Application of Lignin-Derived Carbon Materials in Adsorption and Separation
Abstract
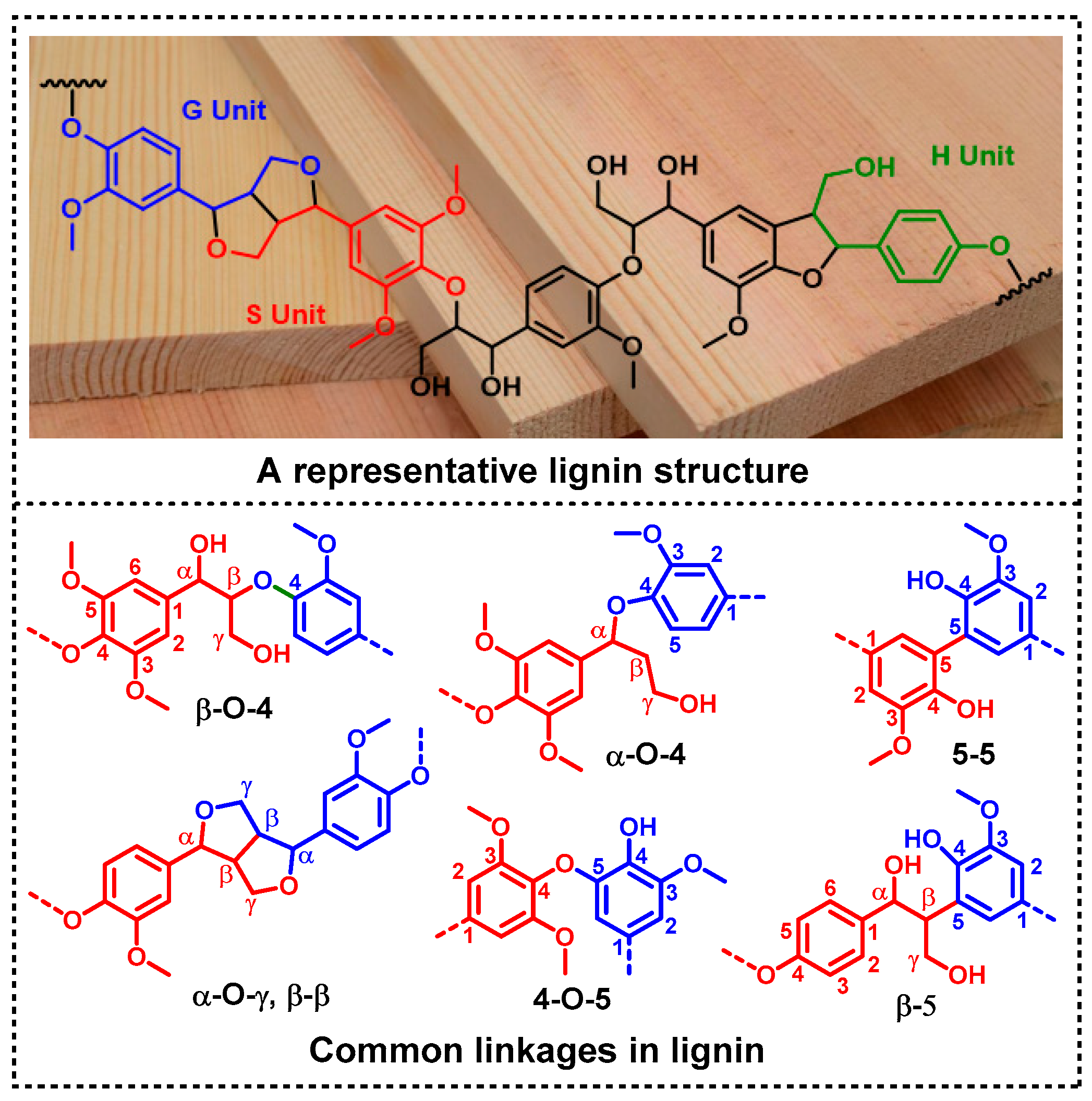
:1. Introduction
2. Type of Carbon Materials
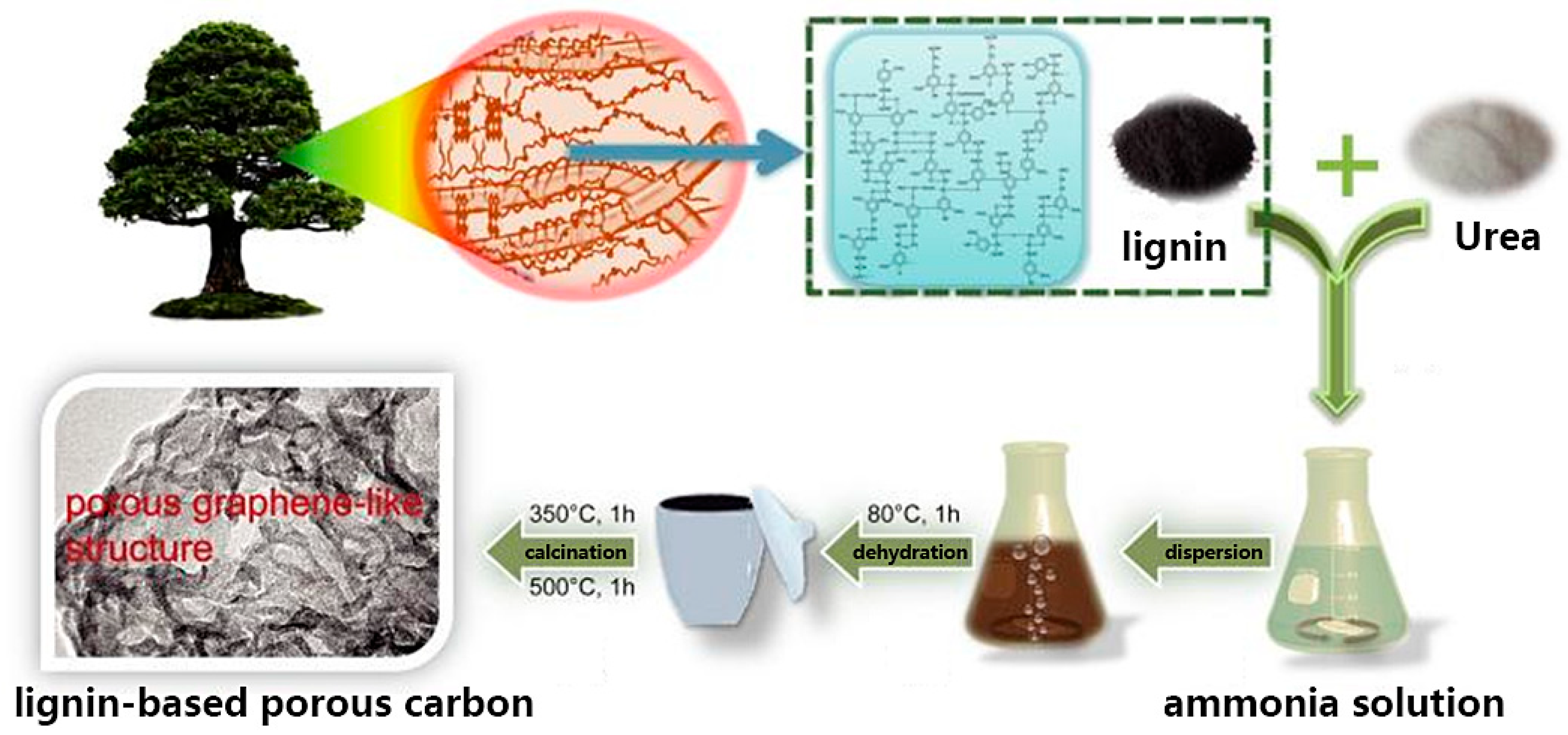
2.1. Porous Carbon
2.2. Activated Carbon
2.3. Carbon Aerogel
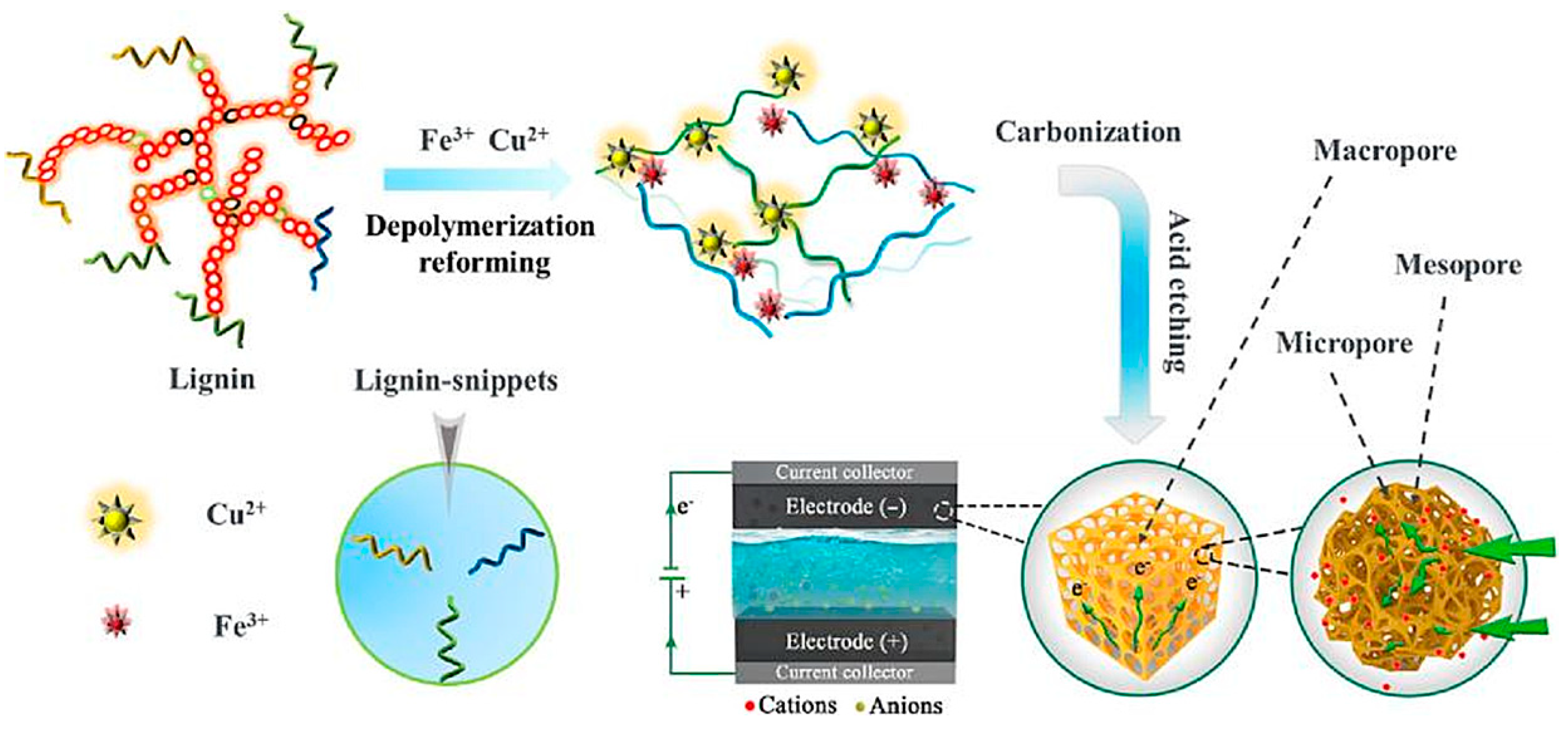
2.4. Carbon Foam
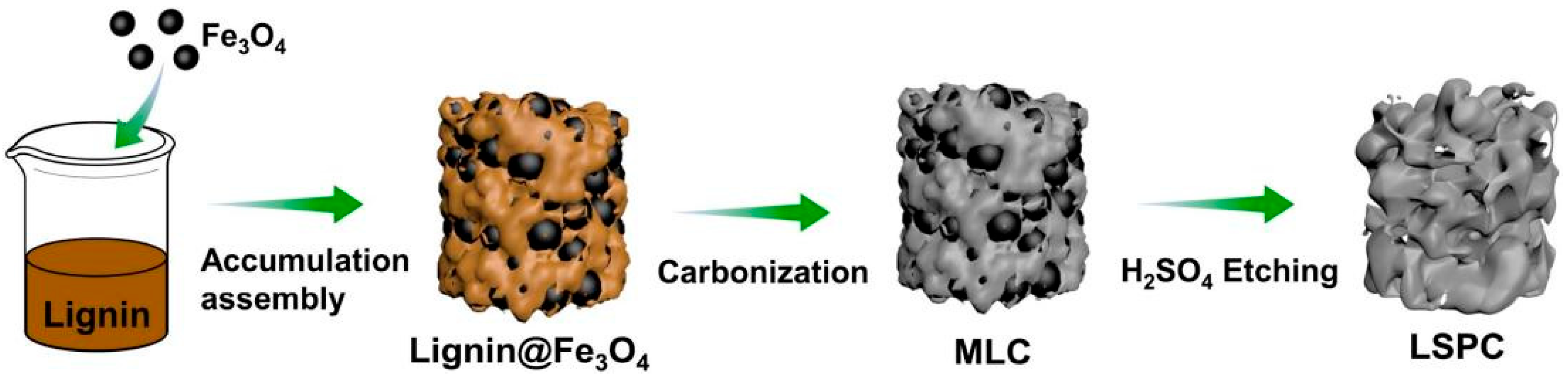
2.5. Carbon Fiber
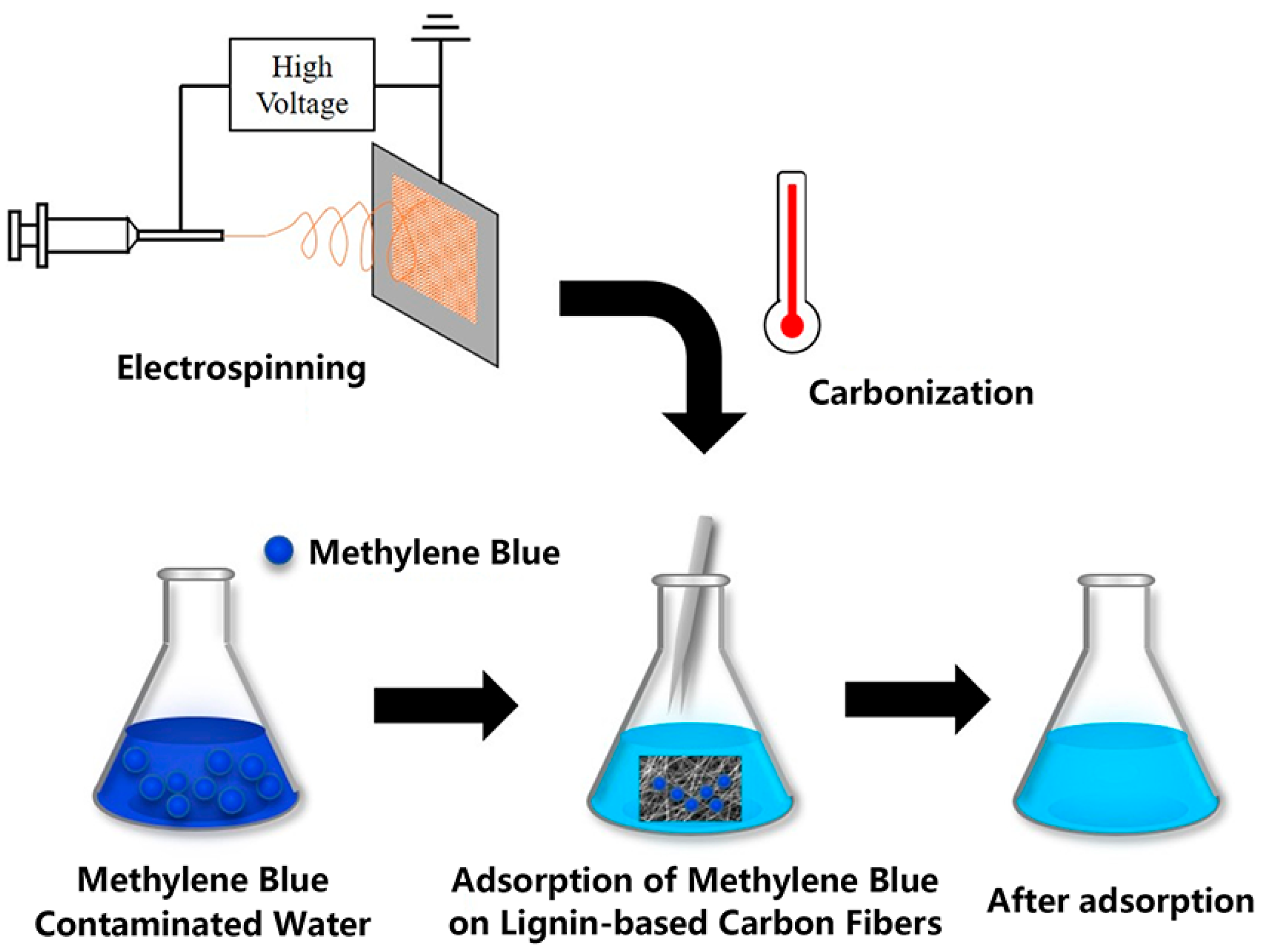
3. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acrylic acid |
| ACFF | Lignin carbon fibers activated by iron nitrate |
| ACFK | Lignin carbon fibers prepared by chemical activation method |
| ACFN | Lignin carbon fibers activated by nickel nitrate |
| ACFS | Lignin carbon fibers prepared by physical activation method |
| ACFZ | Zinc nitrate activated lignin carbon fibers |
| ACs | Activated carbons |
| ADL | Lignin derivative |
| ADLC | Lignin-derived porous carbon |
| AIBN | Azobisisobutyronitrile |
| AL | Alkali lignin |
| APS | Ammonium persulfate |
| BET | Brunauer-emmett-teller |
| Bio-KLB-C | Bio-clean lignin-based carbon fiber |
| BLAC | Sugarcane bagasse lignin |
| BLL | Black liquor lignin |
| BMC | Basic magnesium carbonate |
| C-BLL-BMC | Black liquor lignin activated by basic magnesium carbonate |
| C-BLL-KOH | KOH-activated black liquor lignin |
| C-BLL-MgO | MgO-activated black liquor lignin |
| C-BLL-ZnCl2 | Black liquor lignin activated by zinc chloride |
| CL | Lignin precursor |
| CSLAC | Corn stover lignin |
| CSLAC-800 | CSLAC carbonized at 800 °C |
| DES | Deep eutectic solvent |
| DETA | Diethylenetriamine |
| DMF | N,N-Dimethylformamide |
| DW | Lignin-based carbon foams prepared by the DES method |
| DW-800 | DW with a carbonization temperature of 800 °C |
| G | Guaiacyl |
| GL | Grass lignin |
| GO | Graphene oxide |
| H | P-hydroxyphenyl |
| HL | Hardwood lignin |
| KL | Sulfated lignin |
| LACGO | Lignin-based carbon aerogel |
| LCF | Lignin-based carbon foam |
| LCFs | Lignin carbon fibers |
| LCFs-Fe | Lignin carbon fibers with the addition of Fe3O4 |
| L-CSs | Lignin-based carbon spheres |
| LF | Lignin fibers |
| LF-Fe | Lignin fibers with the addition of Fe3O4 |
| LHGO | Graphene oxide reinforced lignin-based hydrogel |
| L-HPC | Lignin-based layered porous carbon |
| LPC | Lignin-based porous carbon |
| LPF | Phenolic resin |
| LSAC | Lignin sulfonate lignin |
| LSPC | Lignin-based sulfonated porous carbon |
| LTCAs | Lignin-based carbon aerogel |
| MAC0.5–2 | MACs with 0.5:2 mass ratio of Fenton sludge and KOH |
| MAC1–2 | MACs with 1:2 mass ratio of Fenton sludge and KOH |
| MACs | Lignin-based magnetic activated carbon |
| MB | Methylene blue |
| MBA | N, N′-methylenebisacrylamide |
| NaLS-kACA | Lignin carbon aerogels prepared from κ-carrageenan |
| PAN | Polyacrylonitrile |
| PANCNF | Lignin carbon fiber prepared from polyacrylonitrile |
| PAN/SLACNFs | PAN/SLCNF after nitrification |
| PAN/SLCNF | Lignin carbon fibers prepared from polyacrylonitrile and sago lignin |
| PEO | Polyethylene oxide |
| PU | Polyurethane |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| S | Syringyl |
| SL | Softwood lignin |
| SLs | Sago lignin |
| TEMPO | 2,2,6,6-tetramethylpiperidine-1-oxyl radical |
| TOCNF | Cellulose nanofibers oxidized by TEMPO |
| VOCs | Volatile organic compounds |
References
- Saxena, V. Water quality, air pollution, and climate change: Investigating the environmental impacts of industrialization and urbanization. Water Air Soil Pollut. 2025, 236, 73. [Google Scholar]
- Zahoor; Madadi, M.; Nazar, M.; Shah, S.W.A.; Li, N.N.; Imtiaz, M.; Zhong, Z.J.; Zhu, D.C. Green alkaline fractionation of sugarcane bagasse at cold temperature improves digestibility and delignification without the washing processes and release of hazardous waste. Ind. Crop. Prod. 2023, 200, 116815. [Google Scholar]
- Nazar, M.; Xu, Q.; Zahoor; Ullah, M.W.; Khan, N.A.; Iqbal, B.; Zhu, D.C. Integrated laccase delignification with improved lignocellulose recalcitrance for enhancing enzymatic saccharification of ensiled rice straw. Ind. Crop. Prod. 2023, 202, 116987. [Google Scholar]
- Deng, J.L.; Chen, P.L.; Xia, S.P.; Zheng, M. Advances in oxidative coupling of methane. Atmosphere 2023, 14, 1538. [Google Scholar] [CrossRef]
- Bu, Q.; Chen, K.; Xie, W.; Liu, Y.Y.; Cao, M.J.; Kong, X.H.; Chu, Q.L.; Mao, H.P. Hydrocarbon rich bio-oil production, thermal behavior analysis and kinetic study of microwave-assisted co-pyrolysis of microwave-torrefied lignin with low density polyethylene. Bioresour. Technol. 2019, 291, 121860. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Z.Q.; Zhou, B.L.; Yang, Y.Y.; Chen, D.Y. Enhancement of the production of bio-aromatics from renewable lignin by combined approach of torrefaction deoxygenation pretreatment and shape selective catalytic fast pyrolysis using metal modified zeolites. Bioresour. Technol. 2020, 301, 122754. [Google Scholar]
- Li, M.; Jiang, H.N.; Zhang, L.; Yu, X.J.; Liu, H.; Yagoub, A.E.A.; Zhou, C.S. Synthesis of 5-HMF from an ultrasound-ionic liquid pretreated sugarcane bagasse by using a microwave-solid acid/ionic liquid system. Ind. Crop. Prod. 2020, 149, 112361. [Google Scholar] [CrossRef]
- Ji, Q.Y.; Yu, X.J.; Chen, L.; Yarley, O.P.N.; Zhou, C.S. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crop. Prod. 2021, 172, 114064. [Google Scholar]
- Sompolska-Rzenchula, A.; Bak, I.; Becker, A.; Marjak, J.; Perzynska, J. The use of renewable energy sources and environmental degradation in EU Countries. Sustainability 2024, 16, 10416. [Google Scholar] [CrossRef]
- Ji, Q.H.; Yu, X.J.; Yagoub, A.E.A.; Chen, L.; Zhou, C.S. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crop. Prod. 2020, 149, 112357. [Google Scholar]
- Ma, Q.N.; Ji, Q.H.; Chen, L.; Zhu, Z.L.; Tu, S.S.; Okonkwo, C.E.; Out, P.; Zhou, C.S. Multimode ultrasound and ternary deep eutectic solvent sequential pretreatments enhanced the enzymatic saccharification of corncob biomass. Ind. Crop. Prod. 2022, 188, 115574. [Google Scholar] [CrossRef]
- Ji, Q.H.; Zhou, C.S.; Li, Z.Q.; Boateng, I.D.; Liu, X.M. Is nanocellulose a good substitute for non-renewable raw materials? A comprehensive review of the state of the art, preparations, and industrial applications. Ind. Crop. Prod. 2023, 202, 117093. [Google Scholar] [CrossRef]
- Liang, J.K.; Li, H.X.; Chen, L.; Ren, M.N.; Fakayode, O.A.; Han, J.Y.; Zhou, C.S. Efficient hydrogen evolution reaction performance using lignin-assisted chestnut shell carbon-loaded molybdenum disulfide. Ind. Crop. Prod. 2023, 193, 116214. [Google Scholar] [CrossRef]
- Wang, Y.L.; Sun, J.Z.; Ali, S.S.; Gao, l.; Ni, X.N.; Li, X.; Wu, Y.F.; Jiang, J.X. Identification and expression analysis of sorghum bicolor gibberellin oxidase genes with varied gibberellin levels involved in regulation of stem biomass. Ind. Crop. Prod. 2020, 145, 111951. [Google Scholar] [CrossRef]
- Sun, L.Y.; Han, J.; Wu, J.C.; Huang, W.R.; Li, Y.Y.; Mao, Y.L.; Wang, L.; Wang, Y. Cellulose pretreatment with inorganic salt hydrate: Dissolution, regeneration, structure and morphology. Ind. Crop. Prod. 2022, 180, 114722. [Google Scholar] [CrossRef]
- Lu, J.J.; Cheng, M.Y.; Zhao, C.; Li, B.; Peng, H.H.; Zhang, Y.J.; Shao, Q.J.; Hassan, M. Application of lignin in preparation of slow-release fertilizer: Current status and future perspectives. Ind. Crop. Prod. 2022, 176, 114267. [Google Scholar] [CrossRef]
- Ghimben, G.M.; Zhang, B.; Yuso, A.M.D.; Rety, B.; Tarascon, J.-M. Valorizing low cost and renewable lignin as hard carbon for Na-ion batteries: Impact of lignin grade. Carbon 2019, 153, 634–647. [Google Scholar]
- Yao, M.Z.; Bi, X.Y.; Wang, Z.H.; Yu, P.; Dufresne, A.; Jiang, C. Recent advances in lignin-based carbon materials and their applications: A review. Int. J. Biol. Macromol. 2022, 233, 980–1014. [Google Scholar] [CrossRef]
- Yu, X.N.; Wei, Z.Q.; Lu, Z.X.; Pei, H.S.; Wang, H.L. Activation of lignin by selective oxidation: An emerging strategy for boosting lignin depolymerization to aromatics. Bioresour. Technol. 2019, 291, 121885. [Google Scholar]
- Kulas, D.G.; Thies, M.; Shonnard, D. Techno-economic analysis and life cycle assessment of waste lignin fractionation and valorization using the ALPHA Process. ACS Sustain. Chem. Eng. 2021, 9, 5388–5395. [Google Scholar] [CrossRef]
- Liu, M.Y.; Sun, Z.H.; Elangovan, S. Bioactive molecules from lignin via homogeneous and heterogeneous catalytic pathways. Trends Chem. 2023, 5, 713–716. [Google Scholar]
- Huang, W.R.; He, X.C.; Wu, J.C.; Ma, X.N.; Han, J.; Wang, L.; Wang, Y. The evaluation of deep eutectic solvents and ionic liquids as cosolvents system for improving cellulase properties. Ind. Crop. Prod. 2023, 197, 116555. [Google Scholar]
- Nazir, M.J.; Li, G.L.; Nazir, M.M.; Zulfiqar, F.; Siddique, K.H.M.; Iqbal, B.; Du, D.L. Harnessing soil carbon sequestration to address climate change challenges in agriculture. Soil Tillage Res. 2024, 237, 105959. [Google Scholar]
- Li, H.X.; Liang, J.K.; Chen, L.; Ren, M.N.; Zhou, C.S. Utilization of walnut shell by deep eutectic solvents: Enzymatic digestion of cellulose and preparation of lignin nanoparticles. Ind. Crop. Prod. 2023, 192, 116034. [Google Scholar]
- Han, S.Q.; Xie, H.H.; Zhang, L.; Wang, X.H.; Zhong, Y.; Shen, Y.T.; Wang, H.L.; Hao, C. High-performance polyethylenimine-functionalized lignin/silica porous composite microsphere for the removal of hexavalent chromium, phosphate and Congo red from aqueous solutions. Ind. Crop. Prod. 2023, 194, 116289. [Google Scholar]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafan-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar]
- Tong, Y.; Yang, T.H.; Wang, J.; Li, B.S.; Zhai, Y.M.; Li, R.D. A review on the overall process of lignin to phenolic compounds for chemicals and fuels: From separation and extraction of lignin to transformation. J. Anal. Appl. Pyrolysis 2024, 181, 106663. [Google Scholar]
- Spiridon, L. Extraction of lignin and therapeutic applications of lignin-derived compounds. A review. Environ. Chem. Lett. 2020, 18, 771–785. [Google Scholar]
- Wang, Y.T.; Yu, X.N.; Ma, S.S.; Cao, S.L.; Yuan, X.F.; Zhu, W.B.; Wang, H.L. High-value utilization of lignin: Construction of an intelligent release system for targeting the delivery of pesticides. Green Chem. 2024, 26, 42–56. [Google Scholar]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar]
- Wang, S.C.; Bai, J.X.; Innocent, M.T.; Wang, Q.Q.; Xiang, H.X.; Tang, J.G.; Zhu, M.F. Lignin-based carbon fibers: Formation, modification and potential applications. Green Energy Environ. 2022, 7, 578–605. [Google Scholar] [CrossRef]
- Faix, O.; Beinhoff, O. Determination of phenolic hydroxyl group content of milled wood lignins (MWU’s) from different botanical origins using selective aminolysis, FTIR, 1H-NMR, and UV spectroscopy. Holzforschung 1992, 46, 425–432. [Google Scholar]
- Long, S.Y.; Qin, Q.; Liu, J.L.; Xian, X.Q. Study on the lignin-derived sp2 -sp3 hybrid hard carbon materials and the feasibility for industrial production. Sci. Rep. 2024, 14, 5091. [Google Scholar]
- Ren, M.N.; Kong, F.G.; Zhou, C.S.; Fakayode, O.A.; Liang, J.K.; Li, H.X.; Zhou, M.; Fan, X.Y. Green, one-pot biomass hierarchical utilization strategy for lignin-containing cellulose nanofibrils and fractionated lignin preparation. Ind. Crop. Prod. 2023, 203, 117193. [Google Scholar]
- Wang, C.P.; He, G.; Meng, J.; Wang, S.M.; Kong, Y.Z.; Jiang, J.X.; Hu, R.B.; Zhou, G.K. Improved lignocellulose saccharification of a miscanthus reddish stem mutant induced by heavy-ion irradiation. GCB Bioenergy 2020, 12, 1066–1077. [Google Scholar]
- Pu, Y.; Zhou, Q.Y.; Yu, L.; Li, C.; Dong, Y.W.; Yu, N.N.; Chen, X.H. Longitudinal analyses of lignin deposition in green asparagus by microscopy during high oxygen modified atmosphere packaging. Food Packag. Shelf Life 2020, 25, 100536. [Google Scholar]
- Okonkwo, C.E.; Hussain, S.Z.; Onyeaka, H.; Adeyanju, A.A.; Nwonuma, C.O.; Bashir, A.A.; Farooq, A.; Zhou, C.S.; Shittu, T.D. Lignin polyphenol: From biomass to innovative food applications, and influence on gut microflora. Ind. Crop. Prod. 2023, 206, 117696. [Google Scholar]
- Ji, Q.H.; Yu, X.J.; Wu, P.W.; Yagoub, A.E.A.; Chen, L.; Mustapha, A.T.; Zhou, C.S. Pretreatment of sugarcane bagasse with deep eutectic solvents affect the structure and morphology of lignin. Ind. Crop. Prod. 2021, 173, 114108. [Google Scholar]
- Zhang, W.L.; Qiu, X.Q.; Wang, C.W.; Zhong, L.; Fu, F.B.; Zhu, J.H.; Zhang, Z.J.; Qin, Y.L.; Yang, D.J.; Xu, C.C. Lignin derived carbon materials: Current status and future trends. Carbon Res. 2022, 1, 14. [Google Scholar] [CrossRef]
- Gao, S.; Tian, G.Y.; Fu, Y.J.; Wang, Z.J. Production of cellulose pulp and lignin from high-density apple wood waste by preimpregnation-assisted soda cooking. Polymers 2023, 15, 1693. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.H.; Lin, Q.X.; Yue, F.X. Structural features of lignin fractionated from industrial furfural residue using alkaline cooking technology and its antioxidant performance. Front. Energy Res. 2020, 8, 83. [Google Scholar]
- Friedman, S.H. The four worlds of carbon. Nat. Chem. 2012, 4, 426. [Google Scholar]
- Zhang, L.H.; Chen, X.Y.; Li, L.Y.; Li, X.R.; Wu, H.; Zheng, J.F.; Yao, J.R.; Li, G.F. Porous and high specific surface area carbon material derived from ginkgo leaves for high-performance symmetric supercapacitors. Biomass Bioenerg. 2024, 191, 107481. [Google Scholar]
- Chen, Z.K.; Jiang, X.L.; Boyjoo, Y.; Zhang, L.; Li, W.; Zhao, L.; Liu, Y.X.; Zhang, Y.G.; Liu, J.; Li, X.F. Nanoporous carbon materials derived from biomass precursors: Sustainable materials for energy conversion and storage. Electrochem. Energy Rev. 2024, 7, 26. [Google Scholar]
- Priya, A.K.; Muruganandam, M.; Suresh, S. Bio-derived carbon-based materials for sustainable environmental remediation and wastewater treatment. Chemosphere 2024, 362, 142731. [Google Scholar] [PubMed]
- Tong, Y.; Yang, J.Y.; Li, J.J.; Cong, Z.Y.; Wei, L.; Liu, M.M.; Zhai, S.; Wang, K.; An, Q.D. Lignin-derived electrode materials for supercapacitor applications: Progress and perspectives. J. Mater. Chem. 2023, 11, 1061–1082. [Google Scholar]
- Shao, S.S.; Ma, L.X.; Li, X.H.; Zhang, H.Y.; Xiao, R. Preparation of activated carbon with heavy fraction of bio-oil from rape straw pyrolysis as carbon source and its performance in the aldol condensation for aviation fuel as carrier. Ind. Crop. Prod. 2023, 192, 115912. [Google Scholar]
- Li, W.; Wang, G.H.; Zhang, W.H.; Li, J.K.; Zhang, B.; Si, C.L. Lignin-derived 0–3 dimensional carbon materials: Synthesis, configurations and applications. Ind. Crop. Prod. 2023, 204, 117342. [Google Scholar]
- Yin, X.X.; Tao, J.Y.; Wang, J.L.; Yan, B.B.; Chen, G.Y.; Cheng, Z.J. Prediction of activation energy of lignocellulosic biomass pyrolysis through thermogravimetry-assisted machine learning. Biomass Bioenergy 2025, 194, 107644. [Google Scholar]
- Zhao, J.; Zhang, W.J.; Wang, Q.C.; Shen, D.K.; Wang, Z.H. Lignin-derived porous carbons for efficient CO2 adsorption. Carbon Capture Sci. Technol. 2024, 13, 100233. [Google Scholar]
- Gao, Y.; Yue, Q.Y.; Gao, B.Y.; Sun, Y.Y.; Wang, W.Y.; Li, Q.; Wang, Y. Preparation of high surface area-activated carbon from lignin of papermaking black liquor by KOH activation for Ni(II) adsorption. Chem. Eng. J. 2013, 217, 345–353. [Google Scholar]
- Chen, C.Z.; Chen, W.; Zhou, M.Q.; Xiong, Y.Z.; Ji, X.L.; Zhou, M.H.; Zhang, L.L.; Rao, X.P.; Jiang, J.C. Co-ZIF reinforced kraft lignin biochar as an efficient catalyst for highly selective hydrodeoxygenation of lignin-derived chemicals. Chem. Eng. J. 2024, 492, 152353. [Google Scholar]
- Xue, Z.L.; Sun, H.; Wang, G.H.; Sui, W.J.; Jia, H.Y.; Si, C.L. Fabrication modulation of lignin-derived carbon nanosphere supported Pd nanoparticle via lignin fractionation for improved catalytic performance in vanillin hydrodeoxygenation. Int. J. Biol. Macromol. 2024, 258, 128963. [Google Scholar]
- Liu, S.R.; Li, Q.F.; Zou, S.L.; Xia, H.A. Facile synthesis of lignosulfonate-derived sulfur-doped carbon materials for photocatalytic degradation of tetracycline under visible-light irradiation. Microporous Mesoporous Mater. 2022, 336, 111876. [Google Scholar]
- Munir, M.T.; Naqvi, M.; Li, B.; Raza, R.; Khan, A.; Taqvi, S.A.A.; Nizami, A.-S. From waste to watts: Emerging role of waste lignin-derived materials for energy storage. J. Energy Storage 2024, 82, 110477. [Google Scholar]
- Zhang, C.; Chen, N.; Zhao, M.; Zhong, W.; Wu, W.J.; Jin, Y.C. High-performance electrode materials of heteroatom-doped lignin-based carbon materials for supercapacitor applications. Int. J. Biol. Macromol. 2024, 273, 133017. [Google Scholar]
- Muddasar, M.; Culebras, M.; Collins, M.N. Lignin and its carbon derivatives: Synthesis techniques and their energy storage applications. Mater. Today Sustain. 2024, 28, 100990. [Google Scholar]
- Hu, S.L.; Liao, Y.Q.; Ding, X.R.; Jin, C.; Bi, W.T. One-pot mild fabrication of magnetic lignin-based carbon-rich materials using deep eutectic solvent for efficient dye removal. Sep. Purif. Technol. 2025, 361, 131284. [Google Scholar]
- Sun, J.M.; Wu, Z.W.; Ma, C.H.; Xu, M.C.; Luo, S.; Li, W.; Liu, S.X. Biomass-derived tubular carbon materials: Progress in synthesis and applications. J. Mater. Chem. 2021, 24, 13822–13850. [Google Scholar] [CrossRef]
- Mukheja, Y.; Kaur, J.; Pathania, K.; Sah, S.P.; Salunke, D.B.; Sangamwar, A.T.; Pawar, S.V. Recent advances in pharmaceutical and biotechnological applications of lignin-based materials. Int. J. Biol. Macromol. 2023, 241, 124601. [Google Scholar]
- Wang, T.; Jiang, M.W.; Yu, X.L.; Niu, N.; Chen, L.G. Application of lignin adsorbent in wastewater treatment: A review. Sep. Purif. Technol. 2022, 302, 122116. [Google Scholar]
- Supanchaiyamat, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Tsang, D.C.W.; Hunt, A.J. Lignin materials for adsorption: Current trend, perspectives and opportunities. Bioresour. Technol. 2019, 272, 570–581. [Google Scholar] [CrossRef]
- Ponomarev, N.; Sillanpää, M. Combined chemical-templated activation of hydrolytic lignin for producing porous carbon. Ind. Crop. Prod. 2019, 135, 30–38. [Google Scholar] [CrossRef]
- Zhai, Z.Q.; Lu, Y.M.; Liu, G.Y.; Ding, W.L.; Cao, B.B.; He, H.Y. Recent advances in biomass-derived porous carbon materials: Synthesis, composition and applications. Chem. Res. Chin. Univ. 2024, 40, 3–19. [Google Scholar]
- Shi, Z.J.; Ma, M.G. Synthesis, structure, and applications of lignin-based carbon materials: A review. Sci. Adv. Mater. 2019, 11, 18–32. [Google Scholar] [CrossRef]
- Wang, A.Q.; Zheng, Z.K.; Li, R.Q.; Hu, D.; Lu, Y.R.; Luo, H.X.; Yan, K. Biomass-derived porous carbon highly efficient for removal of Pb(II) and Cd(II). Green Energy Environ. 2019, 4, 414–423. [Google Scholar] [CrossRef]
- Mondal, M.K. Removal of Pb(II) ions from aqueous solution using activated tea waste: Adsorption on a fixed-bed column. J. Environ. Manag. 2009, 4, 414–423. [Google Scholar]
- Zhao, T.; Yao, Y.; Li, D.R.; Wu, F.; Zhang, C.Z.; Gao, B. Facile low-temperature one-step synthesis of pomelo peel biochar under air atmosphere and its adsorption behaviors for Ag(I) and Pb(II). Sci. Total Environ. 2018, 640, 73–79. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Tan, Y.J.; Wang, X.; Xiong, F.Q.; Ding, J.R.; Qing, Y.; Wu, Y.Q. Preparation of lignin-based porous carbon as an efficient absorbent for the removal of methylene blue. Ind. Crop. Prod. 2021, 171, 13980. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Xu, J.; Kuang, Y.S.; Cheng, Z.; Wu, Q.Q.; Xie, J.X.; Wang, B.; Gao, W.H.; Zeng, J.S.; Li, J.; et al. Lignin-derived sulfonated porous carbon from cornstalk for efficient and selective removal of cationic dyes. Ind. Crop. Prod. 2021, 159, 113071. [Google Scholar]
- Liang, H.X.; Ding, W.; Zhang, H.W.; Peng, P.; Geng, Z.C.; She, D.; Li, Y. A novel lignin-based hierarchical porous carbon for efficient and selective removal of Cr(VI) from wastewater. Int. J. Biol. Macromol. 2022, 204, 310–320. [Google Scholar]
- Zhao, J.; Zhang, W.J.; Shen, D.K.; Zhang, H.Y.; Wang, Z.H. Preparation of porous carbon materials from black liquor lignin and its utilization as CO2 adsorbents. J. Energy Inst. 2023, 107, 101179. [Google Scholar]
- Xu, S.P.; Ma, J.C.; Jia, H.G.; Zhang, M.Y.; Qu, Y.Q.; Geng, C.B.; Zhao, X.Z.; Shao, M.; Xu, J.Y.; Wang, X. Preparation of porous carbon derived from a lignin-based polymer through ZnCl2 activation for effective capture of iodine. Int. J. Biol. Macromol. 2025, 294, 139412. [Google Scholar]
- Guo, D.L.; Hu, D.G.; Yan, Z.Y.; Yuan, K.S.; Sha, L.Z.; Zhao, H.F.; Chen, J.B.; Liu, B. Preparation and characteristic of high surface area lignin-based porous carbon by potassium tartrate activation. Microporous Mesoporous Mater. 2021, 326, 111340. [Google Scholar]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar]
- Wang, X.; Wang, S.Y.; Liu, W.; Wang, Y. Preparation and characterization of activated carbon from lignin-rich enzymatically hydrolyzed corncob residues and its adsorption of Cu(II) Ions. Starch-Starke 2019, 72, 1900131. [Google Scholar]
- Zhu, R.Y.; Xia, J.; Zhang, H.J.; Kong, F.G.; Hu, X.; Shen, Y.H.; Zhang, W.H. Synthesis of magnetic activated carbons from black liquor lignin and fenton sludge in a one-step pyrolysis for methylene blue adsorption. J. Environ. Chem. Eng. 2021, 9, 106538. [Google Scholar]
- Li, M.P.; Mu, J.C.; Liu, Y.X.; Wang, H.; Wang, Y.Y.; Song, H. Removal of phenol by lignin-based activated carbon as an efcient adsorbent for adsorption of phenolic wastewater. Res. Chem. Intermed. 2023, 49, 2209–2232. [Google Scholar]
- Cao, P.; Li, Y.T.; Shao, J.L. Experimental study on the preparation of lignin-based activated carbon and the adsorption performance for phenol. ACS Omega 2024, 9, 24453–24463. [Google Scholar]
- Yun, H.; Hwang, S.-W.; Jung, M.J.; Choi, I.-G.; Yeo, H.-M.; Kwak, H. Microwave-assisted utilization of kraft lignin-derived activated carbon for efficient dye removal. Biomass Bioenerg. 2024, 186, 107279. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.M.; Carrott, M.M.L.R. Lignin—From natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Jing, Z.F.; Ding, J.C.; Zhang, T.; Yang, D.Y.; Qiu, F.X.; Chen, Q.Y.; Xu, J.C. Flexible, versatility and superhydrophobic biomass carbon aerogels derived from corn bracts for efficient oil/water separation. Food Bioprod. Process. 2019, 115, 134–142. [Google Scholar] [CrossRef]
- Li, S.W.; Hou, D.M.; Cui, Y.S.; Jia, S.A.; Lan, G.; Sun, W.L.; Li, G.Y.; Li, X.; Feng, W. Highly ordered carbon aerogels: Synthesis, structures, properties and applications. Carbon 2024, 218, 118669. [Google Scholar] [CrossRef]
- Wu, C.W.; Li, P.H.; Wei, Y.M.; Yang, C.; Wu, W.J. Review on the preparation and application of lignin-based carbon aerogels. RSC Adv. 2022, 12, 10755. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.Y.; Wei, J.Y.; Jonasson, S.; Hedlund, J.; Oksman, K. Multifunctional carbon aerogels with hierarchical anisotropic structure derived from lignin and cellulose nanofibers for CO2 capture and energy storage. ACS Appl. Mater. Interfaces 2020, 12, 7432–7441. [Google Scholar] [CrossRef]
- Lv, D.; Li, Y.; Wang, L.J. Carbon aerogels derived from sodium lignin sulfonate embedded in carrageenan skeleton for methylene-blue removal. Int. J. Biol. Macromol. 2020, 148, 979–987. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, T.L.; Yu, S.S.; Cheng, Y.; Lu, J.; Wang, H.S. A lignin-based carbon aerogel enhanced by graphene oxide and application in oil/water separation. Fuel 2020, 278, 118376. [Google Scholar] [CrossRef]
- Shen, Y.F.; Wu, Y.F. Review on synthesis of carbon aerogels for CO2 capture. Fuel 2025, 387, 134370. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar]
- Inagaki, M.; Qiu, J.S.; Guo, Q.G. Carbon foam: Preparation and application. Carbon 2015, 87, 128–152. [Google Scholar]
- Liu, H.G.; Wu, S.Q.; Tian, N.; Yan, F.X.; You, C.Y.; Yang, Y. Carbon foams: 3D porous carbon materials holding immense potential. J. Mater. Chem. 2020, 8, 23699–23723. [Google Scholar]
- Qu, J.Y.; Han, Q.; Gao, F.; Qiu, J.S. Carbon foams produced from lignin-phenol-formaldehyde resin for oil/water separation. New Carbon Mater. 2017, 32, 86–91. [Google Scholar]
- Vannarath, A.; Thalla, A.K. Synthesis and characterisation of an ultra-light, hydrophobic and flame-retardant robust lignin-carbon foam for oil-water separation. J. Clean. Prod. 2021, 325, 129263. [Google Scholar]
- Xu, F.; Gui, Y.C.; Zuo, S.L.; Li, J.T.; Wang, S.S. Preparation of lignin-based carbon foam monoliths with high strength and developed micrometer-sized cell/nano-sized porous structures using a self-bubbling method. J. Anal. Appl. Pyrolysis 2022, 163, 105490. [Google Scholar]
- Bai, Y.H.; Yu, M.J.; Zhang, X.F.; Yao, J.F. Deep eutectic solvent assisted preparation of ZnO deposited carbonized wood for efficient CO2 storage and oil absorption. J. Mol. Liq. 2023, 376, 121409. [Google Scholar]
- Li, C.P.; Wu, Y.Q.; An, J.J.; Gao, L.X.; Zhang, D.Q.; Li, J.; An, Z.X. Preparation of carbon foam from depolymerization-reforming lignin for capacitive deionization. Desalination 2023, 559, 116656. [Google Scholar]
- Seo, J.Y.; Park, H.; Shin, K.; Baeck, S.H.; Rhym, Y.; Shim, S.E. Lignin-derived macroporous carbon foams prepared by using poly(methyl methacrylate) particles as the template. Carbon 2014, 76, 357–367. [Google Scholar]
- Patel, P.M.; Patel, H.N.; Kotecha, S.D. Properties of carbon fiber and its applications. Int. J. Eng. Res. Technol. 2013, 2, 554–557. [Google Scholar]
- Xu, X.T.; Pan, H.; Shen, Q.; Tan, X.Y.; Ding, D.N.; Wang, Y.X. Preparation and application of lignin carbon fiber. J. Phys. Conf. Ser. 2021, 1790, 012071. [Google Scholar]
- Song, M.; Zhang, W.; Chen, Y.S.; Luo, J.M.; Crittenden, J.C. The preparation and performance of lignin-based activated carbon fiber adsorbents for treating gaseous streams. Front. Chem. Sci. Eng. 2017, 11, 328–337. [Google Scholar]
- Song, M.; Yu, L.; Song, B.; Meng, F.Y.; Tang, X.H. Alkali promoted the adsorption of toluene by adjusting the surface properties of lignin-derived carbon fibers. Environ. Sci. Pollut. Res. 2019, 26, 22284–22294. [Google Scholar]
- Nordin, N.A.; Rahamn, N.A.; Abdullah, A.H. Effective removal of Pb(II) Ions by electrospun PAN/Sago lignin-based activated carbon nanofibers. Molecules 2020, 25, 3081. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Ghosh, T.; Ayranci, C.; Tang, T. Bio-cleaned Lignin-based carbon fiber and Its application in adsorptive water treatment. J. Appl. Polym. Sci. 2022, 139, e52054. [Google Scholar]
- Sun, S.C.; Xu, Y.; Wen, J.L.; Yuan, T.Q.; Sin, R.C. Recent advances in lignin-based carbon fibers (LCFs): Precursors, fabrications, properties, and applications. Green Chem. 2022, 24, 5709. [Google Scholar]

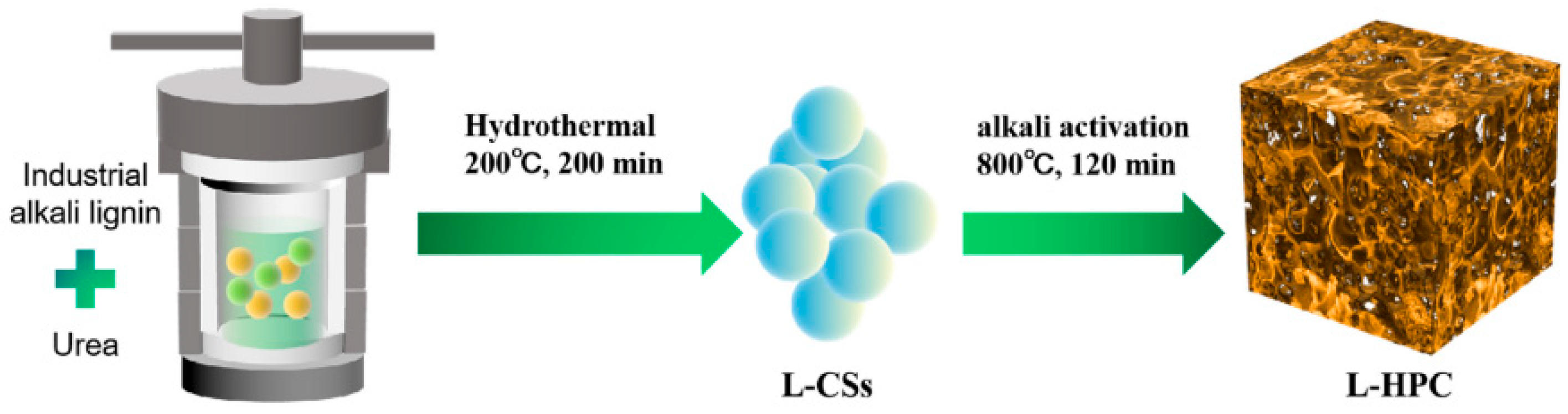
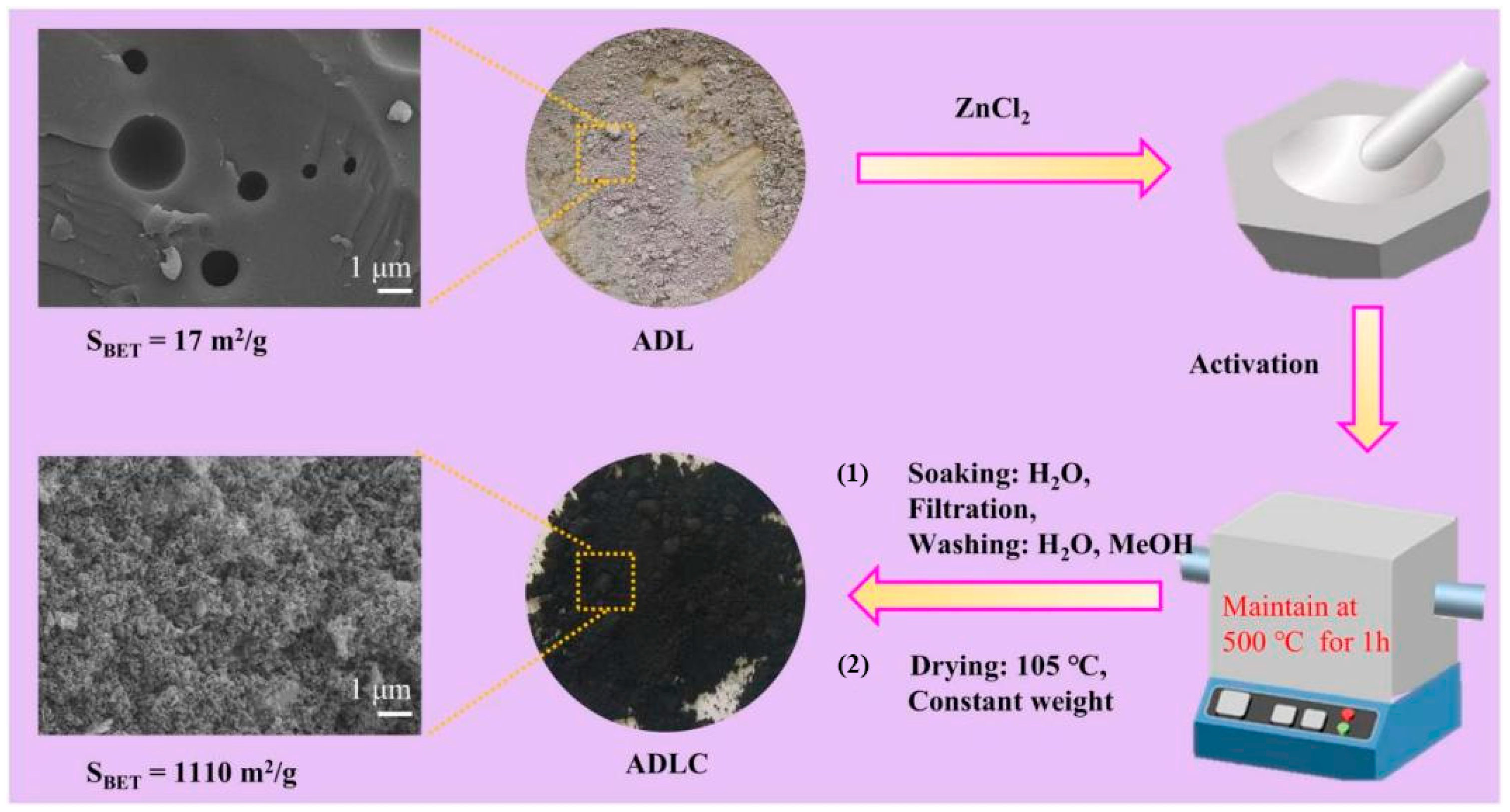
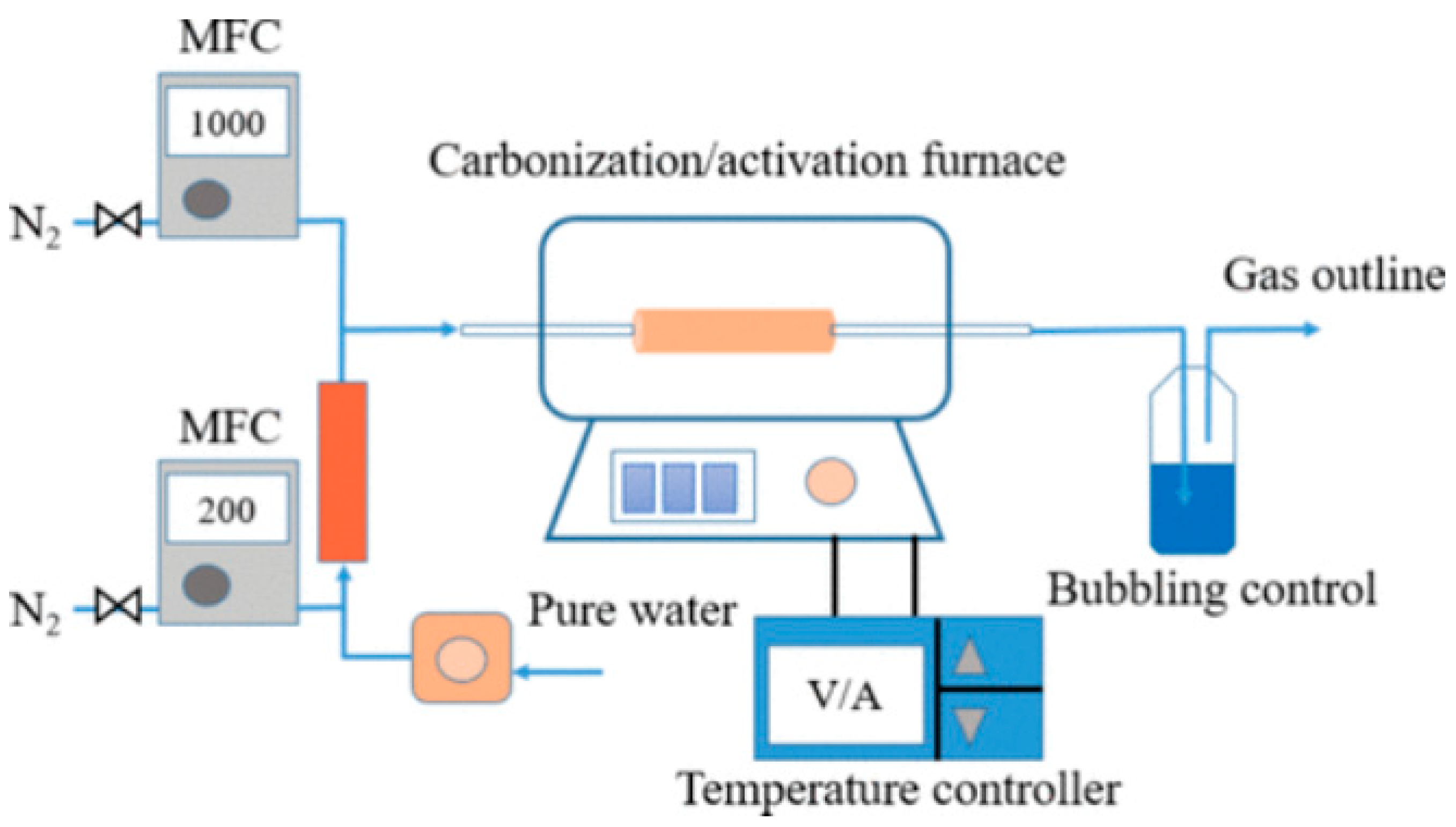
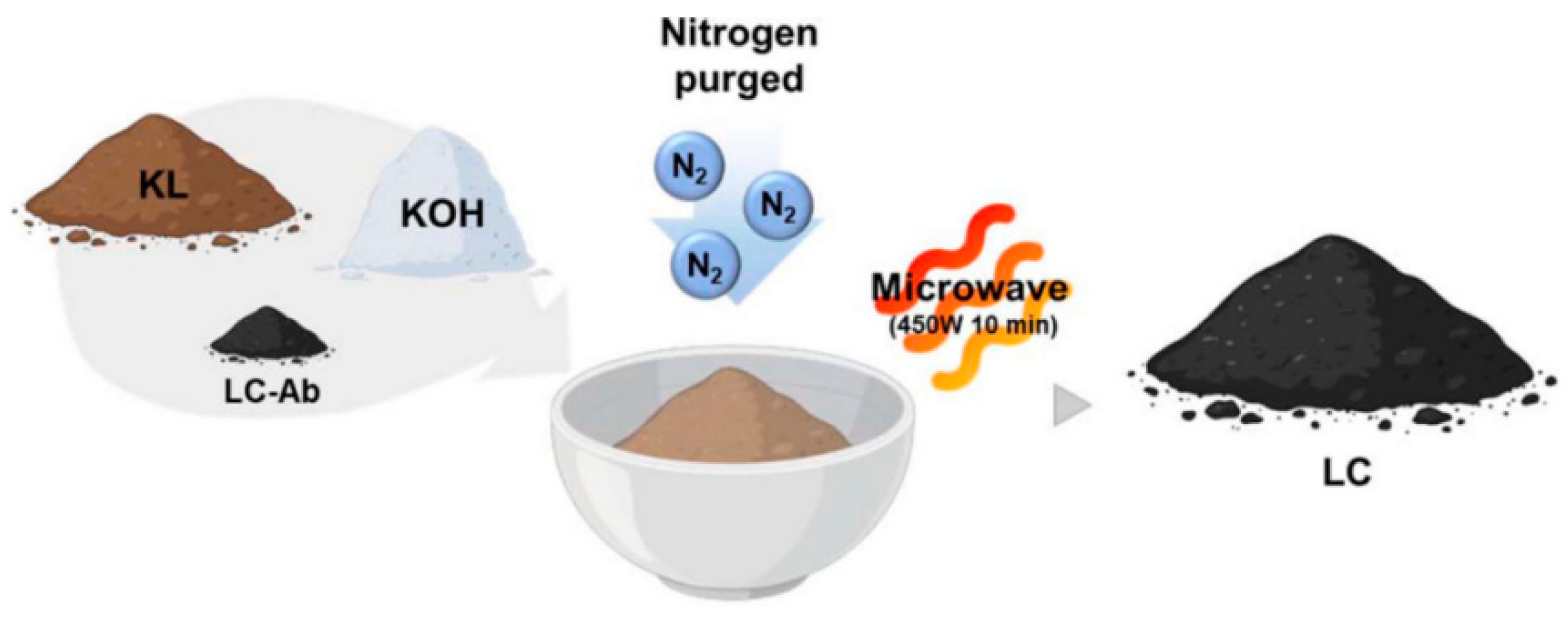
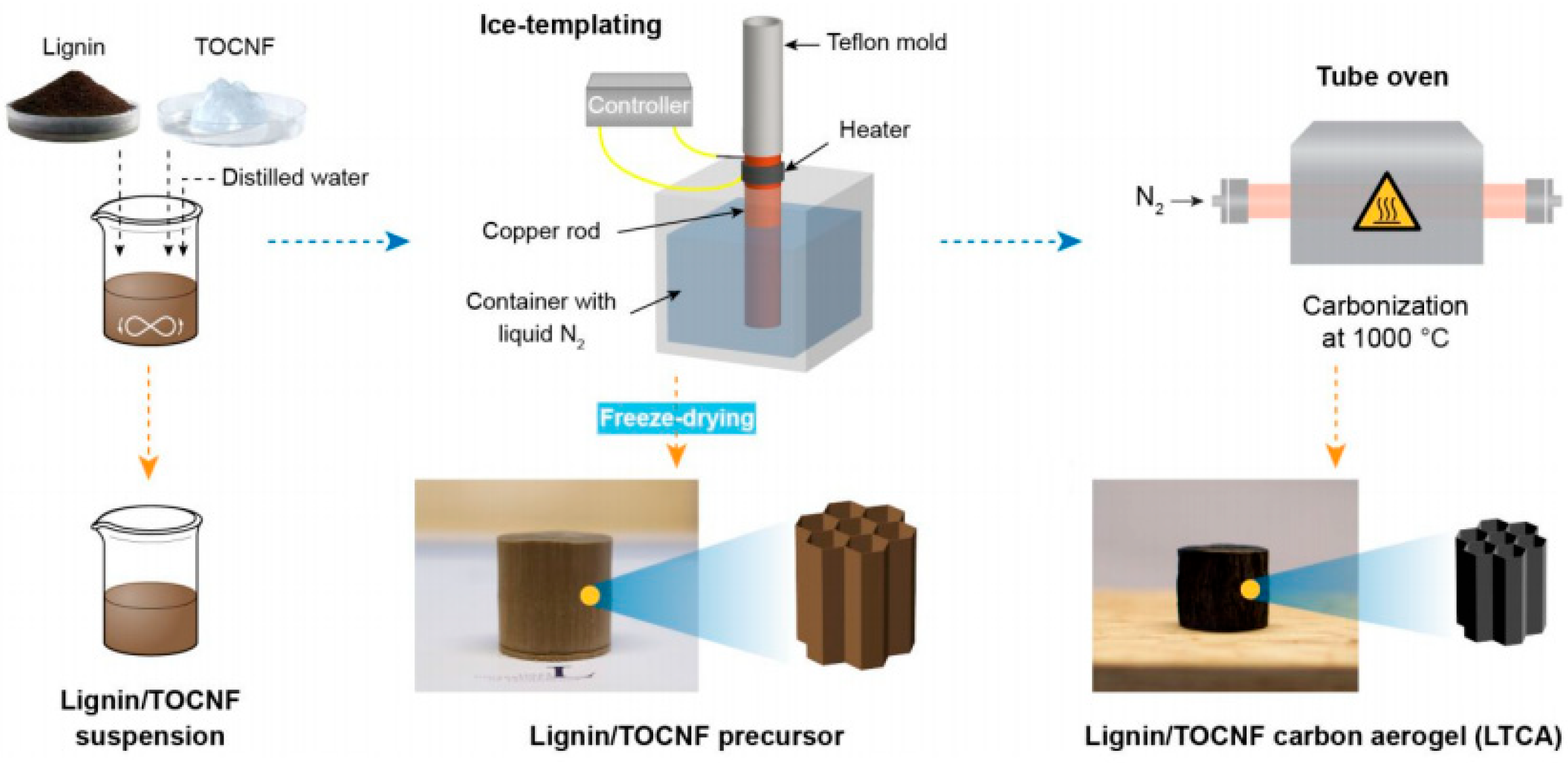
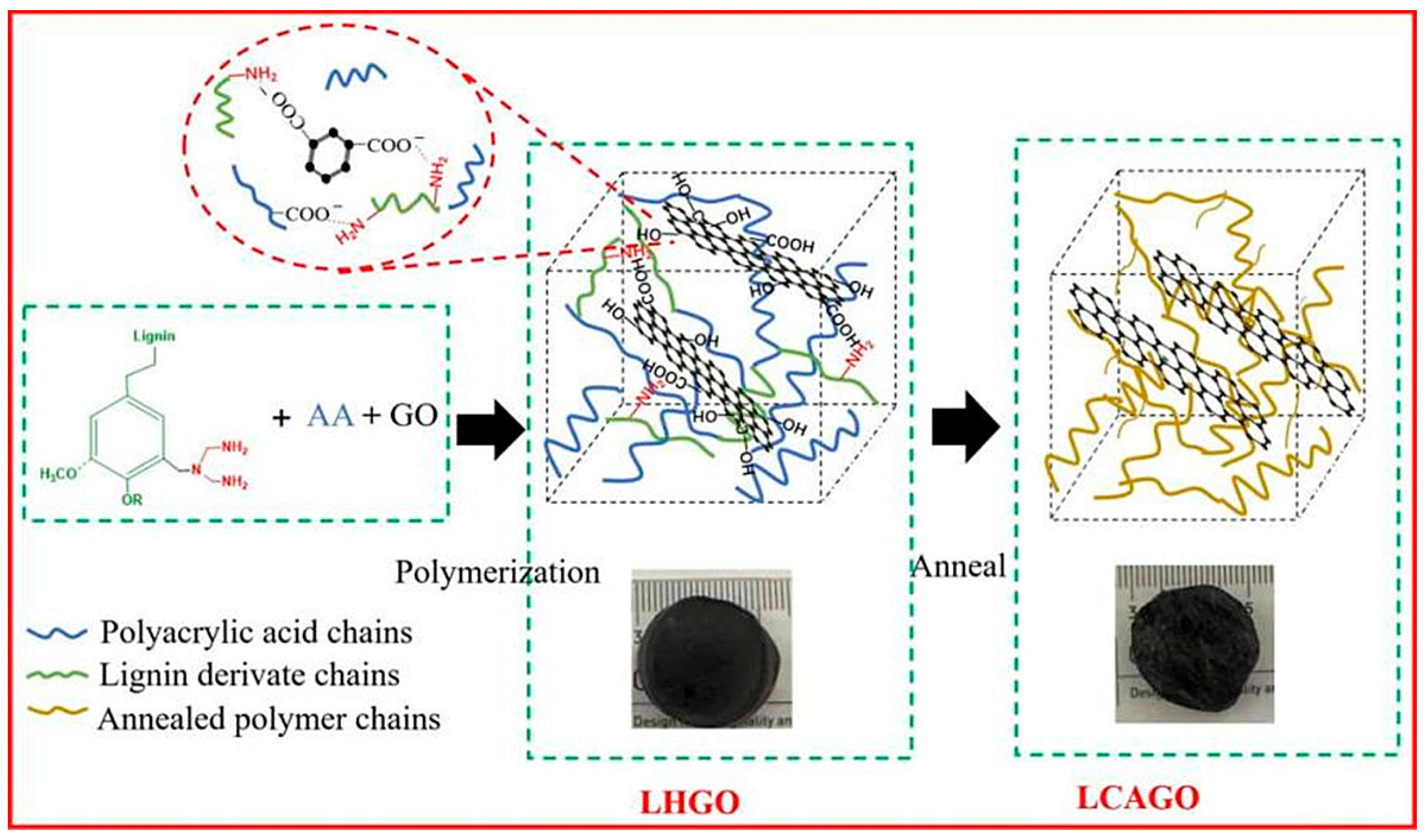
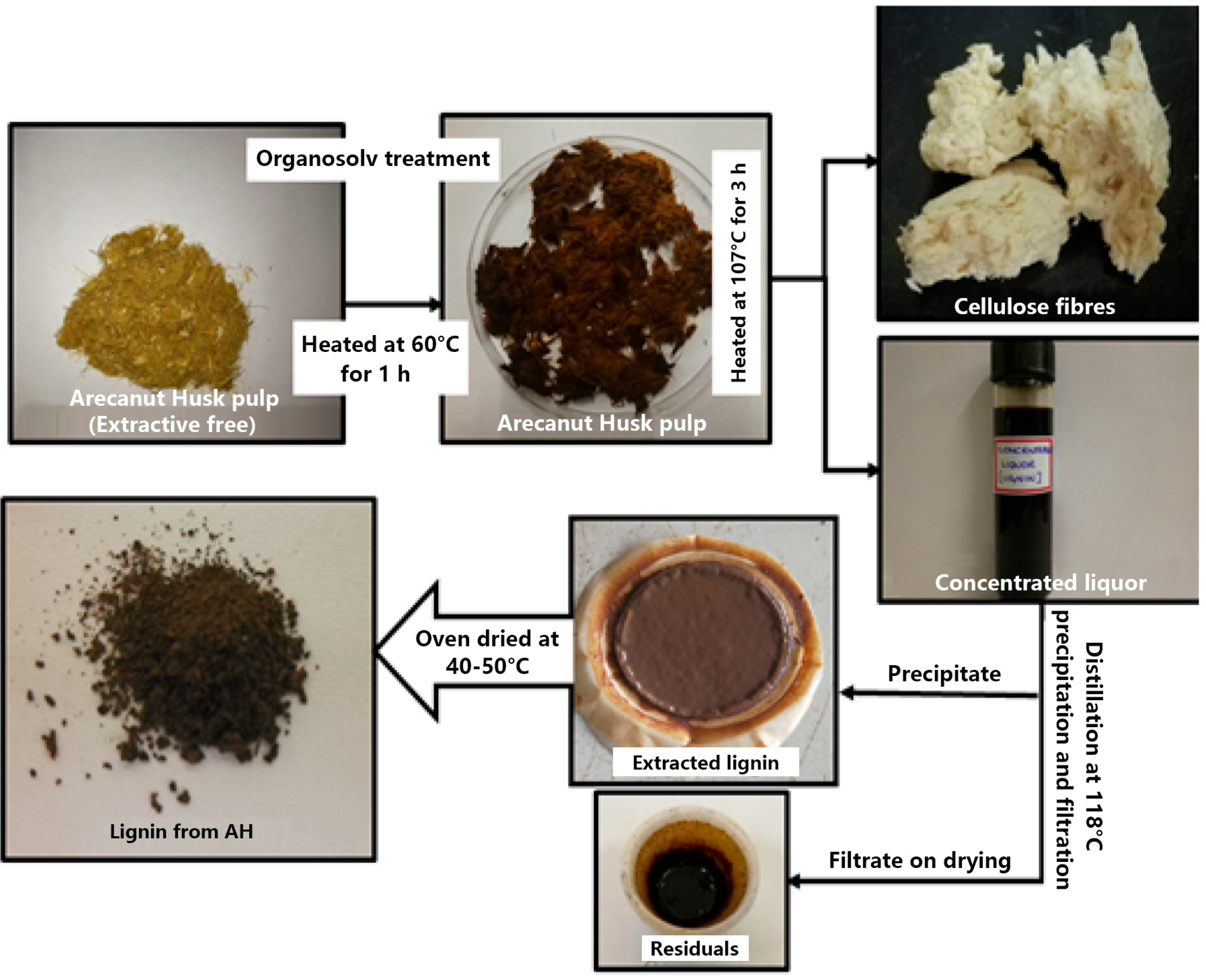
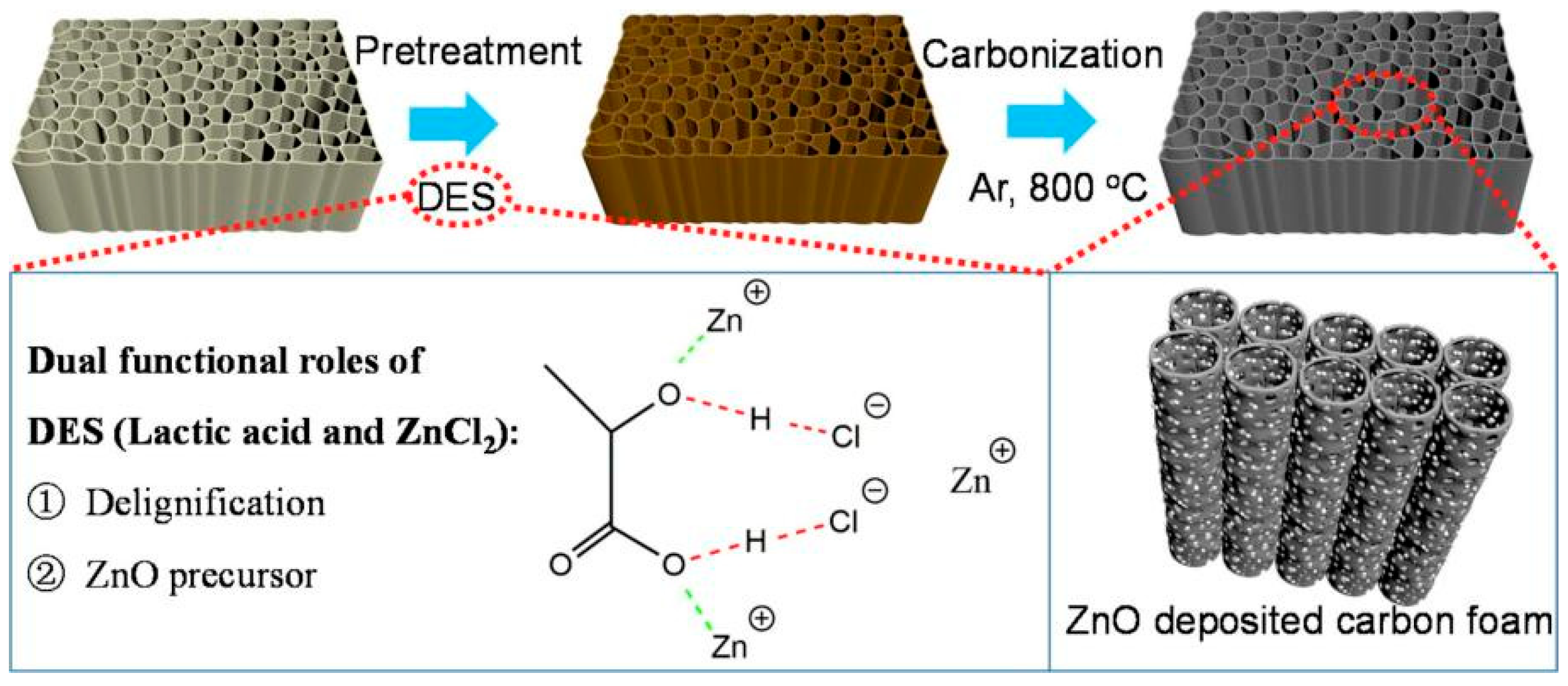
| Adsorbents | Heavy Metals | Adsorption Capacity | Ref. |
|---|---|---|---|
| Activated tea waste | Pd(II) | 81 mg/g | [67] |
| Pomelo peel biochar | Pd(II) | 88.74 mg/g | [68] |
| Mango peel waste | Cd(II) | 68.9 mg/g | [69] |
| Raw Material | Preparation Method | Carbon Material | Adsorption Target | Adsorption Capacity | Cycle Times | Ref. |
|---|---|---|---|---|---|---|
| Native lignin | Chemical solution combustion method | Porous carbon | Pb(II), Cd(II) | 250.5 mg/g, 126.4 mg/g | 5 | [66] |
| Native lignin | Hydrothermal, chemical activation | Porous carbon | Methylene blue, Malachite, Rhodamine B | 1119.18 mg/g, 2467.30 mg/g, 1657.82 mg/g | 4 | [70] |
| Corn stover lignin | Template method | Porous carbon | Methylene blue | 420.40 mg/g | 3 | [71] |
| Industrial alkali lignin | Chemical activation | Porous carbon | Cr(VI) | 887.8 mg/g | 12 | [72] |
| Black liquid lignin | Chemical activation | Porous carbon | CO2 | 5.20 mmol/g | 5 | [73] |
| Graft copolymerization lignin | Chemical activation | Porous carbon | I2 | 2340 mg/g, 354 mg/g | 5 | [74] |
| Corncob residue lignin | Chemical activation | Activated carbon | Cu(II) | 49.21 mg/g | - | [77] |
| Black liquid lignin | Chemical activation | Activated carbon | Methylene blue | 307.2 mg/g | 5 | [78] |
| Corn stover lignin | Chemical activation | Activated carbon | Phenol | 612 mg/g | - | [79] |
| Coconut shell, Alkali lignin | Physical activation, Chemical activation | Activated carbon | Phenol | removal rate 98.22% | 3 | [80] |
| Sulfated lignin | Microwave-assisted heat treatment, chemical activation | Activated carbon | Methylene blue, lime 7 | 543.82 mg/g, 548.54 mg/g | 5 | [81] |
| Sulfated lignin | Ice template method | Carbon aerogels | CO2 | 5.23 mmol/g | - | [86] |
| Sodium lignosulfonate | Chemical activation | Carbon aerogels | Methylene blue | 421.94 mg/g | - | [87] |
| Alkali lignin | Physical activation | Carbon aerogels | Light oil, heavy oil | 32.5 g/g, 34 g/g | - | [88] |
| Sedge grass lignin | Template method | Carbon foam | oil–water separation | about 20 s | 10 | [93] |
| Betel nut shell lignin | Template method | Carbon foam | Diesel oil, sunflower oil | 7842.71 mg/g, 1127.58 mg/g | 5 | [94] |
| hardwood lignin | Self-bubbling method | Carbon foam | Hexane/water | seconds | 20 | [95] |
| Balsa lignin | Deep eutectic solvent assisted method | Carbon foam | CO2, light oil, heavy oil | 3.03 mmol/g, 95 g/g, 157 g/g | 8 | [96] |
| Degradation of lignin | Depolymerization and reorganization | Carbon foam | NaCl | 30.2 mg/g | 30 | [97] |
| Alkali lignin | Electrostatic spinning | Carbon fiber | Toluene | 439 mg/g | - | [101] |
| Alkali lignin | Electrostatic spinning, chemical activation | Carbon fiber | Toluene | 463 mg/g | - | [102] |
| Sago lignin | Electrostatic spinning | Carbon fiber | Pb(II) | 588.24 mg/g | - | [103] |
| Bioclean Lignin | Electrostatic spinning | Carbon fiber | Methylene blue | 548.65 mg/g | 5 | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Zhang, Y.; Shao, S.; Li, H.; Yan, X. Application of Lignin-Derived Carbon Materials in Adsorption and Separation. Separations 2025, 12, 88. https://doi.org/10.3390/separations12040088
Dong X, Zhang Y, Shao S, Li H, Yan X. Application of Lignin-Derived Carbon Materials in Adsorption and Separation. Separations. 2025; 12(4):88. https://doi.org/10.3390/separations12040088
Chicago/Turabian StyleDong, Xiaorui, Yunlei Zhang, Shouyan Shao, Hao Li, and Xingchen Yan. 2025. "Application of Lignin-Derived Carbon Materials in Adsorption and Separation" Separations 12, no. 4: 88. https://doi.org/10.3390/separations12040088
APA StyleDong, X., Zhang, Y., Shao, S., Li, H., & Yan, X. (2025). Application of Lignin-Derived Carbon Materials in Adsorption and Separation. Separations, 12(4), 88. https://doi.org/10.3390/separations12040088









