Abstract
Aspergillus flavus produces dangerous secondary metabolites known as aflatoxins, which are toxic and carcinogenic, and their contamination of agricultural products results in health issues and economic hardships in the U.S. and around the world. Early identification of aflatoxigenic isolates of A. flavus is the key in the management of these fungi. An emerging detection method for specific fungi identification involves the analysis of microbial volatile organic compounds (MVOCs) released by the fungi. Complicating this approach is the understanding that many factors influence metabolic production, including growth parameters, such as growth media, temperature, spore counts and oxidation stress. In addition, analytical and data analysis methods can also influence the results. Several growth and analysis methods were evaluated and optimized in order to better understand the effect of the methods on fungi MVOC signatures. The results indicate that carboxen/polydimethylsiloxane (CAR/PDMS) has the best extraction efficiency for the MVOCs emitted by A. flavus. Both chemical defined agar (CDA) and chemical defined liquid (CDL) are suitable growth media for MVOC emission studies. The highest MVOC production was found at 30 °C. Log transformation was considered one of the best data pretreatment methods when analyzing MVOC data and resulted in the best principal component analysis (PCA) clustering in the experiments with different growth media. This study aims to elucidate fungal volatile organic compounds (VOCs) differences due to variations in growth parameters as a first step in the development of an analytical method for the monitoring of aflatoxigenic A. flavus contamination in crop storage facilities.
1. Introduction
Aflatoxins are secondary metabolic products produced primarily by Aspergillus flavus and Aspergillus parasiticus [1]. Aflatoxin contamination of corn, peanut and other agricultural commodities has a significant impact on health and the agricultural economy, especially in the southeastern United States, where aflatoxin contamination costs farmers, buyers and sellers an annual average of $22.7 million [2]. The production of aflatoxins is always associated with the production of other metabolites, some of which are volatile. These volatile metabolites are produced during both primary and secondary metabolism and are often collectively referred to as microbial volatile organic compounds (MVOCs) [3]. MVOCs are widely investigated as the indicator of fungal growth [4,5,6,7], mycotoxins’ production [8,9,10] and for fungal taxonomy [11,12,13].
In recent years, metabolomics approaches have been widely used for the investigation of the metabolites of biological samples for identifying biomarkers that correlate to a disease [14,15], drug toxicity [16,17] or genetic or environmental variation [18]. Metabolites can belong to a wide variety of compound classes, such as amino acids, lipids, organic acids, nucleotides, alcohols, esters and hydrocarbons [19]. These compounds are very diverse in their physical and chemical properties and occur in a wide concentration range. Some of these metabolites are volatile enough for headspace sampling. Metabolic profiling and fingerprinting methods are used to elucidate a microorganism’s life processes [19]. Metabolic profiling is a determination of the chemicals and their concentrations produced by the specific biosynthesis pathway of organisms. Metabolic fingerprinting is the screening approach to classify samples based on metabolite patterns or “fingerprints”. The metabolomics study process often includes sample preparation, sample collection, instrumental analysis, data pretreatment and data analysis.
Relative humidity, temperature, substrate (growth medium), number of fungal spores inoculated and oxidation stress are the main factors influencing fungal growth, metabolism and MVOCs’ production in a laboratory setting [20,21,22]. For example, López-Malo et al. [23] studied the effect of incubation temperature (10–30 °C), pH (3.0–4.0) and vanillin concentration (350–1200 ppm) on the growth of A. flavus. They concluded that the germination time and radial growth rate were significantly affected by the three studied variables. Joffe and Lisker [24] studied the effects of light, temperature and pH value for aflatoxins’ production. They indicated that 24 °C was the optimal temperature for aflatoxin production. Polizzi et al. [21] studied the influence of various growth parameters on fungal growth and volatile metabolite production by indoor molds. They proved that the range of MVOCs and the quantities were larger on malt extract agar than on wallpaper and plasterboard. VOCs and aflatoxin production have also been shown to be associated with oxidative stress [25]. Clearly, fungal growth conditions are an important consideration when conducting a metabolomic fingerprinting study involving the production of MVOCs.
Sampling methods, such as thermal desorption tube (Tenax TA) [4,26,27], purge and trapping of headspace gases [28,29], headspace sorptive extraction [12,30] and solid phase microextraction (SPME) [31,32,33,34], have been used for the collection of MVOCs. SPME is a popular technique because it has the advantages of low cost per analysis and portability. Volatile chemicals can be selectively enriched on SPME fibers depending on fiber coating selection. Therefore, the SPME fiber coating selection is important and should be tailored for specific applications. Optimization of fiber selection for 15 volatile and semivolatile analytes representing 13 organic classes was performed, and extraction efficiencies of the fibers for each of the analytes were compared [35]. This study illustrated key considerations involved in the selection of a SPME fiber, including: (1) the polarity and functionality of the polymer absorbent; and (2) the volatility and functional groups of the target analyte.
The separation of MVOCs is efficiently accomplished with the use of gas chromatography (GC) [33,36]. Detection methods include flame ionization detection (FID) [34,37,38] and mass spectrometry (MS) [8,36,39,40,41,42] for the quantification and qualification of metabolic profiles. Among the MS techniques, single quadrupole mass detection is most widely used; however, more advanced techniques, such as triple quadrupole (MS/MS) [43,44], time of flight (TOF) [45] and ion mobility mass spectrometry (IMS-MS) [46,47], are utilized depending on the purpose of the analysis.
In metabolomics research, different data pretreatment methods are applied in order to generate “clean” data in the form of normalized peak areas that reflect metabolite concentrations. These clean data can then be used as the input for data analysis. Data pretreatment aids to enhance relevant (biological) information and to reduce the influence of confounding factors from random error and spurious chemicals from column and absorbent bleeding [48]. Three classes of data pretreatment methods are normally utilized, including centering, scaling and transformations. Centering converts all of the concentrations to fluctuations on zero [49]. Scaling enables the adjustment of fold differences between the metabolites, increasing the importance of low abundant metabolites. Transformations including log and power transformations are generally applied to correct for heteroscedasticity [50].
Statistical data analysis methods including multivariate data analysis (MVDA) can be used for extracting important features from large or small datasets containing a number of variables and observations. MVDA includes multivariate ANOVA (MANOVA), linear discriminant analysis (LDA), cluster analysis (CA), principal component analysis (PCA) and partial least square analysis (PLS). These methods are widely used in fungal detection and classification [11,51,52,53].
In this study, the effect of sample collection strategy using SPME fibers and sample preparation (fungal growth parameters) on the MVOCs’ production from A. flavus was investigated. For SPME fiber evaluation, the extraction efficiencies of three commercially available SPME fibers coated with carboxen/polydimethylsiloxane (CAR/PDMS), divinylbenzene/polydimethylsiloxane (DVB/PDMS) and carboxen/divinylbenzene /PDMS were compared. A single A. flavus isolate was selected for this study in an attempt to reduce variations due to phenotype differences associated with different isolates [32]. For the growth parameters’ effect study, the A. flavus isolate was grown under varied conditions using different temperatures and numbers of spores inoculated and on different substrates to evaluate the influence of these factors on MVOCs’ production. The aim of this study was to optimize fungal growth conditions for large MVOCs’ production and to determine MVOCs’ variability within a single isolate. Different data pretreatment methods, including scaling, centering and transformations, were evaluated using MVOCs’ datasets from A. flavus grown on five different media to select the proper method for PCA analysis. To our knowledge, this is the first study focused on the evaluation of the growth parameters and data analysis methods for fungal MVOCs analysis.
2. Materials and Methods
2.1. Chemicals and Growth Media
An alkane mixture standard, methanol (≥99.5%), sucrose, FeSO4·7H2O (99+%) and L-asparagine monohydrate were purchased from Sigma-Aldrich (St. Louis, MO, USA). (NH4)2SO4 (99.7%), KH2PO4 (99.7%), MgSO4 anhydrous, CaCl2·2H2O, ZnSO4·7H2O, MnCl2 (97%) and Tween 20 solution were bought from Thermo Fisher Scientific (Pittsburgh, PA, USA).
Malt extract agar (MEA), Czapek solution agar (CSA) and corn meal agar (CMA) were purchased from Becton, Dickinson and Company (Franklin Lakes, NJ, USA). The ingredients of chemical defined agar (CDA) was mixed based on the literature [54]. The ingredients of the growth medium are listed in Table 1.

Table 1.
Growth substrate and their ingredients used in the study.
2.2. Fungal Isolates and Growth
The aflatoxigenic isolate K73 was collected from corn sampled in Sunflower County, MS. This isolate was used for an SPME fiber comparison study. Two aflatoxigenic isolates 5-3B and 4-3A were isolated from pig feed in Maben, MS. These isolates were used for the growth parameters’ (growth substrate, concentration of spore suspension and temperature) effects study.
In a typical experiment, the fungal isolate was cultured in a Petri dish containing MEA medium at 30 °C in the incubator for 7 days and was subcultured every two weeks to maintain fresh spores. Fresh spores were extracted using 0.02% Tween 20 solution and diluted to the desired concentration using sterile distilled water while the number of spores was counted using the hemocytometer (C. A. Hausser and Sons, Philadelphia, PA, USA). A spore suspension (10 µL) was injected into a 50-mL Erlenmeyer flask containing 30 mL of the sterile growth medium. The Erlenmeyer flasks (Fisher Scientific Inc., Pittsburgh, PA, USA) were then covered with aluminum foil and sealed with parafilm.
2.3. Selection of SPME Fibers
Carboxen/polydimethylsiloxane (CAR/PDMS), divinylbenzene/polydimethylsiloxane (DVB/PDMS) and DVB/CAR/PDMS SPME fibers were purchased from Sigma-Aldrich (St. Louis, MO, USA). The aflatoxigenic isolate K73 was used for the SPME fiber comparison study. The growth medium used was MEA, and the spores’ concentration was maintained at 1 × 106 spores/mL. Fungal cultures were incubated in the absence of light at 30 °C for 7 days. Fungal cultures were prepared in 6 replicates for each type of SPME fiber for a total of 18 experiments. After 7 days of culture incubation, SPME fibers were exposed to the headspace of the Erlenmeyer flasks containing the fungus at 30 °C for 5 h. After the sampling period, the fiber was pulled into the needle sheath; the SPME device was removed from the flask and then inserted into the hot injection port of the GC-MS for thermal desorption.
2.4. Effects of Growth Parameters on the MVOCs’ Production
The aflatoxigenic isolate, 5-3B, was used for growth media, the concentration of the spore suspension and temperature effects’ study. The growth parameters evaluated are listed in Table 2. CAR/PDMS fibers were used for the effects of growth parameters’ study.

Table 2.
Growth parameters evaluated for the effects on MVOCs’ production.
The ingredients and the amount used are listed in Table 1 for the fungal growth media test. The growth substrates were prepared by dissolving the agar in 1 L of deionized water followed by autoclaving at 121 °C for 15 min. The chemical defined liquid medium (CDL) was prepared using the same ingredients as in the chemical defined agar (CDA) medium without adding 15 g agar. Fungal cultures were prepared with 6 replicates for each growth medium for a total of 30 experiments. The fungi were grown in the absence of light at 30 °C for 7 days, and MVOCs were extracted using the PDMS/CAR fiber for 5 h at 30 °C.
The aflatoxigenic isolate 4-3A was used for the spores’ concentration effect evaluation study. The concentration of spore suspension used is listed in Table 2; otherwise, the conditions were identical to those described above in Section 2.2, where only 1 × 106 spores/mL were used. The toothpick method involves taking a small sample of spores and mycelium from the growing fungi using a sterile toothpick and directly inserting it on the surface of agar medium. The fungi was grown in the absence of light at 30 °C for 7 days. The fungal cultures were prepared in 6 replicates for each concentration inoculum for a total of 24 experiments. The MVOCs were extracted using PDMS/CAR fiber for 5 h at 30 °C.
In the temperature effect study, each flask was incubated in the absence of light at four temperatures (15 °C, 30 °C, 37 °C and 45 °C) for 7 days. The concentration of spores was 1 × 106 spores/mL, and the fungi were grown on MEA media. The growth conditions were identical to the previously described method, except for the use of 4 different temperatures. The fungal isolates were prepared in 6 replicates at each temperature level for a total of 24 experiments. CAR/PDMS fibers were used to extract MVOCs for 5 h at 30 °C.
2.5. GC-MS Analysis
The analysis of collected MVOCs was performed with a GC-MS. Extracted volatiles were thermally desorbed from the SPME fiber in the injection port (at 270 °C), equipped with a 78.5 mm × 6.5 mm × 0.75 mm SPME inlet liner. Thermal desorption was setup for 5 min, and the SPME fiber was conditioned for 1 h at 270 °C following the manufacturer’s instructions before the next usage. The gas chromatography capillary column used for separation was a 60-m DB-1 capillary column with an internal diameter of 320 µm and a film thickness of 1 µm. Helium was used as a carrier gas with a flow velocity of 1.2 mL min−1. The following GC oven temperature program was applied: 45 °C for 9 min, 10 °C min−1 to 85 °C, hold for 3 min, 3 °C min−1 to 110°C, hold for 3 min, 3 °C min−1 to 120 °C, hold for 3 min, and 10 °C min−1 to 270 °C, hold for 5 min. The MS analysis was carried out in full scan mode (scan range from 35–350 atomic mass unit (amu)) with ionization energy of 70 eV. Ion source and quadrupole temperatures were 230 °C and 150 °C, respectively.
2.6. GC-MS MVOCs Data Manipulation
Tentative chromatographic peak identification was made by library matching using the NIST 08 MS Library (Gaithersburg, MD, USA). Compounds were considered positively identified when both mass spectra and the retention index (RI) led to the same identification. Quantitative data for each analyte were determined using peak area. Peak alignment adjustments were required due to instrument drift and experimental error. Peak alignment procedures for samples from GC-MS measurements play an important role in biomarker detection and metabolomic studies in general [55]. The peak alignment procedures are illustrated in the Supplementary Material (Figures S1–S7).
Additional data processing required that peak areas of zero were replaced with values equal to 1 count to allow for log transformation [48]. The lowest peak areas in the rest of the data are on the order of 105. Any MVOCs detected less than three times in the 6 replication experiments were removed from further data treatment. Silicon-containing peaks with mass to charge ratio (m/z) of 73, 207 and 281 are believed to have originated from the column stationary phase and were also removed from the processed data.
Systematic data pretreatment can be used to enhance the results of follow-on classification methods, including PCA and PLS. The data pretreatment methods including centering, autoscaling, Pareto scaling, log transformation, power transformation, and area normalization were compared using PCA to evaluate the classification results of five media types with six replications. The overview of the pretreatment methods were described by Van Den Berg et al. [48]. In the SPME fiber selection study, log transformation was applied to achieve better group separations. Each MVOC detected represents the dependent variable in PCA, and each replication is the observation.
2.7. Data Analysis
MANOVA was performed to examine whether there is a significant difference in the quantities of MVOCs emitted by the fungal culture inoculated with different spore doses. Fifteen MVOCs, commonly emitted by the fungi, were selected to compare the quantitative variation caused by the change in spore dose. These 15 compounds are ethanol, 1,4-pentadiene, 2-methylfuran, 2-methyl-1-propanol, 3-methylbutanol, 2-methylbutanol, toluene, (-)-aristolene, β-elemene, α-farnesene, cubenene, δ-cadinene, β-germacrene, β-panasinsene and β-cadinene. The data were treated by log transformation, and then, MANOVA was performed using SAS 9.3 software (SAS Institute Inc., Cary, NC, USA). PCA was performed using the software program SIMCA-P+ 11.0 (Umetrics, Umea, Sweden). PCA classification results were evaluated using score plots.
3. Results and Discussion
3.1. Evaluation of SPME Fiber on Metabolic Profiling
Extracted MVOC profiles and quantities were determined, and the information was used to select the best SPME fiber for metabolic fingerprinting. Experiment precision (repeatability) was evaluated based on the Relative Standard Deviation (RSD%) of the six replicates of three SPME fiber types: CAR/PDMS, DVB/PDMS and DVB/CAR/PDMS. These fibers were evaluated in terms of their efficiency in extracting volatile metabolites emitted by A. flavus K73 growth on an MEA substrate. The fungal culture was incubated for 7 days at 30 °C with an initial inoculation spore concentration of 1 × 106 spores/mL. The SPME extraction was maintained at 30 °C for 5 h. The extraction efficiency evaluation included two aspects, MVOC selectivity and quantity (peak area).
Three evaluated fibers showed different abilities to extract volatile metabolites of A. flavus, as shown in Figure 1A,B. We took steps to reduce the impact of this natural variation for collecting Figure 1 data. The 18 samples (six replicates for each fiber) that were analyzed were grown under identical conditions at the same time. The large error bars indicate the degree of natural variation. However, even with the large error bars, we are able to determine that CAR/PDMS is superior at extracting VOC, resulting in a larger total ion count. The CAR/PDMS fiber not only extracted the largest number of MVOCs (Figure 1A), but also extracted the largest amount of MVOCs based on the total peak area of all of the volatile metabolites (Figure 1B). A closer look at the data revealed MVOC functional group selectivity. Identified MVOCs were divided into nine chemical classes, including alcohols, aldehydes, furans, hydrocarbons, ketones, organic acids, organosulfur compounds, sesquiterpenes and other compounds. Among the chemical classes, hydrocarbons were divided into Hydrocarbon 1 (fewer than ten carbons) and Hydrocarbon 2 (ten or more carbons). Other compounds include seven unknown compounds, one ether and one ester.
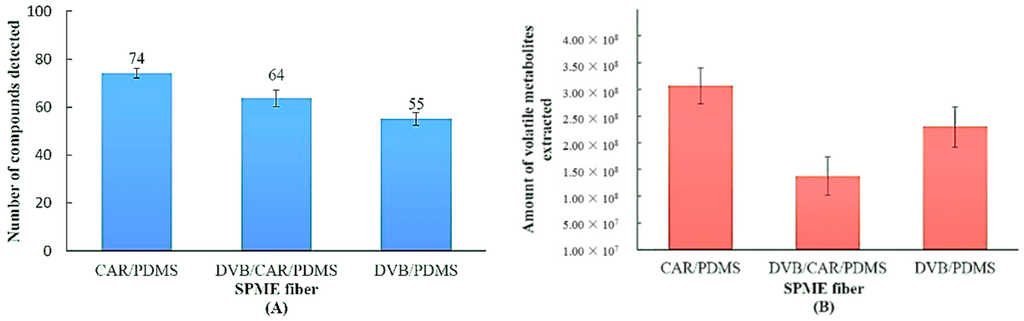
Figure 1.
Solid phase microextraction (SPME) fibers’ comparison through the number of (A) and amount of (B) volatile metabolites extracted from A. flavus culture using three types of SPME fibers, carboxen (CAR)/PDMS, divinylbenzene (DVB)/CAR/PDMS and DVB/PDMS, in six replications. The error bars indicate the standard deviation of six replicates.
The CAR/PDMS fiber extracted greater amounts of alcohols, furans, Hydrocarbon 1, Hydrocarbon 2 and ketones, while DVB/PDMS extracted larger amounts of high molecular weight compounds containing the organosulfur compounds, sesquiterpenes and other compounds (Figure 2). These results agree with the literature, which describes the CAR/PDMS as likely to extract low molecular weight compounds, while DVB/PDMS is better at extracting high molecular weight compounds [56].
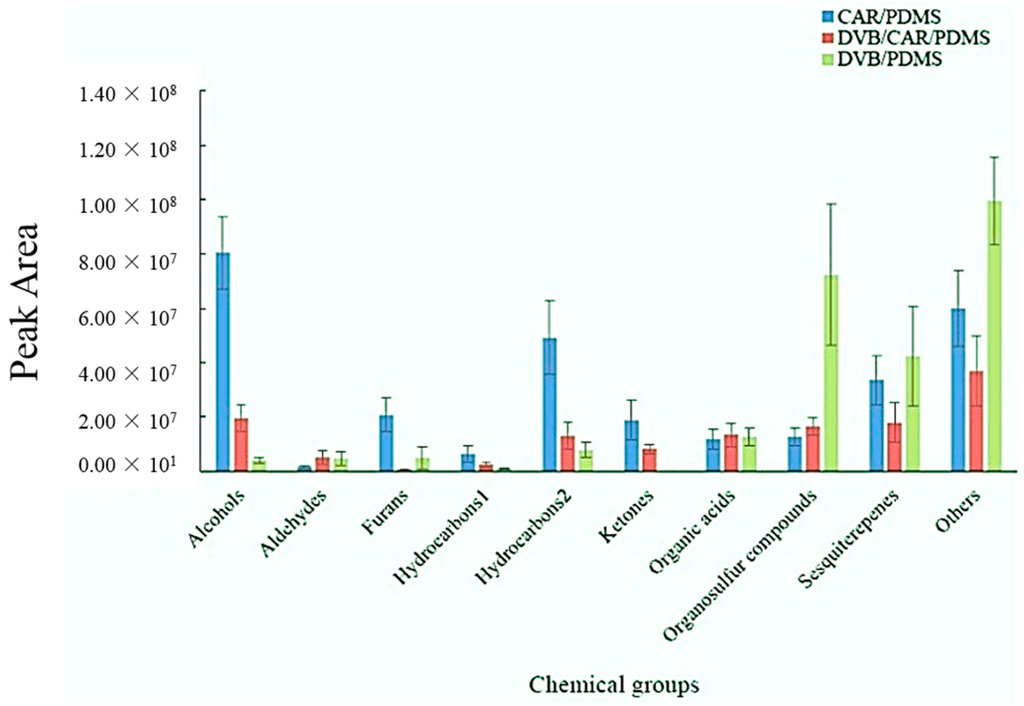
Figure 2.
SPME fibers’ comparison though the amount of volatiles in chemical groups extracted from A. flavus culture using three types of SPME fibers, CAR/PDMS, DVB/CAR/PDMS and DVB/PDMS, in six replications. The error bars indicate the standard deviation of the peak areas of six replicates. Among the chemical classes, hydrocarbons were divided into Hydrocarbon 1 (fewer than ten carbons) and Hydrocarbon 2 (ten or more carbons).
Since the CAR/PDMS fiber has difficulty adsorbing higher molecular weight analytes and DVB/PDMS has difficulty extracting analytes with low molecular weights, a DVB/CAR/PDMS fiber was developed by the manufacturer [57]. The advertised extraction advantage of the extended molecular weight range of VOCs was not observed based on our results (Figure 1A,B). CAR/PDMS was determined to be an excellent SPME fiber coating choice for fungal MVOC profiling based on its ability to collect the largest number and greatest quantity of MVOCs.
The precision or repeatability of this method was examined using the relative standard deviation percentage (RSD%) of each extracted and identified fungal MVOC. The RSD% of each metabolite was calculated using both peak area and peak area percentage data. An internal standard (IS) was not used in this study for two reasons: (1) concerns that an internal standard would compete with absorption sites on the SPME fibers with fungal VOCs; and (2) the ultimate goal of this program is the development of an electronic nose for early detection of A. flavus-producing aflatoxins in crop storage facilities; for this and related real-world field applications, we will not be able to easily add an IS to aid in analysis. Table 3 lists 15 common MVOCs detected and their RSD% for each SPME fiber. The peak area percentage was obtained by dividing peak area ion currents of each compound by chromatograms total ion current (TIC) times one hundred. This is referred to as the area normalization method. This method is the earliest and most straightforward of the data pretreatment methods and requires no reference standards or calibration to be prepared. The average RSD% for each SPME fiber type is listed in Table 3.

Table 3.
Fifteen selected MVOCs and their RSD% (using both peak area and peak area percentage) obtained using three types of SPME fibers, CAR/PDMS, DVB/PDMS, and DVB/CAR/ PDMS, with 6 replications.
The average peak areas RSD% for CAR/PDMS, DVB/CAR/PDMS and DVB/PDMS are 56.7%, 40.9% and 49.8%, respectively. Using peak area %, the RSD%’s of CAR/PDMS, DVB/CAR/PDMS and DVB/PDMS are 56.7%, 52.1% and 39.5%, respectively. Previous studies show that RSD%’s determined using analytical standards calculated by peak area were 18.4%, 14.9% and 13.1%, for CAR/PDMS, DVB/CAR/PDMS and DVB/PDMS, respectively [32]. Several volatile metabolites show large fluctuations in concentration under identical experimental condition; this is due in part to uninduced biological variation [48]. In this study, the uninduced biological variation added between 26% and 38% to our experimental variability.
3.2. Effect of the Growth Substrates on MVOCs’ Production
Growth factors affecting aflatoxin production by Aspergillus parasiticus have been reported by Reddy et al. [54]. They found the amino acid asparagine to be essential for aflatoxin production. A number of authors have reported how substrate composition influences volatile production by fungi. For example, Larsen and Frisvad [58] found that while volatile profiles from Penicillium isolates were generally similar when the isolates were grown on either yeast extract sucrose agar or malt extract agar, fewer VOCs were produced by the same isolates when grown on Czapek yeast autolysate agar. Kahlos et al. [59] found that VOCs produced by the brown rot fungus Gloeophyllum odoratum varied, depending on the presence in the media of different growth elicitors, indicating the importance of media in studies of this nature. Hence, precedence exists for expecting MVOC variability due to growth media. The relationship between growth medium and A. flavus MVOC profiles is explored in the section below.
Five growth media substrates (Table 1) were evaluated. CMA contains the least amount of organic nutrients (around two grams); MEA is most commonly used in the MVOC studies [31,60,61]; while CSA is typically used for A. flavus cultures. CDA and CDL are chemically-defined media that have previously been used for an aflatoxin production study [54]. The fungal culture was incubated for seven days at 30 °C with an initial inoculation spore concentration of 1 × 106 spores/mL. CAR/PDMS fiber was used for extracting the MVOCs for 5 h at 30 °C. Table 4 lists a subset of the identified MVOCs and their relative quantity (expressed in peak area) produced by A. flavus isolate 5-3B on the five different incubation media (CMA, CSA, CDA, CDL and MEA).

Table 4.
Forty one c selected common MVOCs and their relative quantities (expressed in peak area) produced by A. flavus isolate 5-3B on the five different incubation media (CMA, CSA, CDA, CDL and MEA).
A total of 132 MVOCs are detected in fungal cultures grown on all substrates. Forty one of the MVOCs were produced by the fungus in at least 4 out of 5 growth media substrates. Ten MVOCs were produced on all growth media, including ethanol (1), 2-methyl-furan (18), toluene (10), dodecane (54), decanal (67), (Z)-3-Hexadecene (84) and some sesquiterpenes (trans-α-bergamotene (82), (−)-aristolene (88), β-elemene (89) and valencene (104). These common fungal MVOCs have the potential to be considered as a group of indicators of fungal growth.
Different MVOC profiles are produced when the fungus is grown on different media. This difference can be seen when MVOCs are divided into different chemical classes (alcohols, aldehydes, alkanes, alkenes, esters, ethers, furans, hydrocarbons, aromatic hydrocarbons, ketones, organic acids and sesquiterpenes), as shown in Figure 3. The 132 MVOCs were grouped by chemical class and their raw peak areas summed for Figure 3. MANOVA was performed to test whether there are significant differences among the total quantities of MVOCs in each chemicals’ class across the five growth media.
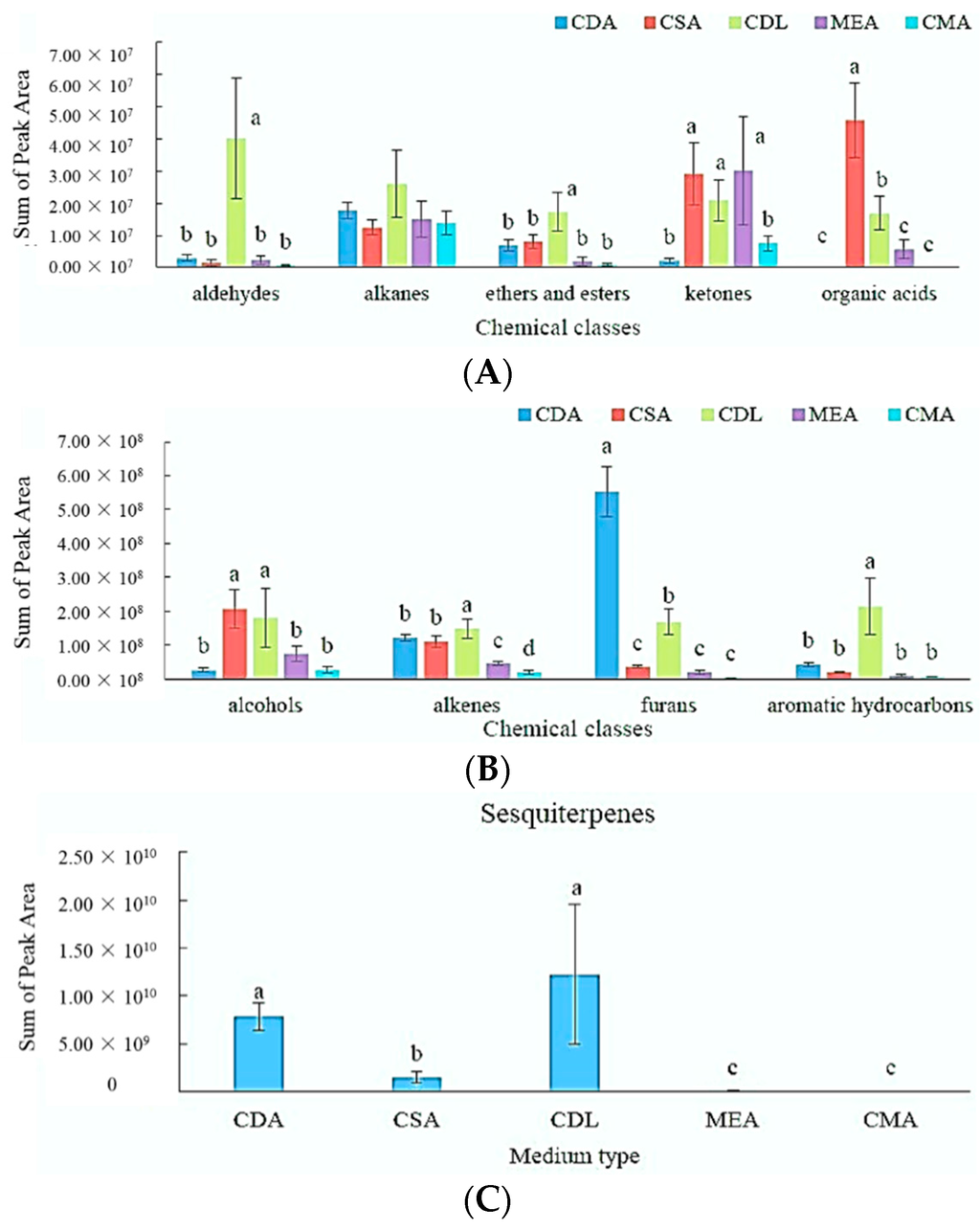
Figure 3.
Comparison of the amount of volatile metabolites (sum of peak area with SD (six replicates)) emitted by A. flavus 5-3B on growth media CDA, CSA, CDL, MEA and CMA. Different letters above the bars indicate significant differences (MANOVA, p < 0.05). The data with the highest total ion current (TIC) for each chemical class starts with label a. The TIC of each medium shows no significant difference in the alkane group. (A): Chemical classes of aldehydes, alkanes, ethers and esters, ketones and organic acids; (B): Chemical classes of alcohols, alkenes, furans, aromatic hydrocarbons; (C): Medium type.
Figure 3 illustrates that the MVOCs’ production in CDA media has larger amounts of furans than the other media. Among these growth media, the fungi growth on the CDL media produces the largest amount of aldehydes, a combination of ether and esters, aromatic hydrocarbons and alkenes. CDL and CDA have an identical list of ingredients, except for the addition of 15 g of agar to the CDA media, which helps to produce a gel-like media. The quantities of MVOCs produced by the fungi growth on the liquid media (CDL) are much greater than those grown on the agar medium (CDA) for all chemical classes, expect the furans. Interestingly, both CDL and CDA produced significantly more sesquiterpenes than the other growth media (Figure 3C). One possible explanation is the addition of L-asparagine, which has been shown to enhance the production of sesquiterpenes and aflatoxins [54]. MVOCs’ production is clearly affected by growth media.
3.3. Effect of the Concentration of Spore Suspension on MVOCs’ Production
Traditional fungal inoculation and subculture methods are quite simple. A small piece of fungal culture is removed with a sterile blade and then transferred to the surface of the growth medium. For A. flavus, it typically takes seven days for the fungi to cover the entire agar surface of the 100 mm diameter petri dish. In this study, differing spore suspensions were precisely prepared. Using these suspensions, a controlled spore dose could be added to the growth media in order to elucidate the relationship between dose and MVOC production.
Spores of A. flavus were collected with a Tween 20 solution, diluted as needed with water and counted using a hemocytometer. Then, 10 µL of a specific concentration of the spore suspension were injected on the malt extract agar (MEA) media surface before the standard seven days of incubation at 30 °C. CAR/PDMS fiber was used to extract MVOCs for 5 h at 30 °C. Four concentrations of the spore suspension were used, including a high concentration (1.2 × 107 spores/mL), medium concentration (2.4 × 106 spores/mL) and low concentration (4.8 × 105 spores/mL). A toothpick inoculation method was also used for comparison.
The peak areas of 15 commonly-detected MVOCs emitted by A. flavus 5-3B were selected to compare the different spore doses (Table 5). No consistent trends were observed that correlate spore count with specific MVOC peak ion counts.

Table 5.
Quantities of 15 selected common MVOCs of fungi culture inoculated with four different spore concentrations.
3.4. Effect of Temperature on MVOCs’ Production
Temperature has been proven to affect A. flavus growth and aflatoxin production. MVOC production from A. flavus was investigated using four different temperatures: 15, 30, 37 and 45 °C. Again, spores were grown on MEA media for seven days; the inoculated spore count was 10 µL of a 106 spores/mL mixture, and the MVOCs were collected with a CAR/PDMS SPME fiber for 5 h at 30 °C. The TIC chromatograms obtained from analyzing the MVOCs emitted by A. flavus 5-3B grown at different temperatures were compared to select the preferred temperature (Figure 4). The fungi grown at 15 and 30 °C produced more amounts of sesquiterpenes compared to the fungi grown at 37 and 45 °C (Figure 4B). The morphologies of the fungi grown in 15, 30 and 37 °C are similar after seven days; however, the fungi grows slowly at 45 °C. The compound 1-butanol can be used as the indicator of fungi growth condition, because 1-butanol is produced largely from MEA medium. The presence of a large amount of 1-butanol (Figure 4A) in the fungi culture indicated the slow growth rate of fungi at 45 °C.
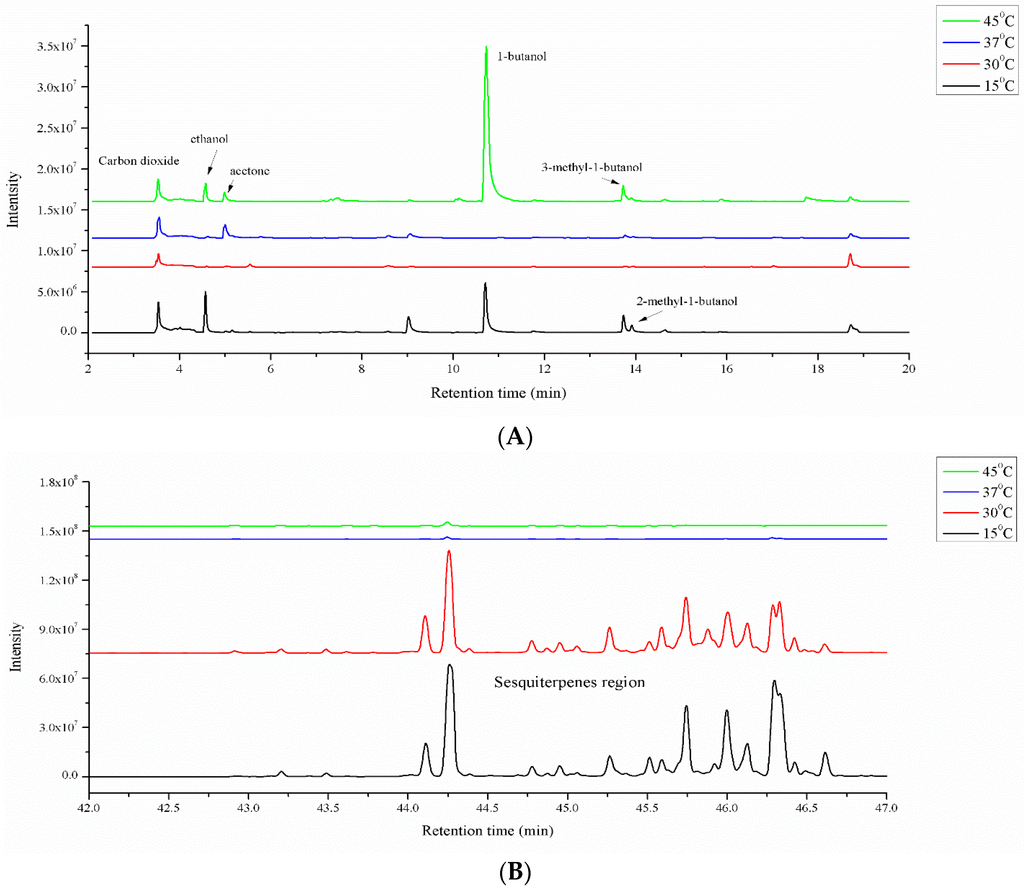
Figure 4.
TIC chromatogram comparison of MVOCs’ profiles obtained from A. flavus 5-3B grown at different temperatures (15, 30, 37 and 45 °C). (A): Retention time from 2 to 20 min; (B): Retention time on sesquiterpenes region.
Schindler et al. [62] reported that the optimum temperature for aflatoxin production occurred at 24 °C, and the maximal growth of A. flavus isolates occurred at 29 and 35 °C. However, the optimum growth and aflatoxin production temperature may vary when growing on the different substrates. Karunaratne and Bullerman [63] reported that the mycelial growth and sporulation of A. flavus occurred faster at 35 °C at all spore levels than 28 °C on rice. At 28 °C, high amounts of aflatoxin B1 were produced, while the lower and the higher spore levels produced comparatively lower levels of aflatoxin. A suitable temperature for fungal growth and aflatoxins’ production should be used when selecting the optimum temperature for the MVOCs study. A growth temperature of approximately 30 °C was determined to be the best temperature for this MVOC profiling study.
3.5. Effect of Data Pretreatment Methods
Data pretreatment methods can be utilized to convert raw data to a different scale (for instance, logarithmic scale or relative scale), which reduces unwanted biases to more clearly depict important biological signals. The effect of data pretreatment has been illustrated through the application of six data pretreatment methods on the MVOCs’ data of A. flavus grown on the five different media substrates. The results of these methods are shown in Figure 5, where (A) is the raw data. The other graphs represent: (B) centering; (C) autoscaling; (D) Pareto scaling; (E) log transformation; (F) power transformation; and (G) area normalization. The pretreatment methods were performed according to the equations listed in Table 3.
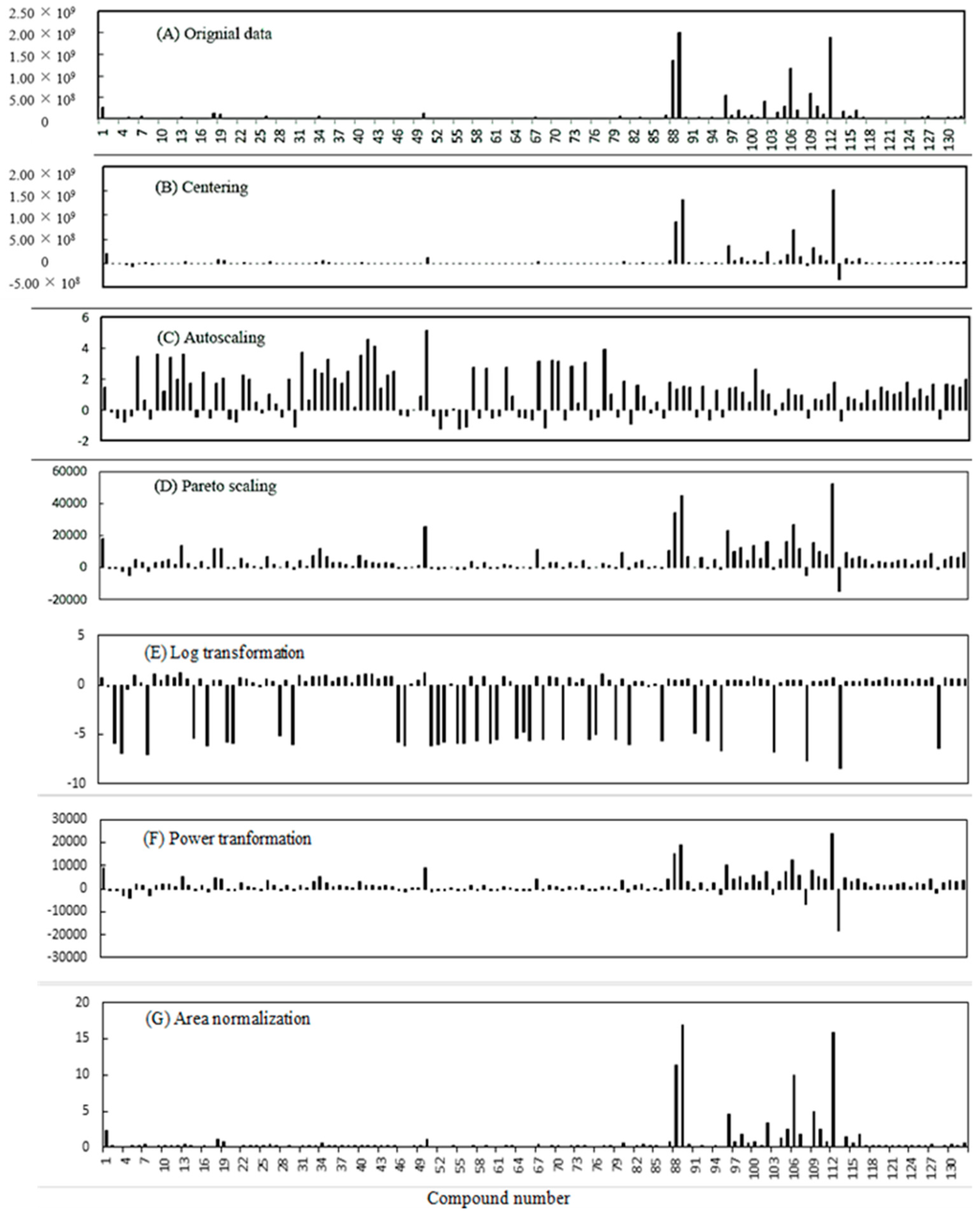
Figure 5.
Effect of data pretreatment on the original data.
The MVOCs’ raw data obtained from the five growth media study was used for the data pretreatment methods’ evaluation. The raw peak area data for the 132 identified MVOCs are shown in Figure 5A (MVOC profile from CDL media Replication 1). Mean centering was applied to obtain a mean value of zero in order to improve the interpretability of the model (Figure 5B). Autoscaling is a combination of mean centering and scaling to unit variance where the scaling weight employed is 1/s, and s represents the standard deviation of the variable (peak area of a specific MVOC). After autoscaling, “long” variables are “shrunk”, and “short” variables are “stretched” (Figure 5C). In Pareto scaling, the scaling weight is 1/√s, and it is intermediate between the extremes of no scaling and autoscaling (Figure 5D). The data do not become dimensionless after autoscaling, so this method stays closer to the original measurement than autoscaling.
Another objective of data pre-treatment is converting a non-normal distribution of the specific variable into a normal one. One way to accomplish this is through log transformation (Figure 5E). The benefits of this sort of transformation include: (1) simplifying the response function by linearizing a non-linear response-factor relationship; (2) stabilizing the variance of the residuals; and (3) making the distribution of the residuals more normal, which can serve to eliminate outliers. Power transformation plots the square root of the data (Figure 5F) and is similar to the Pareto scaling method. Finally, the area normalization method (Figure 5G) showed similar results when compared to the original data (Figure 5A). The area normalization method is a semi-quantitative approach using the relative percentage of each compound of the total MVOCs extracted.
Each of the six data pretreatment methods was applied to the entire dataset. PCA was used to analyze the effect of each method (Figure 6). PCA can also identify important MVOCs contributing to classification by analysis of the loadings. Suitable data pretreatment methods help provide good cluster separation where the distance within the cluster of a specific category (media type) and the distance between the clusters of the categories are favorable. The application of log transformation (Figure 6E) provided the best clustering results in the score plots. PCA analysis of the centering (Figure 6B), Pareto scaling (Figure 6D) and the original data (Figure 6A) provided poor clustering results compared to the other pretreatment methods. Power transformed data showed intermediate cluster separation. Tight clusters were produced with the area normalization method (Figure 6G); however, the CDA, CSA and CDL clusters were not well separated.
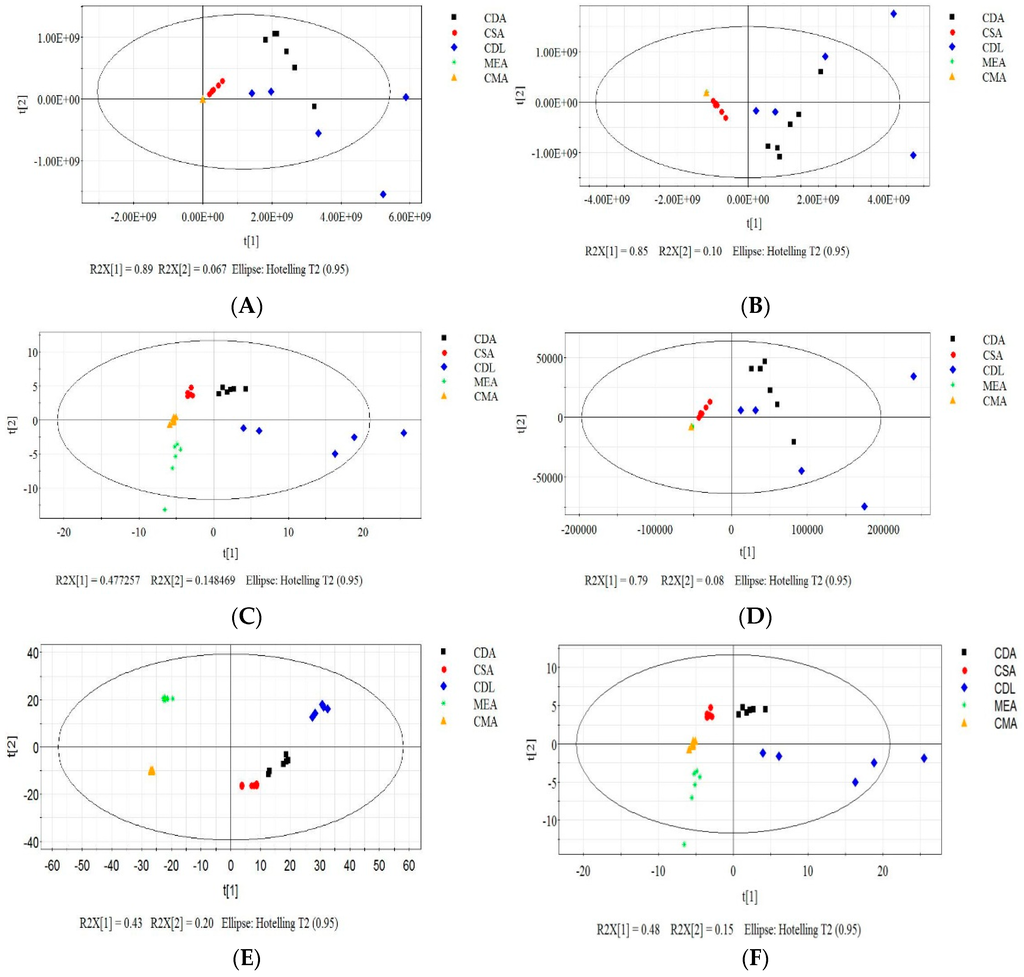
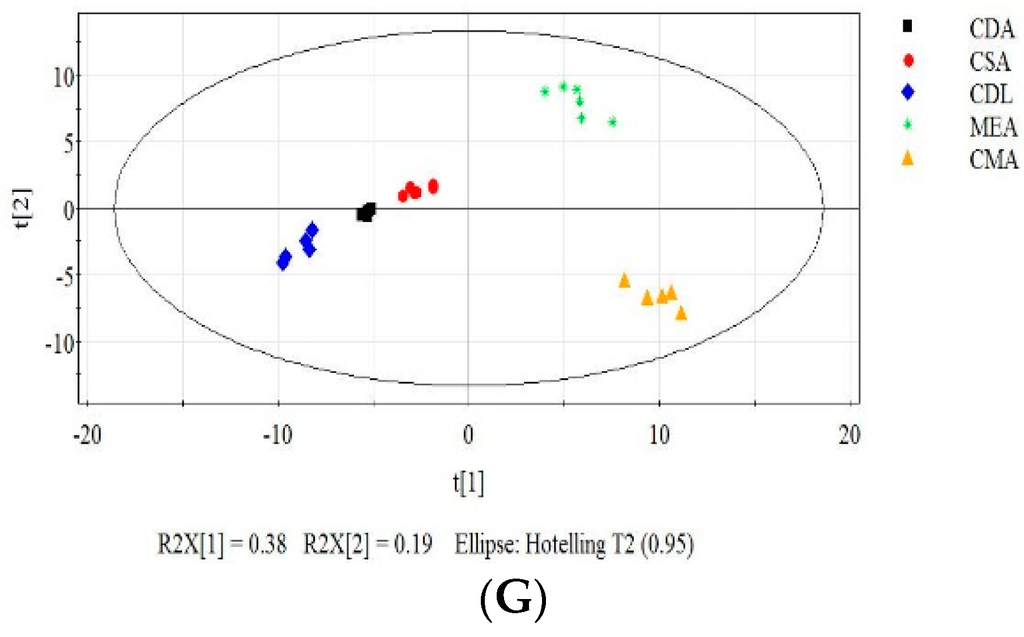
Figure 6.
Effect of data pretreatment on the PCA results. PCA results of original data (A), centered data (B), autoscaled data (C), Pareto scaled data (D), log transformed data (E), power transformed data (F) and area normalized data (G).
In the original (Figure 6A) and centering data (Figure 6B), MEA, CMA and CSA clusters “squeeze” together because CDA and CDL have much larger variances in the data caused by a higher concentration of MVOCs. Large variances play an important role for the classification of different categories (media) in PCA analysis. Poor cluster separation resulted from data pretreatment without a “hard” scaling method, such as autoscaling. Pareto scaling is the intermediate between no scaling and autoscaling, which also showed unfavorable classification.
4. Conclusions
This study demonstrates that the experimental parameters used for MVOCs’ fingerprinting are crucial to the outcomes of MVOCs profiles and the data analysis. The identity and quantity of MVOCs extracted can be affected by many factors. MVOC profile trends were observed for: (1) the selection of SPME fiber; (2) fungal growth medium; and (3) growth temperature. The original spore dose also changes the MVOC profiles; however, no clear trends were observed. The CAR/PDMS fiber seems to perform better than the other SPME fibers by collecting a larger variety and quantity of MVOC. Fungi grown on the CDL media produced much larger quantities of MVOCs compared to CSA, CDA, CMA and MEA medium. The highest MVOC production was found at 30 °C (this is known to be the optimal temperature for aflatoxin production). The data pretreatment method is also a key component of data analysis. Proper pretreatment methods will lead to better cluster separation, which will aid in the discovery of relevant biomarkers.
Many of the results presented here highlight the difficulties associated with the chemotaxonomy of species from MVOC profiles even in a controlled laboratory environment. The goal of the development of an aflatoxigenic A. flavus monitoring system for food storage is extremely challenging. Our results indicate that changes in growth media and conditions will result in significantly different profiles than those produced in a laboratory environment. However, the methods used, both for MVOC profiling and analysis, along with aflatoxin analysis can be applied to generate new isolate profiles. These new profiles can then be used to develop monitoring strategies for the early identification of aflatoxigenic A. flavus contamination in an industrial setting.
Supplementary Materials
The following are available online at http://www.mdpi.com/2297-8739/3/2/13/s1.
Acknowledgments
The authors thank the Mississippi Corn Promotion Board for partial funding of this research and Mary Scruggs for excellent technical assistance. The authors would also like to acknowledge support from the University Grants Commission for the Joint Research Program entitled “Indo-US initiatives on Cleaner Energy and Water Research” between Jawaharlal Nehru University and Mississippi State University and the Mississippi State University Chemistry Department for financial support of this project. This article reports the results of research only. The mention of trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by Mississippi State University.
Author Contributions
Dongdi Sun, Todd Mlsna and Richard Baird conceived of and designed the experiments. Dongdi Sun, Jinyan She, Julie L. Gower and C. Elizabeth Stokes performed the experiments. Gary L. Windham provided the materials. Dongdi Sun analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gourama, H.; Bullerman, L.B. Aspergillus flavus and Aspergillus parasiticus: Aflatoxigenic fungi of concern in foods and feeds: A review. J. Food Prot. 1995, 58, 1395–1404. [Google Scholar]
- Lamb, M.C.; Sternitzke, D.A. Cost of aflatoxin to the farmer, buying point, and sheller segments of the southeast united states peanut industry. Pean. Sci. 2001, 28, 59–63. [Google Scholar] [CrossRef]
- Korpi, A.; Järnberg, J.; Pasanen, A.-L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.J.; Beaucham, C. Dominant microbial volatile organic compounds in 23 US homes. Chemosphere 2013, 90, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Moularat, S.; Hulin, M.; Robine, E.; Annesi-Maesano, I.; Caillaud, D. Airborne fungal volatile organic compounds in rural and urban dwellings: Detection of mould contamination in 94 homes determined by visual inspection and airborne fungal volatile organic compounds method. Sci. Total. Environ. 2011, 409, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kimura, T.; Tanaka, H.; Kaneko, S.; Ichii, S.; Kiuchi, M.; Suzuki, T. Analysis of volatile metabolites emitted by soil-derived fungi using head space solid-phase microextraction/gas chromatography/mass spectrometry: I. Aspergillus fumigatus, Aspergillus nidulans, Fusarium solani and Penicillium paneum. Surf. Interface Anal. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Kuske, M.; Romain, A.-C.; Nicolas, J. Microbial volatile organic compounds as indicators of fungi. Can an electronic nose detect fungi in indoor environments? Build. Environ. 2005, 40, 824–831. [Google Scholar] [CrossRef]
- Van Lancker, F.; Adams, A.; Delmulle, B.; de Saeger, S.; Moretti, A.; van Peteghem, C.; de Kimpe, N. Use of headspace SPME-GC-MS for the analysis of the volatiles produced by indoor molds grown on different substrates. J. Environ. Monitor. 2008, 10, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Jurjevic, Z.; Rains, G.C.; Wilson, D.M.; Lewis, W. Volatile metabolites associated with one aflatoxigenic and one nontoxigenic Aspergillus flavus strain grown on two different substrates. Phytopathol. Mediterr. 2009, 47, 266–271. [Google Scholar]
- Moularat, S.; Robine, E. Process for determining mycotoxin production from a specific chemical fingerprint. Google Patents US 20130244272 A1, 2012. [Google Scholar]
- Polizzi, V.; Adams, A.; Malysheva, S.V.; de Saeger, S.; van Peteghem, C.; Moretti, A.; Picco, A.M.; de Kimpe, N. Identification of volatile markers for indoor fungal growth and chemotaxonomic classification of Aspergillus species. Fungal Biol. 2012, 116, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Faubert, P.; Hagen, M.; zu Castell, W.; Polle, A.; Schnitzler, J.-P.; Rosenkranz, M. Volatile profiles of fungi—Chemotyping of species and ecological functions. Fungal Genet. Biol. 2013, 54, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lavine, B.K.; Mirjankar, N.; LeBouf, R.; Rossner, A. Prediction of mold contamination from microbial volatile organic compound profiles using head space gas chromatography/mass spectrometry. Microchem. J. 2012, 103, 119–124. [Google Scholar] [CrossRef]
- Griffin, J.L.; Nicholls, A.W. Metabolomics as a functional genomic tool for understanding lipid dysfunction in diabetes, obesity and related disorders. Pharmgenomics Pers. Med. 2006, 7, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Quinones, M.P.; Kaddurah-Daouk, R. Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol. Dis. 2009, 35, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Kaddurah-Daouk, R.; Kristal, B.S.; Weinshilboum, R.M. Metabolomics: A global biochemical approach to drug response and disease. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 653–683. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Applications of metabolomics in drug discovery and development. Drugs. R D 2008, 9, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Viant, M.R.; Tjeerdema, R.S. Metabolomics: Methodologies and applications in the environmental sciences. J. Pestic. Sci. 2006, 31, 245–251. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef] [PubMed]
- Holmquist, G.; Walker, H.; Stahr, H. Influence of Temperature, pH, Water Activity and Antifungal Agents on Growth of Aspergillus Flavus and A. Parasiticus. J. Food Sci. 1983, 48, 778–782. [Google Scholar] [CrossRef]
- Polizzi, V.; Adams, A.; de Saeger, S.; van Peteghem, C.; Moretti, A.; de Kimpe, N. Influence of various growth parameters on fungal growth and volatile metabolite production by indoor molds. Sci. Total Environ. 2012, 414, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Grintzalis, K.; Vernardis, S.I.; Klapa, M.I.; Georgiou, C.D. Role of Oxidative Stress in Sclerotial Differentiation and Aflatoxin B1 Biosynthesis in Aspergillus Flavus. Appl. Environ. Microbiol. 2014, 80, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- López-Malo, A.; Alzamora, S.M.; Argaiz, A. Effect of vanillin concentration, pH and incubation temperature on Aspergillus flavus, Aspergillus niger, Aspergillus ochraceus and Aspergillus parasiticus growth. Food Microbiol. 1997, 14, 117–124. [Google Scholar] [CrossRef]
- Joffe, A.; Lisker, N. Effects of light, temperature, and pH value on aflatoxin production in vitro. Appl. Environ. Microbiol. 1969, 18, 517–518. [Google Scholar]
- Calenic, B.; Miricescu, D.; Greabu, M.; Andrey, V.K.; Troppmair, J.; Ruzsanyi, V.; Amann, A. Oxidative stress and volatile organic compounds: Interplay in pulmonary, cardio-vascular, digestive tract systems and cancer. Open Chem. 2015, 13, 1020–1030. [Google Scholar] [CrossRef]
- Sunesson, A.; Vaes, W.; Nilsson, C.; Blomquist, G.; Andersson, B.; Carlson, R. Identification of volatile metabolites from five fungal species cultivated on two media. Appl. Environ. Microbiol. 1995, 61, 2911–2918. [Google Scholar] [PubMed]
- Betancourt, D.A.; Krebs, K.; Moore, S.A.; Martin, S.M. Microbial volatile organic compound emissions from Stachybotrys chartarum growing on gypsum wallboard and ceiling tile. BMC Microbiol. 2013, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.O.; Frisvad, J.C. Comparison of different methods for collection of volatile chemical markers from fungi. J. Microbiol. Methods 1995, 24, 135–144. [Google Scholar] [CrossRef]
- Camara, M.; Gharbi, N.; Cocco, E.; Guignard, C.; Behr, M.; Evers, D.; Orlewski, P. Fast screening for presence of muddy/earthy odorants in wine and in wine must using a hyphenated gas chromatography-differential ion mobility spectrometry (GC/DMS). Int. J. Ion. Mobil. Spectrom. 2011, 14, 39–47. [Google Scholar] [CrossRef]
- Demyttenaere, J.C.R.; Moriña, R.M.; Sandra, P. Monitoring and fast detection of mycotoxin-producing fungi based on headspace solid-phase microextraction and headspace sorptive extraction of the volatile metabolites. J. Chromatogr. A 2003, 985, 127–135. [Google Scholar] [CrossRef]
- Jeleń, H. Use of solid phase microextraction (SPME) for profiling fungal volatile metabolites. Lett. Appl. Microbiol. 2003, 36, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wood-Jones, A.; Wang, W.; Vanlangenberg, C.; Jones, D.; Gower, J.; Simmons, P.; Baird, R.; Mlsna, T. Monitoring MVOC profiles over time from isolates of Aspergillus flavus using SPME GC-MS. J. Agric. Chem. Environ. 2014, 3, 48–63. [Google Scholar] [CrossRef]
- Kluger, B.; Zeilinger, S.; Wiesenberger, G.; Schöfbeck, D.; Schuhmacher, R. Detection and identification of fungal microbial volatile organic compounds by HS-SPME-GC-MS. In Laboratory Protocols in Fungal Biology; Springer: Berlin, Geramny, 2013; pp. 455–465. [Google Scholar]
- Deshmukh, Y.; Khare, P.; Patra, D.; Nadaf, A.B. HS-SPME-GC-FID method for detection and quantification of Bacillus cereus ATCC 10702 mediated 2-acetyl-1-pyrroline. Biotechnol. Prog. 2014, 30, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Shirey, R.E. Optimization of extraction conditions and fiber selection for semivolatile analytes using solid-phase microextraction. J. Chromatogr. Sci. 2000, 38, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Stoppacher, N.; Kluger, B.; Zeilinger, S.; Krska, R.; Schuhmacher, R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods 2010, 81, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, M.T.; Mayahi, S.; Denning, D.W. A study on Aspergillus species in houses of asthmatic patients from Sari City, Iran and a brief review of the health effects of exposure to indoor Aspergillus. Environ. Monit. Assess. 2010, 168, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.; Larsen, K.; Simkus, M. Volatile metabolites from indoor molds grown on media containing wood constituents. Environ. Sci. Pollut. Res. 2003, 10, 206–208. [Google Scholar] [CrossRef]
- Radványi, D.; Gere, A.; Jókai, Z.; Fodor, P. Rapid evaluation technique to differentiate mushroom disease-related moulds by detecting microbial volatile organic compounds using HS-SPME-GC-MS. Anal. Bioanal. Chem. 2015, 407, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Zscheppank, C.; Wiegand, H.; Lenzen, C.; Wingender, J.; Telgheder, U. Investigation of volatile metabolites during growth of Escherichia coli and Pseudomonas aeruginosa by needle trap-GC-MS. Anal. Bioanal. Chem. 2014, 406, 6617–6628. [Google Scholar] [CrossRef] [PubMed]
- Koek, M.M.; Muilwijk, B.; van der Werf, M.J.; Hankemeier, T. Microbial metabolomics with gas chromatography/mass spectrometry. Anal. Chem. 2006, 78, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Matysik, S.; Herbarth, O.; Mueller, A. Determination of microbial volatile organic compounds (MVOCs) by passive sampling onto charcoal sorbents. Chemosphere 2009, 76, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Syhre, M.; Scotter, J.M.; Chambers, S.T. Investigation into the production of 2-Pentylfuran by Aspergillus fumigatus and other respiratory pathogens in vitro and human breath samples. Med. Mycol. 2008, 46, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.; Nielsen, K.F.; Din, S.U. Patterns of volatile metabolites and nonvolatile trichothecenes produced by isolates of Stachybotrys, Fusarium, Trichoderma, Trichothecium and Memnoniella. Environ. Sci. Pollut. Res. 2003, 10, 162–166. [Google Scholar] [CrossRef]
- Meruva, N.; Penn, J.; Farthing, D. Rapid identification of microbial VOCs from tobacco molds using closed-loop stripping and gas chromatography/time-of-flight mass spectrometry. J. Ind Microbiol. Biotechnol. 2004, 31, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Ruzsanyi, V.; Baumbach, J.; Eiceman, G. Detection of the mold markers using ion mobility spectrometry. Int. J. Ion. Mobil. Spectrom. 2003, 6, 53–57. [Google Scholar]
- Tiebe, C.; Hübert, T.; Koch, B.; Ritter, U.; Stephan, I. Investigation of gaseous metabolites from moulds by ion mobility spectrometry (IMS) and gas chromatography-mass spectrometry (GC-MS). Int. J. Ion Mobil. Spectrom. 2010, 13, 17–24. [Google Scholar] [CrossRef]
- Van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Bro, R.; Smilde, A.K. Centering and scaling in component analysis. J. Chemom. 2003, 17, 16–33. [Google Scholar] [CrossRef]
- Kvalheim, O.M.; Brakstad, F.; Liang, Y. Preprocessing of analytical profiles in the presence of homoscedastic or heteroscedastic noise. Anal. Chem. 1994, 66, 43–51. [Google Scholar] [CrossRef]
- Thorn, R.M.; Reynolds, D.M.; Greenman, J. Multivariate analysis of bacterial volatile compound profiles for discrimination between selected species and strains in vitro. J. Microbiol. Methods 2011, 84, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Claeson, A.S.; Nordin, S.; Sunesson, A.L. Effects on perceived air quality and symptoms of exposure to microbially produced metabolites and compounds emitted from damp building materials. Indoor Air 2009, 19, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Pinzari, F.; Fanelli, C.; Canhoto, O.; Magan, N. Electronic nose for the early detection of moulds in libraries and archives. Indoor Built Environ. 2004, 13, 387–395. [Google Scholar] [CrossRef]
- Reddy, T.; Viswanathan, L.; Venkitasubramanian, T. Factors affecting aflatoxin production by Aspergillus parasiticus in a chemically defined medium. Microbiology 1979, 114, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Gullberg, J.; Nordström, A.; Kusano, M.; Kowalczyk, M.; Sjöström, M.; Moritz, T. A strategy for identifying differences in large series of metabolomic samples analyzed by GC/MS. Anal. Chem. 2004, 76, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Elsevier: Philadelphia, PA, USA, 2011. [Google Scholar]
- Rega, B.; Fournier, N.; Guichard, E. Solid phase microextraction (SPME) of orange juice flavor: Odor representativeness by direct gas chromatography olfactometry (D-GC-O). J. Agric. Food Chem. 2003, 51, 7092–7099. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.O.; Frisvad, J.C. Characterization of volatile metabolites from 47 Penicillium taxa. Mycol. Res. 1995, 99, 1153–1166. [Google Scholar] [CrossRef]
- Kahlos, K.; Kiviranta, J.L.; Hiltunen, R.V. Volatile constituents of wild and in vitro cultivated Gloeophyllum odoratum. Phytochemistry 1994, 36, 917–922. [Google Scholar] [CrossRef]
- Wheatley, R.; Hackett, C.; Bruce, A.; Kundzewicz, A. Effect of substrate composition on production of volatile organic compounds from Trichoderma spp. inhibitory to wood decay fungi. Int. Biodeterior. Biodegrad. 1997, 39, 199–205. [Google Scholar] [CrossRef]
- Gao, P.; Korley, F.; Martin, J.; Chen, B.T. Determination of unique microbial volatile organic compounds produced by five Aspergillus species commonly found in problem buildings. Am. Ind. Hyg. Assoc. J. 2002, 63, 135–140. [Google Scholar] [CrossRef]
- Schindler, A.F.; Palmer, J.G.; Eisenberg, W.V. Aflatoxin production by Aspergillus flavus as related to various temperatures. J. Appl. Microbiol. 1967, 15, 1006–1009. [Google Scholar]
- Karunaratne, A.; Bullerman, L.B. Interactive effects of spore load and temperature on aflatoxin production. J. Food Prot. 1990, 53, 227–236. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).