Study of the Oxidative Forced Degradation of Glutathione in Its Nutraceutical Formulations Using Zone Fluidics and Green Liquid Chromatography
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Reagents and Solutions
2.3. HPLC Conditions
2.4. ZF Conditions
2.5. Oxidative Forced Degradation of GSH
3. Results and Discussion
3.1. HPLC Method
3.2. OPA-Based Zone Fluidics Method
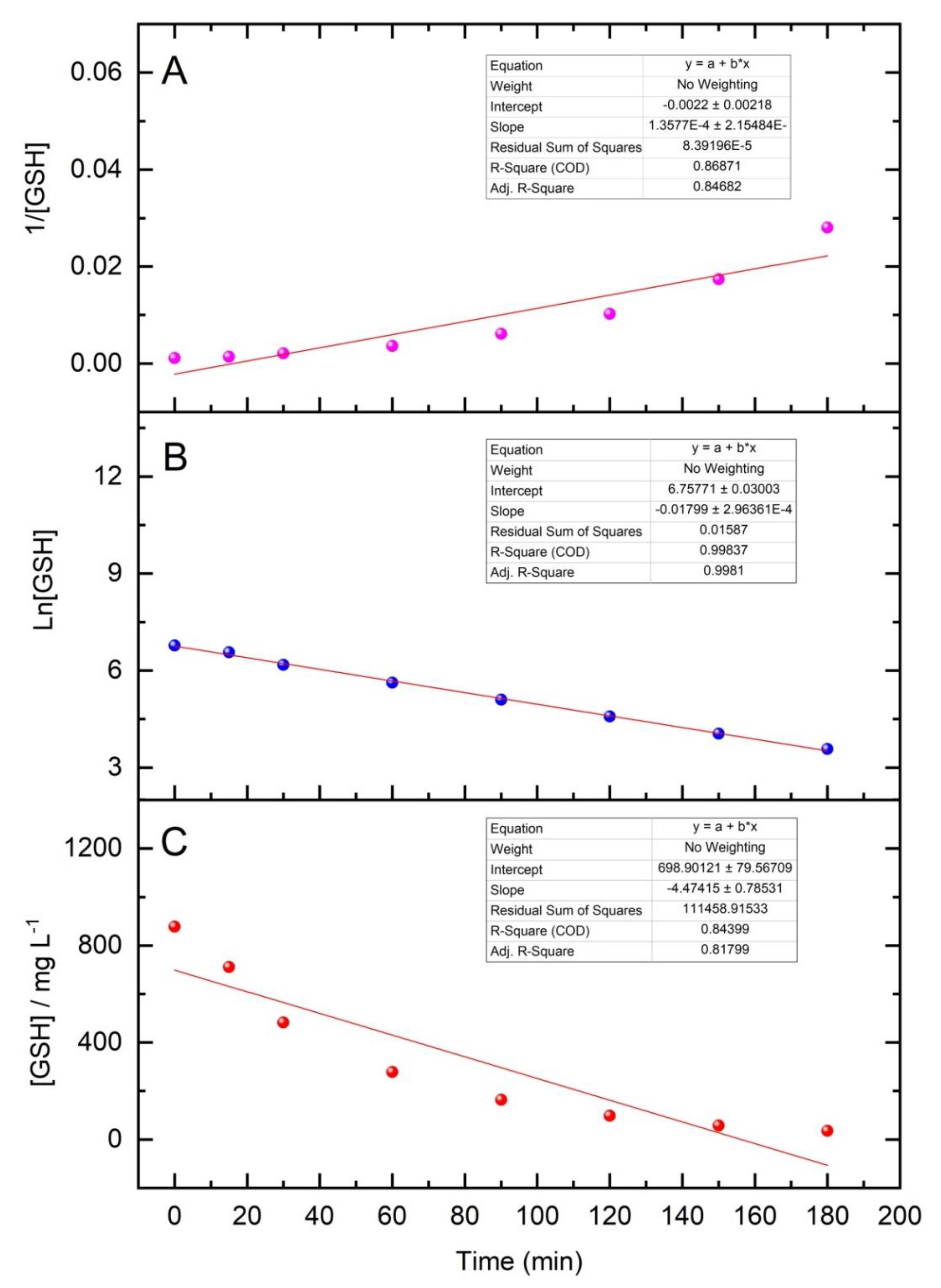
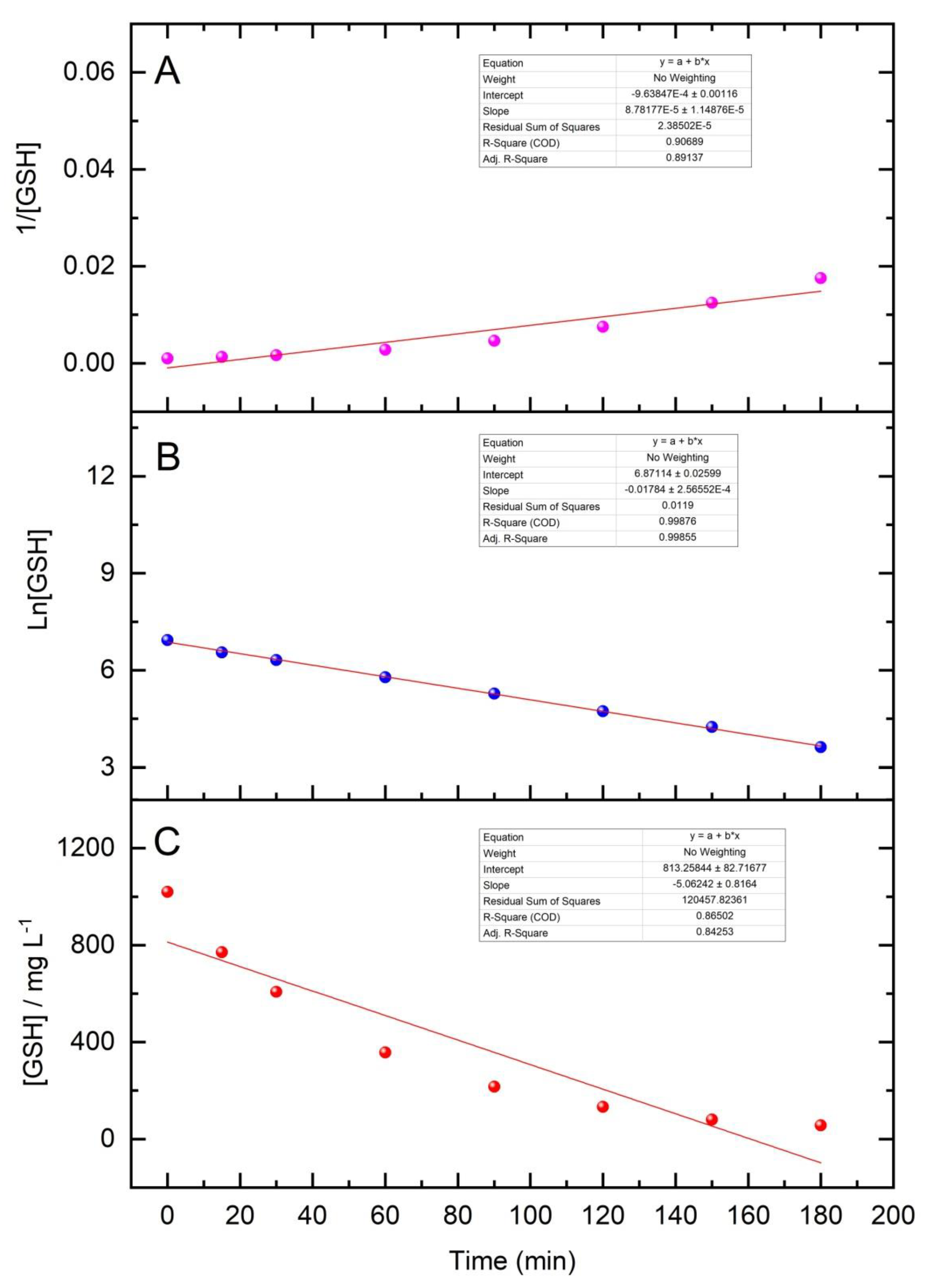
3.3. Oxidative Forced Degradation Results and Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rawat, T.; Pandey, I.P. Forced degradation studies for drug substances and drug products- scientific and regulatory considerations. J. Pharm. Sci. Res. 2015, 7, 238–241. [Google Scholar]
- FDA. Guidance for Industry Q1A(R2) Stability Testing of New Drug Substances and Products; ICH: Geneva, Switzerland, 2003.
- Snape, T.J.; Astles, A.M.; Davies, J. Understanding the chemical basis of drug stability and degradation. Pharm. J. 2010, 285, 416–417. [Google Scholar]
- Basak, A.K.; Raw, A.S.; Yu, L.X.; Alsante, K.M.; Ando, A.; Brown, R.; Ensing, J.; Hatajik, T.D.; Kong, W.; Tsuda, Y. The role of degradant profiling in active pharmaceutical ingredients and drug products. Adv. Drug Deliv. Rev. 2007, 59, 29–37. [Google Scholar]
- Camera, E.; Picardo, M. Analytical methods to investigate glutathione and related compounds in biological and pathological processes. J. Chromatogr. B 2002, 781, 181–206. [Google Scholar] [CrossRef]
- Tsiasioti, A.; Iakovidou, I.; Zacharis, C.K.; Tzanavaras, P.D. Automated fluorimetric sensor for glutathione based on zone fluidics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019. [Google Scholar] [CrossRef] [PubMed]
- Begic, A.; Djuric, A.; Gobeljic, B.; Stevanovic, I.; Lukic, V.; Stanojevic, I.; Ninkovic, M.; Saso, L.; Vojvodic, D.; Djukic, M. The simple isocratic HPLC—UV method for the simultaneous determination of reduced and oxidized glutathione in animal tissue. Acta Chromatogr. 2017, 29, 67–84. [Google Scholar] [CrossRef]
- Tzanavaras, P.D.; Papadimitriou, S.; Zacharis, C.K. Automated Stopped-Flow Fluorimetric Sensor for Biologically Active Adamantane Derivatives Based on Zone Fluidics. Molecules 2019, 24, 3975. [Google Scholar] [CrossRef] [PubMed]
- Zacharis, C.K.; Tzanavaras, P.D.; Zotou, A. Ethyl propiolate as a post-column derivatization reagent for thiols: Development of a green liquid chromatographic method for the determination of glutathione in vegetables. Anal. Chim. Acta 2011, 690, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.; Magerus, T.; Langland, J.O. The use of intravenous glutathione for symptom management of Parkinson’s disease: A case report. Altern. Ther. Health Med. 2018, 24, 56–60. [Google Scholar] [PubMed]
- Dawson, R.M.C. Data for Biochemical Research, 3rd ed.; Oxford science publications: New York, NY, USA, 1987. [Google Scholar]
- Krezel, A.; Bal, W. Coordination chemistry of glutathione. Acta Biochim. Pol. 1999, 46, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Bidlingmeyer, B.; Wang, Q. Additives for Reversed-Phase Hplc Mobile Phases. U.S. Patent 7,125,492 B2, 24 October 2006. [Google Scholar]
- Gritti, F.; Gilar, M.; Walter, T.H.; Wyndham, K. Retention loss of reversed-phase chromatographic columns using 100% aqueous mobile phases from fundamental insights to best practice. J. Chromatogr. A 2020, 1612. [Google Scholar] [CrossRef] [PubMed]
- Alltech® PrevailTM Introduction. Available online: www.discoverysciences.com (accessed on 12 December 2019).
- Przybyciel, M.; Majors, R.E. Phase Collapse in Reversed-Phase LC. LC GC Eur. 2002, 15, 1–5. [Google Scholar]
- Tang, D.; Shafer, M.M.; Vang, K.; Karner, D.A.; Armstrong, D.E. Determination of dissolved thiols using solid-phase extraction and liquid chromatographic determination of fluorescently derivatized thiolic compounds. J. Chromatogr. A 2003, 998, 31–40. [Google Scholar] [CrossRef]
- Angeli, V.; Chen, H.; Mester, Z.; Rao, Y.; D’Ulivo, A.; Bramanti, E. Derivatization of GSSG by pHMB in alkaline media. Determination of oxidized glutathione in blood. Talanta 2010, 82, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Matsuda, R.; Ito, K.; Nishimura, W.; Imai, K.; Maeda, M. Detection limit estimated from slope of calibration curve: An application to competitive ELISA. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2005, 21, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Moussa, B.A.; Hashem, H.M.A.; Mahrouse, M.A.; Mahmoud, S.T. Experimental Design Approach in HPLC Method Development: Application for the Simultaneous Determination of Sacubitril and Valsartan in Presence of Their Impurities and Investigation of Degradation Kinetics. Chromatographia 2018, 81, 139–156. [Google Scholar] [CrossRef]
- Drug Stability and Kinetics. Available online: https://www.scribd.com/doc/76640703/Drug-Stability-and-Kinetics (accessed on 21 February 2020).
- Abedinzadeh, Z.; Gardes-Albert, M.; Ferradini, C. Kinetic study of the oxidation mechanism of glutathione by hydrogen peroxide in neutral aqueous medium. Can. J. Chem. 1989, 67, 1247–1255. [Google Scholar] [CrossRef]
- Finley, J.W.; Wheeler, E.L.; Witt, S.C. Oxidation of glutathione by hydrogen peroxide and other oxidizing agents. J. Agric. Food Chem. 1981, 29, 404–407. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.A.; da Silva, G.D.; Campos, M.S.T. Chemical degradation kinetics of fibrates: Bezafibrate, ciprofibrate and fenofibrate. Braz. J. Pharm. Sci. 2016, 52, 545–553. [Google Scholar] [CrossRef]
- Zar, J. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper New Jersey River, NJ, USA, 1999. [Google Scholar]
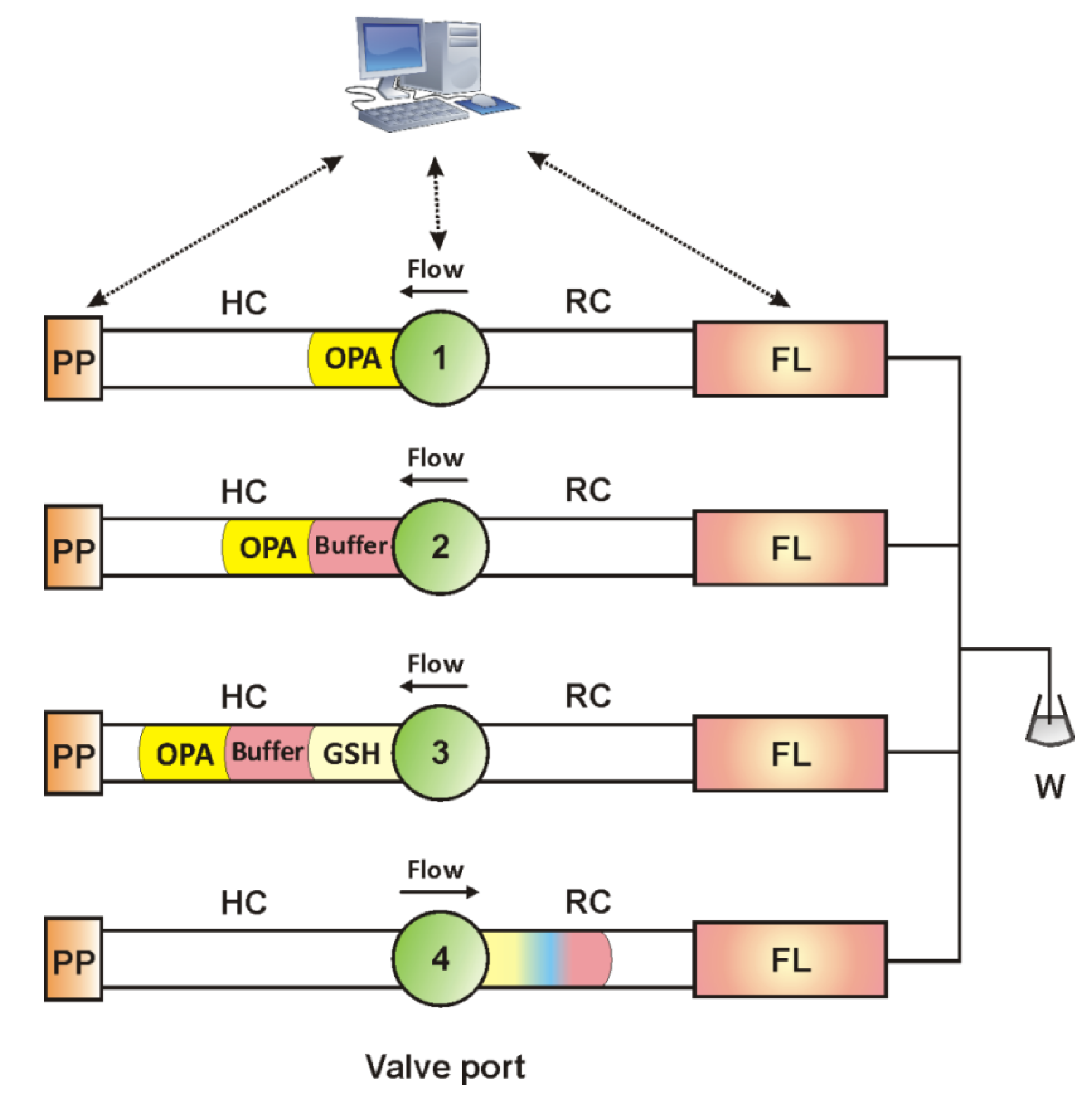

| Time (s) | Pump Action | Flow Rate | Volume (μL) | Valve Position | Action Description |
|---|---|---|---|---|---|
| (mL min−1) | |||||
| 0 | Off | — | — | 1 | Selection of OPA reagent port |
| 10 | Aspirate | 0.6 | 100 | 1 | Aspiration of OPA in the holding coil |
| 1 | Off | — | — | 2 | Selection of buffer port |
| 7 | Aspirate | 0.6 | 70 | 2 | Aspiration of buffer in the holding coil |
| 1 | Off | — | — | 3 | Selection of sample port |
| 15 | Aspirate | 0.6 | 150 | 3 | Aspiration of sample in the holding coil |
| 1 | Off | — | — | Selection of detector port | |
| 120 | Deliver | 0.6 | 1200 | 4 | Propulsion of reaction mixture to detector |
| 0 | Off | — | — | 4 | End of measuring cycle |
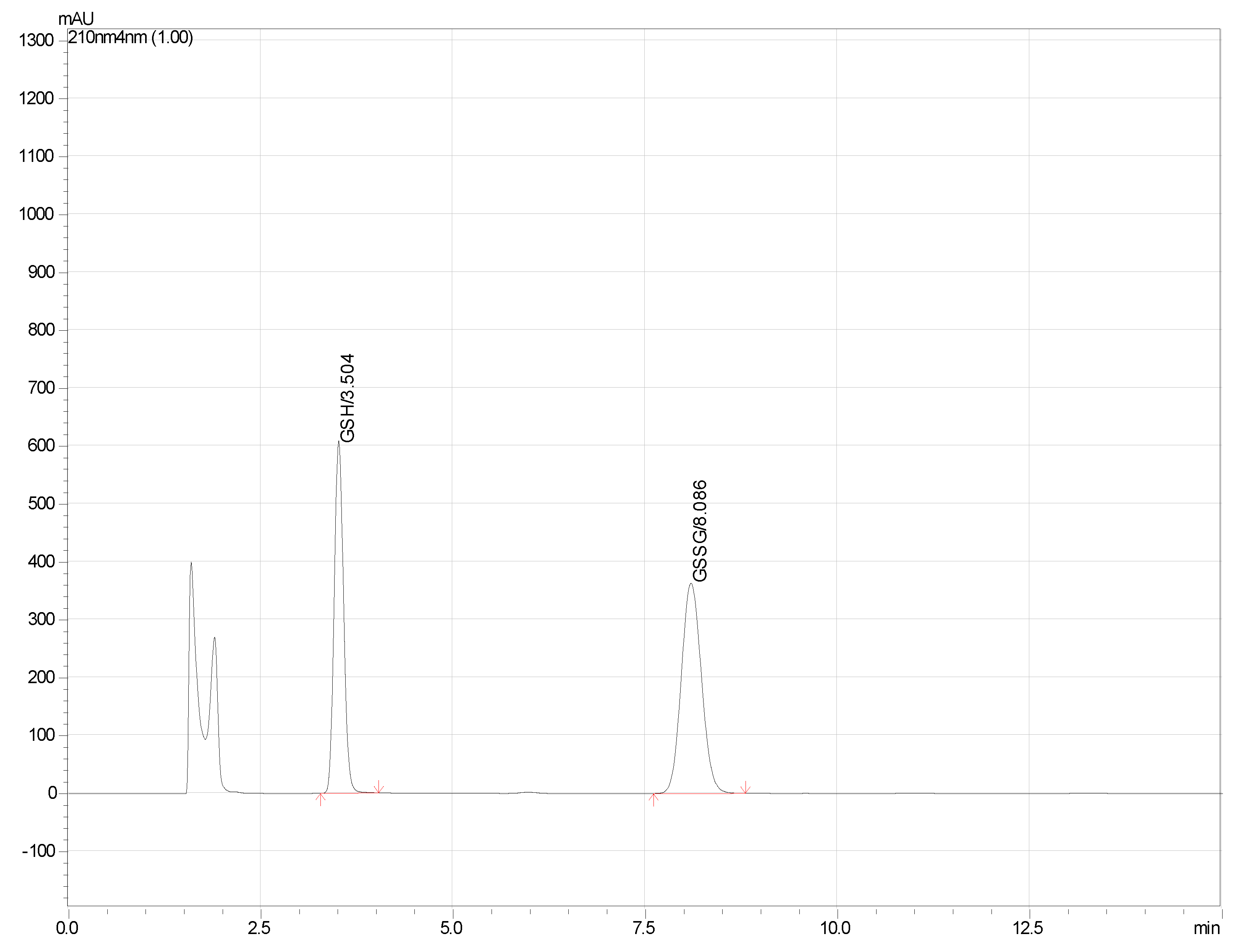
| HPLC Parameter | GSH | GSSG |
|---|---|---|
| Retention time (min) | 3.5 | 8.1 |
| Retention factor | 1.19 | 4.1 |
| Theoretical plates/m | 43,000 | 34,100 |
| Tailing factor | 1.15 | 1.11 |
| Resolution factor | — | 12.6 |
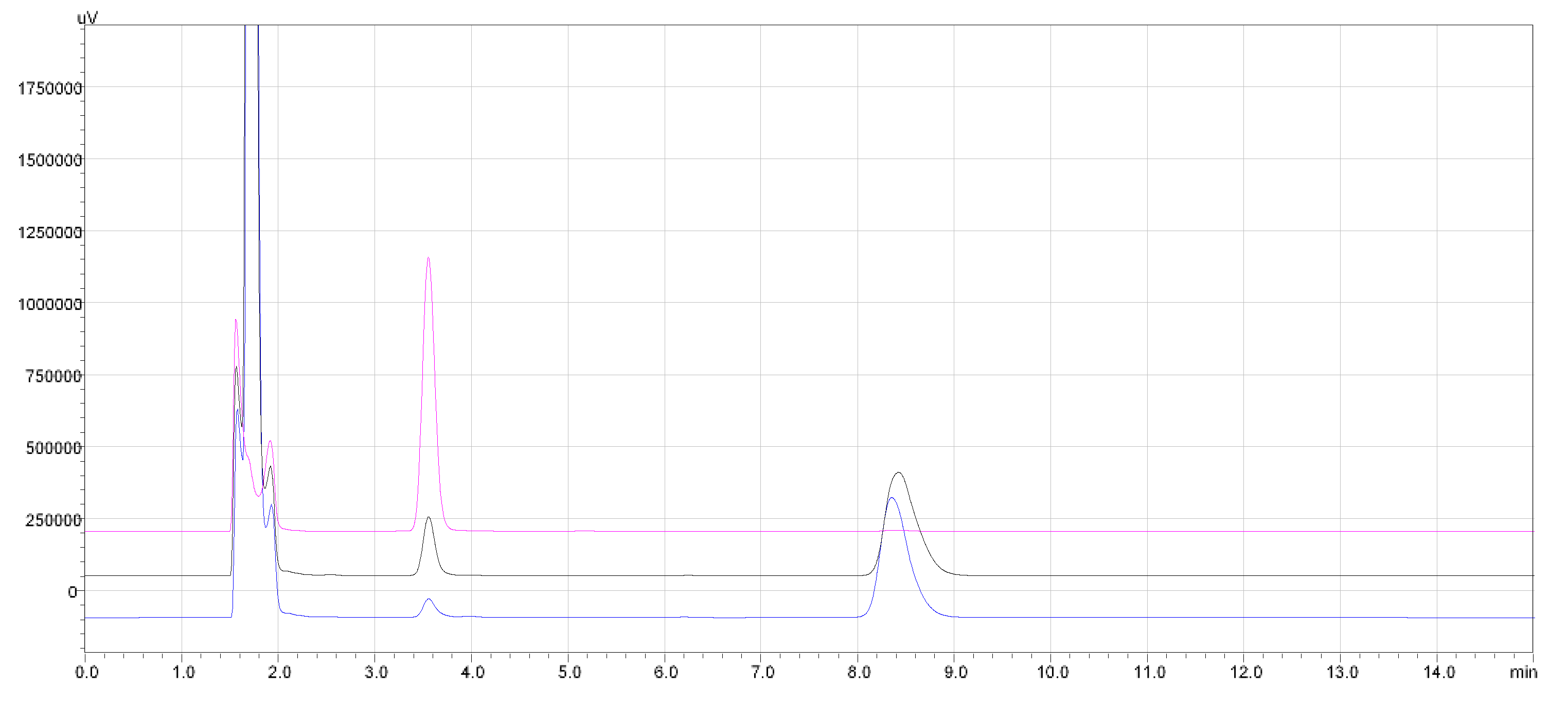
| GSH (mg L−1) | GSSG (mg L−1) | Recovery (%) |
|---|---|---|
| 10 | — | 100.0 |
| 10 | 10 | 101.8 |
| 10 | 50 | 100.5 |
| 10 | 100 | 98.2 |
| 10 | 250 | 97.4 |
| 10 | 500 | 102.7 |
| 10 | 1000 | 103.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiasioti, A.; Zacharis, C.K.; Zotou, A.-S.; Tzanavaras, P.D. Study of the Oxidative Forced Degradation of Glutathione in Its Nutraceutical Formulations Using Zone Fluidics and Green Liquid Chromatography. Separations 2020, 7, 16. https://doi.org/10.3390/separations7010016
Tsiasioti A, Zacharis CK, Zotou A-S, Tzanavaras PD. Study of the Oxidative Forced Degradation of Glutathione in Its Nutraceutical Formulations Using Zone Fluidics and Green Liquid Chromatography. Separations. 2020; 7(1):16. https://doi.org/10.3390/separations7010016
Chicago/Turabian StyleTsiasioti, Apostolia, Constantinos K. Zacharis, Anastasia-Stella Zotou, and Paraskevas D. Tzanavaras. 2020. "Study of the Oxidative Forced Degradation of Glutathione in Its Nutraceutical Formulations Using Zone Fluidics and Green Liquid Chromatography" Separations 7, no. 1: 16. https://doi.org/10.3390/separations7010016
APA StyleTsiasioti, A., Zacharis, C. K., Zotou, A.-S., & Tzanavaras, P. D. (2020). Study of the Oxidative Forced Degradation of Glutathione in Its Nutraceutical Formulations Using Zone Fluidics and Green Liquid Chromatography. Separations, 7(1), 16. https://doi.org/10.3390/separations7010016








