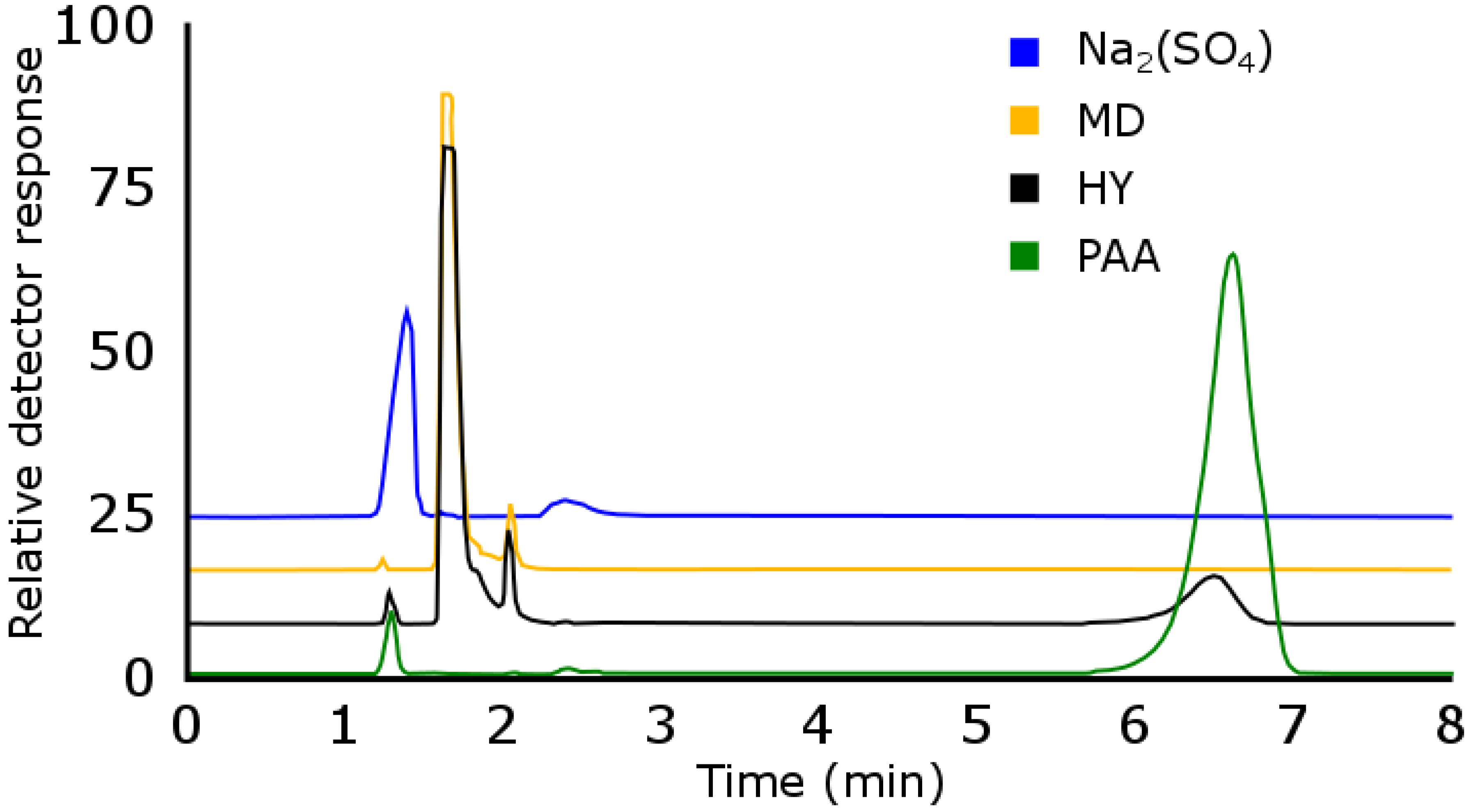
3.1.2. Reversed-Phase Liquid Chromatography (RPLC)
Extended Polar Selectivity C18 Reversed-Phase Liquid Chromatography (EPS RPLC)
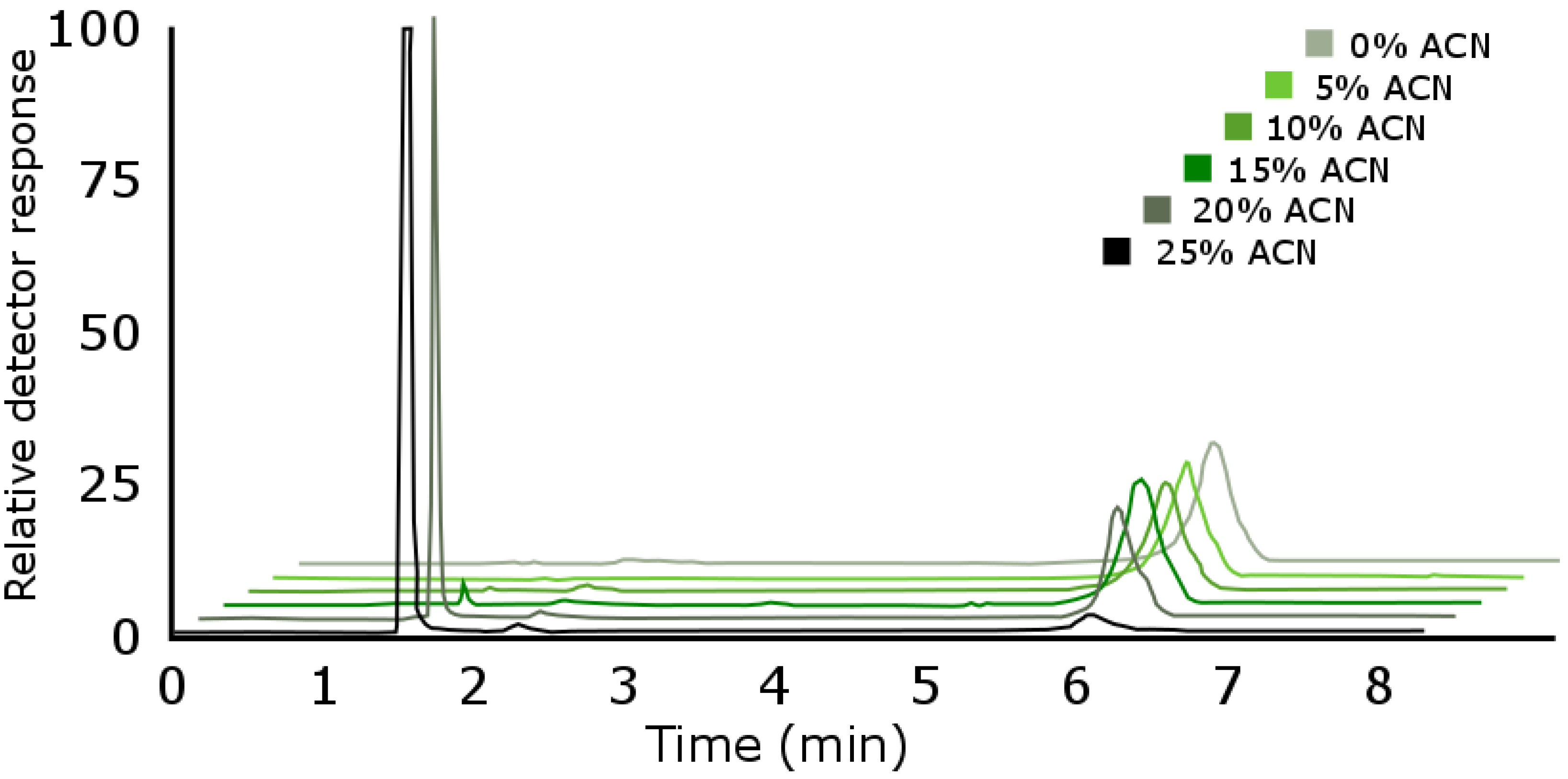
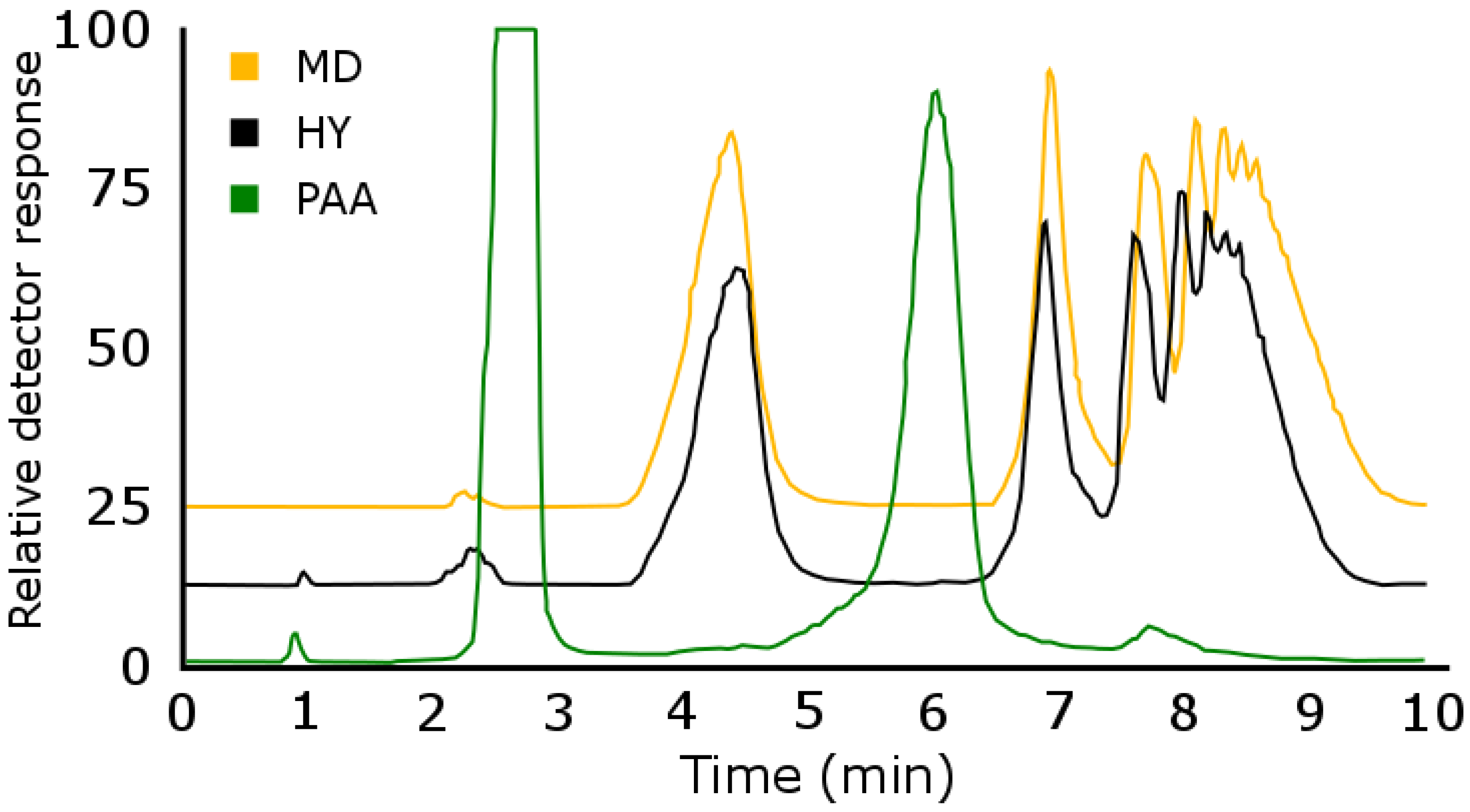
Breakthrough in Extended Polar Selectivity C18 Reversed-Phase Liquid Chromatography
Titan C18 Reversed Phase-Chromatography for 2D
3.1.3. Hydrophilic-Interaction Liquid Chromatography (HILIC)
3.2. LC × LC Separations
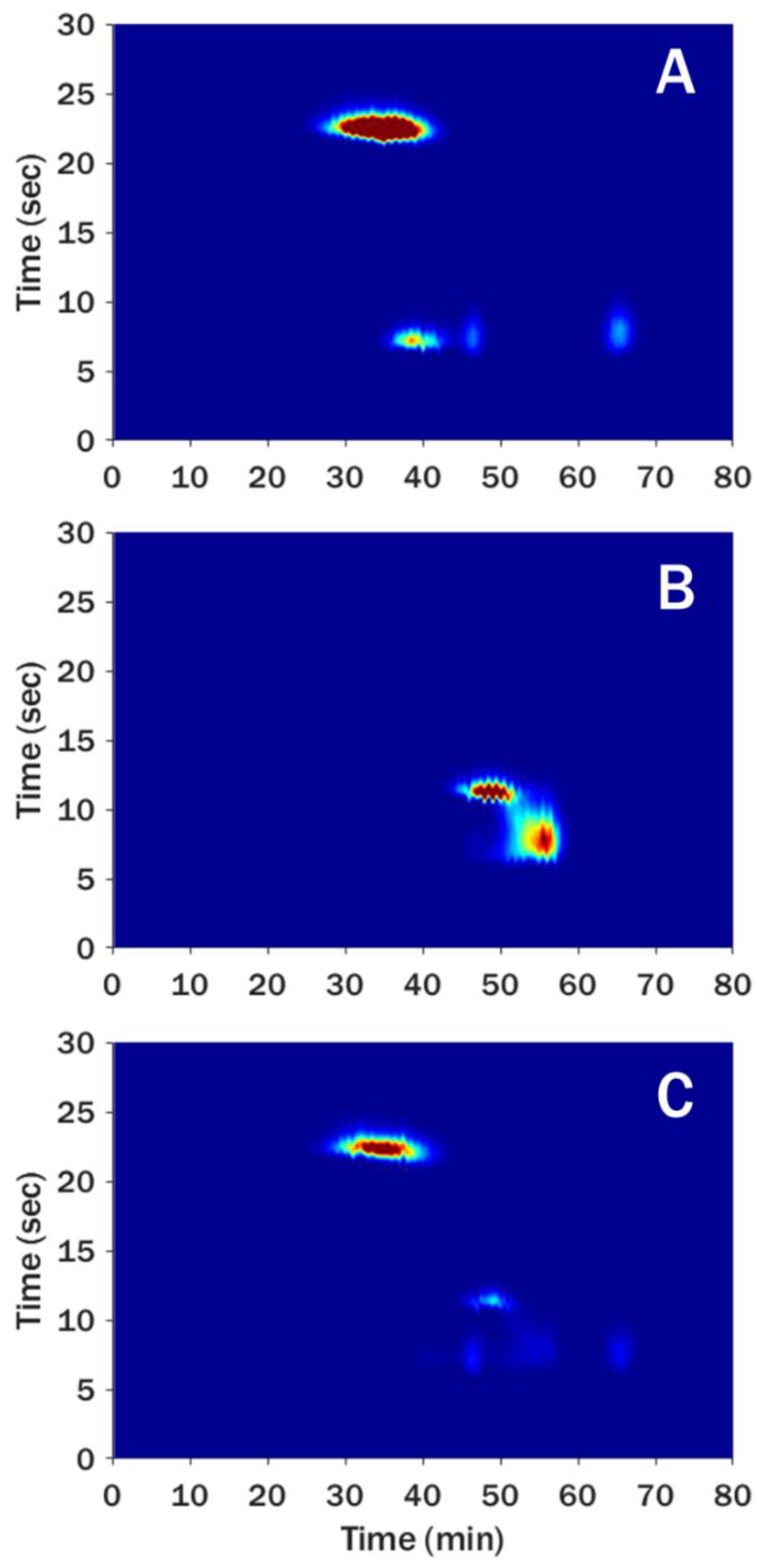
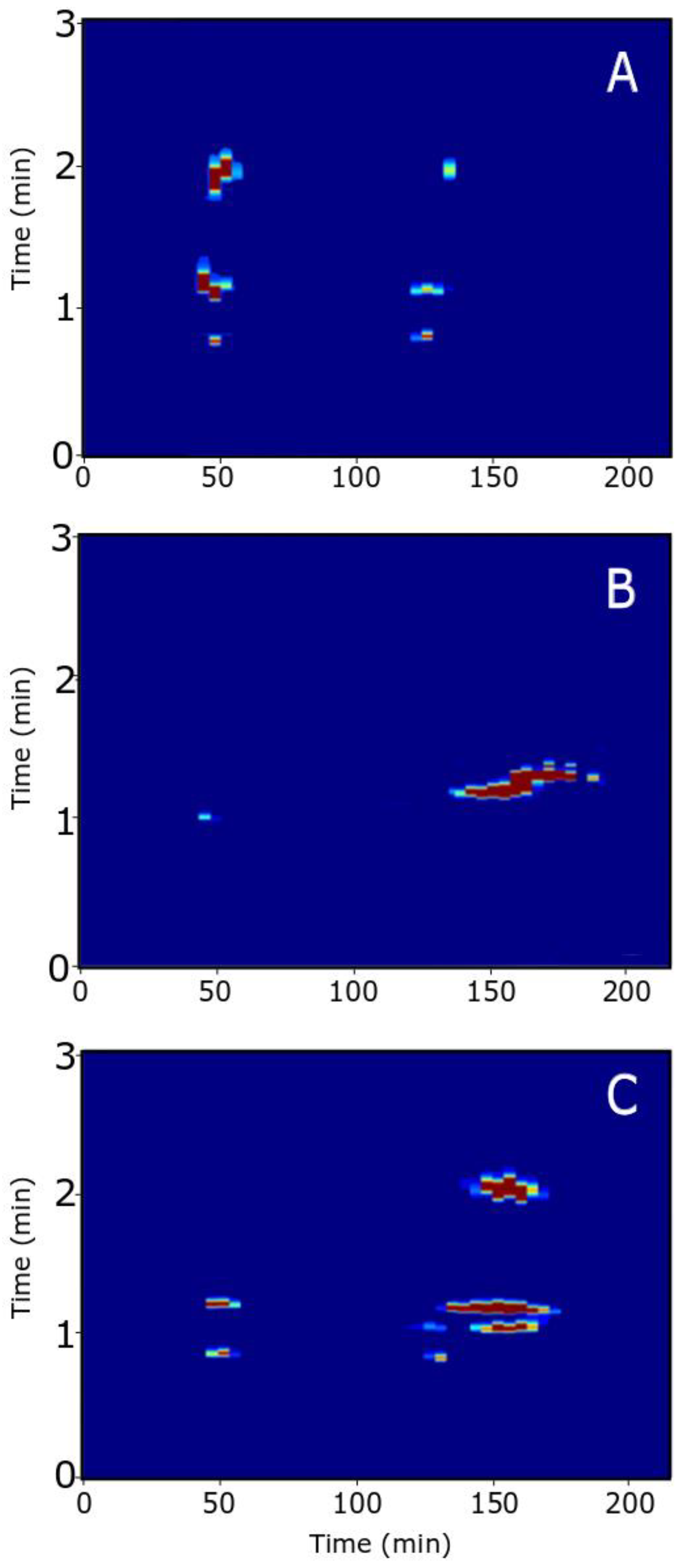
3.2.1. Aqueous-SEC × RPLC
3.2.2. HILIC × RPLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giddings, J. Sample dimensionality: A predictor of order-disorder in component peak distribution in multidimensional separation. J. Chromatogr. A 1995, 703, 3–15. [Google Scholar] [CrossRef]
- Pirok, B.W.; Stoll, D.R.; Schoenmakers, P.J. Recent developments in two-dimensional liquid chromatography: Fundamental improvements for practical applications. Anal. Chem. 2018, 91, 240–263. [Google Scholar] [CrossRef] [PubMed]
- Pirok, B.W.; Gargano, A.; Schoenmakers, P.J. Optimizing separations in online comprehensive two-dimensional liquid chromatography. J. Sep. Sci. 2017, 41, 68–98. [Google Scholar] [CrossRef]
- Pirok, B.W.; Pous-Torres, S.; Ortiz-Bolsico, C.; Vivó-Truyols, G.; Schoenmakers, P. Program for the interpretive optimization of two-dimensional resolution. J. Chromatogr. A 2016, 1450, 29–37. [Google Scholar] [CrossRef]
- Groeneveld, G.; Pirok, B.W.J.; Schoenmakers, P. Perspectives on the future of multi-dimensional platforms. Faraday Discuss. 2019, 218, 72–100. [Google Scholar] [CrossRef]
- Schure, M.R.; Davis, J.M. Orthogonal separations: Comparison of orthogonality metrics by statistical analysis. J. Chromatogr. A 2015, 1414, 60–76. [Google Scholar] [CrossRef]
- Jiang, X.; Van Der Horst, A.; Schoenmakers, P. Breakthrough of polymers in interactive liquid chromatography. J. Chromatogr. A 2002, 982, 55–68. [Google Scholar] [CrossRef]
- Moreno, F.J.; Montilla, A.; Villamiel, M.; Corzo, N.; Olano, A. Analysis, structural characterization, and bioactivity of oligosaccharides derived from lactose. Electrophoresis 2014, 35, 1519–1534. [Google Scholar] [CrossRef]
- Nagy, G.; Peng, T.; Pohl, N.L.B. Recent liquid chromatographic approaches and developments for the separation and purification of carbohydrates. Anal. Methods 2017, 9, 3579–3593. [Google Scholar] [CrossRef]
- Ortiz, A.M.; Matute, A.I.R.; Sanz, M.L.; Moreno, F.J.; Herrero, M. Separation of di- and trisaccharide mixtures by comprehensive two-dimensional liquid chromatography. Application to prebiotic oligosaccharides. Anal. Chim. Acta 2019, 1060, 125–132. [Google Scholar] [CrossRef]
- Al Samman, M.; Radke, W.; Khalyavina, A.; Lederer, A. Retention behavior of linear, branched, and hyperbranched polyesters in interaction liquid chromatography. Macromolecules 2010, 43, 3215–3220. [Google Scholar] [CrossRef]
- Loiseau, J.; Doerr, N.; Suau, J.M.; Egraz, J.B.; Llauro, M.F.; Ladavière, C.; Claverie, J.P. Synthesis and characterization of poly(acrylic acid) produced by raft polymerization. application as a very efficient dispersant of CaCO3, kaolin, and TiO2. Macromolecules 2003, 36, 3066–3077. [Google Scholar] [CrossRef]
- Viktor, Z.; Farcet, C.; Moire, C.; Brothier, F.; Pfukwa, H.; Pasch, H. Comprehensive two-dimensional liquid chromatography for the characterization of acrylate-modified hyaluronic acid. Anal. Bioanal. Chem. 2019, 411, 3321–3330. [Google Scholar] [CrossRef]
- Gaborieau, M.; Castignolles, P. Size-exclusion chromatography (SEC) of branched polymers and polysaccharides. Anal. Bioanal. Chem. 2010, 399, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Dong, K.; Zhu, M.; Zhang, Q.; Xie, B.; Li, D.; Gan, H.; Linhardt, R.J.; Zhang, Z. Development of a method to analyze the complexes of enoxaparin and platelet factor 4 with size-exclusion chromatography. J. Pharm. Biomed. Anal. 2019, 164, 668–671. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zeng, Y.; Rong, Y.; Song, Y.; Shi, L.; Chen, B.; Yang, X.; Xu, N.; Linhardt, R.J.; Zhang, Z. Profiling analysis of low molecular weight heparins by multiple heart-cutting two dimensional chromatography with quadruple time-of-flight mass spectrometry. Anal. Chem. 2015, 87, 8957–8963. [Google Scholar] [CrossRef] [PubMed]
- Witono, J.R.; Marsman, J.; Noordergraaf, W.; Heeres, H.; Janssen, L.P.B.M. Improved homopolymer separation to enable the application of 1H NMR and HPLC for the determination of the reaction parameters of the graft copolymerization of acrylic acid onto starch. Carbohydr. Res. 2013, 370, 38–45. [Google Scholar] [CrossRef]
- Yang, P.; Gao, W.; Zhang, T.; Pursch, M.; Luong, J.; Sattler, W.; Singh, A.; Backer, S. Two-dimensional liquid chromatography with active solvent modulation for studying monomer incorporation in copolymer dispersants. J. Sep. Sci. 2019, 42, 2805–2815. [Google Scholar] [CrossRef]
- Stoll, D.R.; Talus, E.S.; Harmes, D.C.; Zhang, K. Evaluation of detection sensitivity in comprehensive two-dimensional liquid chromatography separations of an active pharmaceutical ingredient and its degradants. Anal. Bioanal. Chem. 2014, 407, 265–277. [Google Scholar] [CrossRef]
- Schmitz, S.; Dona, A.C.; Castignolles, P.; Gilbert, R.G.; Gaborieau, M. Assessment of the extent of starch dissolution in dimethyl sulfoxide by1H NMR spectroscopy. Macromol. Biosci. 2009, 9, 506–514. [Google Scholar] [CrossRef]
- Potts, L.W.; Stoll, D.R.; Li, X.; Carr, P.W. The impact of sampling time on peak capacity and analysis speed in on-line comprehensive two-dimensional liquid chromatography. J. Chromatogr. A 2010, 1217, 5700–5709. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, F.; Zaia, J.; Linhardt, R.J. Top-down approach for the direct characterization of low molecular weight heparins using LC-FT-MS. Anal. Chem. 2012, 84, 8822–8829. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Steppich, J.; Wang, Z.; Sun, Y.; Xue, C.; Linhardt, R.J.; Li, L. Bottom-up low molecular weight heparin analysis using liquid chromatography-fourier transform mass spectrometry for extensive characterization. Anal. Chem. 2014, 86, 6626–6632. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Ibáñez, E.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M. Focusing and non-focusing modulation strategies for the improvement of on-line two-dimensional hydrophilic interaction chromatography × reversed phase profiling of complex food samples. Anal. Chim. Acta 2017, 985, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Sánchez-Camargo, A.P.; Garcia-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E.; et al. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, G.; Dunkle, M.N.; Rinken, M.; Gargano, A.; De Niet, A.; Pursch, M.; Mes, E.P.; Schoenmakers, P. Characterization of complex polyether polyols using comprehensive two-dimensional liquid chromatography hyphenated to high-resolution mass spectrometry. J. Chromatogr. A 2018, 1569, 128–138. [Google Scholar] [CrossRef]
- Van De Ven, H.; Gargano, A.; Van Der Wal, S.; Schoenmakers, P. Switching solvent and enhancing analyte concentrations in small effluent fractions using in-column focusing. J. Chromatogr. A 2016, 1427, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wach, W.; Fornefett, I.; Buttersack, C.; Buchholz, K. Adsorption and HPLC of carbohydrates and related hydroxy compounds on zeolites. Anal. Methods 2018, 10, 1817–1832. [Google Scholar] [CrossRef]
- Stoll, D.R.; Shoykhet, K.; Petersson, P.; Buckenmaier, S. Active solvent modulation: A Valve-based approach to improve separation compatibility in two-dimensional liquid chromatography. Anal. Chem. 2017, 89, 9260–9267. [Google Scholar] [CrossRef] [PubMed]
- Vonk, R.J.; Gargano, A.; Davydova, E.; Dekker, H.L.; Eeltink, S.; De Koning, L.J.; Schoenmakers, P. Comprehensive two-dimensional liquid chromatography with stationary-phase-assisted modulation coupled to high-resolution mass spectrometry applied to proteome analysis of saccharomyces cerevisiae. Anal. Chem. 2015, 87, 5387–5394. [Google Scholar] [CrossRef]
- Ianovska, M.A.; Mulder, P.P.M.F.A.; Verpoorte, E. Development of small-volume, microfluidic chaotic mixers for future application in two-dimensional liquid chromatography. RSC Adv. 2017, 7, 9090–9099. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van de Ven, H.C.; Purmova, J.; Groeneveld, G.; Bos, T.S.; Gargano, A.F.G.; van der Wal, S.; Mengerink, Y.; Schoenmakers, P.J. Living with Breakthrough: Two-Dimensional Liquid-Chromatography Separations of a Water-Soluble Synthetically Grafted Bio-Polymer. Separations 2020, 7, 41. https://doi.org/10.3390/separations7030041
van de Ven HC, Purmova J, Groeneveld G, Bos TS, Gargano AFG, van der Wal S, Mengerink Y, Schoenmakers PJ. Living with Breakthrough: Two-Dimensional Liquid-Chromatography Separations of a Water-Soluble Synthetically Grafted Bio-Polymer. Separations. 2020; 7(3):41. https://doi.org/10.3390/separations7030041
Chicago/Turabian Stylevan de Ven, H.C., J. Purmova, G. Groeneveld, Tijmen S. Bos, A.F.G. Gargano, Sj. van der Wal, Y. Mengerink, and Peter J. Schoenmakers. 2020. "Living with Breakthrough: Two-Dimensional Liquid-Chromatography Separations of a Water-Soluble Synthetically Grafted Bio-Polymer" Separations 7, no. 3: 41. https://doi.org/10.3390/separations7030041
APA Stylevan de Ven, H. C., Purmova, J., Groeneveld, G., Bos, T. S., Gargano, A. F. G., van der Wal, S., Mengerink, Y., & Schoenmakers, P. J. (2020). Living with Breakthrough: Two-Dimensional Liquid-Chromatography Separations of a Water-Soluble Synthetically Grafted Bio-Polymer. Separations, 7(3), 41. https://doi.org/10.3390/separations7030041





