Abstract
Edge-capping modified MXene membranes with new channels created by lateral nanosheets are of great research significance. After introducing tripolyphosphate (STPP) to Ti edges of Ti3C2Tx nanosheets and fabricating the STPP-MXene membranes edge-capping method, this research investigated the performance optimization mechanism of STPP-modified MXene membranes in terms of salt permeability (NaCl, Na2SO4, MgCl2, and MgSO4) and transmembrane energy barriers (Esalt) through the concentration gradient permeation test. Experimental results demonstrated an approximately 1.86-fold enhancement in salt flux (Js) compared to the MXene membranes. The solution–diffusion model was also introduced to evaluate the salt solubility (Ks) and diffusivity (Ds) during permeation. Furthermore, analysis of transmembrane energy barriers revealed that STPP modification induced significantly larger reductions in activation energy for magnesium salts (MgSO4: 55.1%; MgCl2: 47.4%) compared to sodium salts (NaCl: 30.5%; Na2SO4: 30.9%). This phenomenon indicated the weakened electrostatic interactions between high-valent Mg2+ and the modified lateral membrane Ti edges, whereas the limited charge density of Na+ resulted in relatively modest optimization. The results highlight the contribution of STPP capping on the edges of adjacent lateral nanosheets. Therefore, the modification increased the transportation rate of cations across the MXene membrane by more than twice, thus advancing the application of 2D MXene membranes in resource recovery.
1. Introduction
In the field of nanofiltration membrane separation, two-dimensional membranes (such as graphene, graphene oxide, molybdenum disulfide, etc.) are attracting increasing attention due to their convenient membrane preparation, excellent membrane-forming properties, and unique interlayer channels [1,2,3,4]. Among these, transition metal carbides/nitrides (MXenes), exemplified by those synthesized through HF or LiF etching of Ti3AlC2, stand out as prominent representatives [5,6]. The MXene membranes exhibit advantageous characteristics, such as superior hydrophilicity, excellent mechanical strength, and abundant terminal functional groups (e.g., -O, -OH, -F) [7]. The stacking of Ti3C2Tx nanosheets endows the MXene membranes with inherent multiscale Ti-C-Ti structures, enabling the formation of stacked sub-nanometer channels with remarkable uniformity [8,9]. Under dry conditions, these channels exhibit interlayer spacings of approximately 0.6–0.8 nm, a size that is close to that of most hydrated ions (<1 nm) and larger than that of most solvent molecules (<2 nm) [10]. Consequently, under the domain confinement effect within sub-nanometer channels, MXene membranes demonstrate the ability of molecular/ion sieving, making them particularly suitable for pollutant remediation in aqueous environments [11,12,13]. However, due to the limitation of the inherently low porosity of the MXene surface, the pristine MXene material needs to be modified to construct more efficient mass transfer pathways.
To address this challenge, current research has focused on modifying the sub-nanometer interlayer channels. Strategies include covalent organic framework intercalation, electrostatic interactions, and thermal crosslinking. These approaches aim to either expand interlayer spacing to >1 nm for efficient molecular/ion sieving (e.g., organic dye separation) [14,15,16] or reduce interlayer spacing to sub-nanometer dimensions for highly selective ion/ion separation (e.g., heavy metal adsorption of Pb2+, Cu2+) [17,18,19,20]. Although the strategies serve distinct purposes, relying solely on the dimensional modification of MXene interlayer channels presents inherent limitations. Specifically, the expanded interlayer channels are prone to suffer from swelling-induced loss of separation selectivity in aqueous environments [13,21,22]. On the other hand, the narrowed interlayer spacing compromises membrane permeability [17,23,24]. This inevitable trade-off between selectivity and permeability fundamentally restricts researchers from fully exploiting the inherent potential of MXene in the nanofiltration separation process.
The sodium tripolyphosphate (STPP) that was initially designed to absorb the edge Ti3C2Tx nanosheets to enhance their stability in an aqueous environment, has been demonstrated to achieve efficient dye/salt separation in cross-flow devices in recent research [25,26,27]. This outcome can be attributed to the moderately expanded interlayer spacing (from 1.44 to 1.63 nm) and enhanced hydrophilicity of the modified STPP-MXene membranes. More importantly, research has shown that the successful termination of Ti functional groups on the adjacent lateral Ti3C2Tx nanosheets by STPP not only rendered the edges uncharged but also created lateral spaces (shoulder spacing) between the nanosheets, which serve as channels to assist interlayer spacing during sieving processes. Molecules and ions must enter the entrance of adjacent transverse nanosheets before transferring through the interlaminar channels. However, under pressure-driven conditions, the cross-flow configuration used in the research fails to simulate the natural permeation interaction between ions and membranes. It is worth noting that this perspective has rarely been explored in previous studies [28,29]. Furthermore, the newly created shoulder spacing channels from lateral nanosheet modification in STPP-MXene membranes have only been simulated using density functional theory (DFT) for cation sieving, leaving the mechanism behind permeability enhancement inadequately explained. Clearly, the influence of the edge-capping method on the Ti edges of the MXene nanosheets for ion transport mechanisms requires further clarification.
In order to make up for the defeciencies of previous research, this research continues to employ STPP for fabricating STPP-MXene membranes by absorbing it on the edges of adjacent Ti3C2Tx nanosheets. Moreover, a concentration-gradient diffusion cell was utilized to compare salt permeance between MXene and STPP-MXene membranes. To analyze salt permeation mechanisms, the solution–diffusion model was applied to evaluate ion transport processes across MXene and STPP-MXene membranes in terms of salt permeability and diffusivity. Finally, based on transition state (TST) theory, the activation energy of cation entry into the membranes was assessed to evaluate the contribution of newly created shoulder spacing channels to salt permeation. The experiment proved that by combining STPP non-covalently with the edges of MXene nanosheets through electrostatic adsorption, the new lateral shoulder spacing could be activated. Then, the efficiency of transmembrane ion transport was increased by approximately twofold.
2. Materials and Methods
2.1. Materials and Chemicals
The hydrophilic PVDF membranes (0.1 μm pore size) for the experiment were purchased from De Lv Technology Co., Ltd. (from Jiaxing, Zhejiang province, China). XinXi Technology Co., Ltd. from Foshan, Guangdong province, China supplied the MXene (Ti3C2Tx) dispersion (concentration: 5.00 mg/mL). Sodium tripolyphosphate (STPP) was purchased from Shanghai MACKLIN Biochemical Technology Co., Ltd. (Shanghai, China). Sodium sulfate (Na2SO4), sodium chloride (NaCl), magnesium chloride (MgCl2), and magnesium sulfate (MgSO4) were acquired from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Fabrication of Membranes
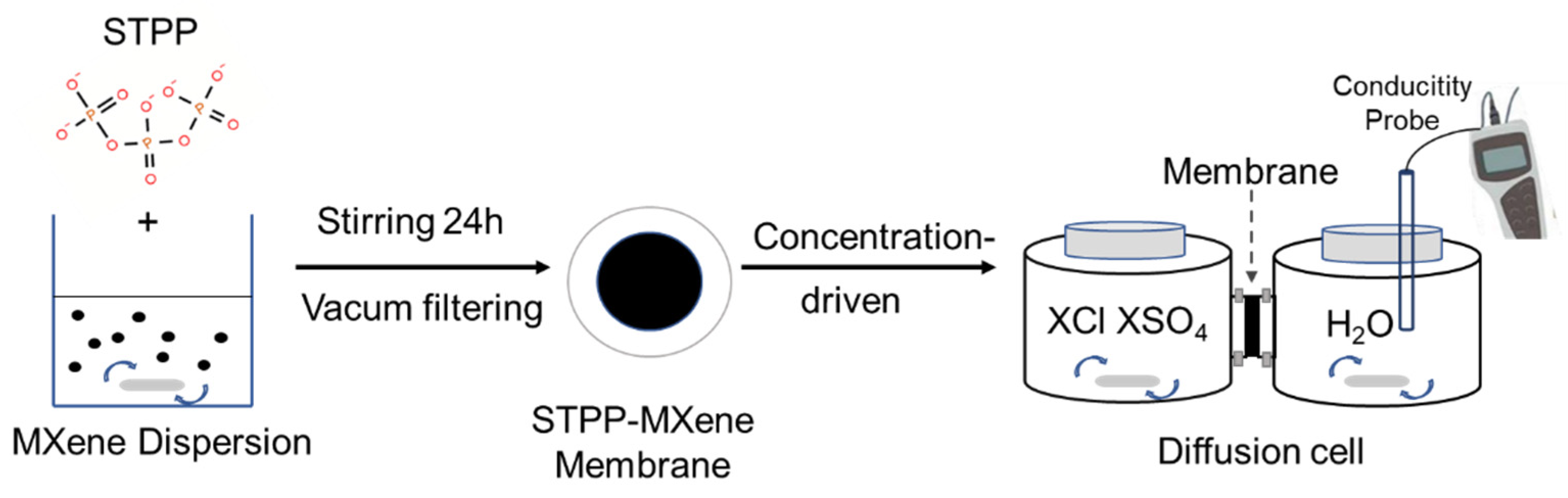
The Ti3C2Tx dispersion with a colloidal concentration of 5 mg/mL was first diluted 100 times using deionized water. Then, STPP was introduced into the Ti3C2Tx dispersion at a concentration of 0.25 mol/L. The abovementioned dispersions were magnetically stirred at room temperature (25 °C) for 24 h to acquire the STPP-Ti3C2Tx dispersions. To fabricate the composite membrane, we loaded the dispersions onto the PVDF membranes directly via vacuum filtration (Figure 1). Lastly, both of the newly fabricated MXene and STPP-MXene membranes were placed in the fume hood for 2–3 min to remove moisture from their surfaces, and then the subsequent experiments on the membranes were carried out.
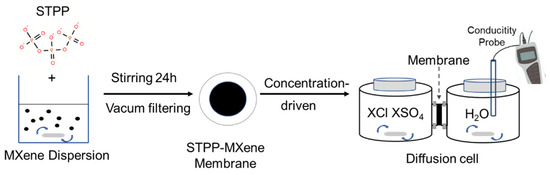
Figure 1.
Schematic of the synthesis and test process for STPP-MXene membrane.
2.3. Characterization of Membranes
The surface and cross-section morphologies of the membranes were observed by using scanning electron microscopy (SEM, Quanta250, Kutná Hora, Czech Republic, and ZEISS Gemini SEM 300, Berlin, Germany). Ti3C2Tx nanosheets and the roughness of the membrane surface were characterized using an atomic force microscopy device (AFM, Bruker Dimension Icon, Cologne, Germany).
2.4. Salt Flux for Mxene and STPP-MXene Membranes
The MXene and STPP-MXene membrane were fabricated to test salt flux for NaCl, MgCl2, Na2SO4, and MgSO4 utilizing a two-compartment gradient diffusion cell [30] with an effective area of 2.54 cm2. The concentration of the feed solutions of NaCl, MgCl2, Na2SO4, and MgSO4 on the feed side was 0.1 mol/L. The permeate side contained deionized water. The salt flux was determined using the following formula [31,32]:
where , , , , , and are the salt flux (mol m−2 h−1), the volume of solution in the compartments (cm3), the initial ion concentration in the permeation solution (mol/L), the ion concentration in the permeation solution at time t (mol/L), the effective membrane area (cm2), and the time (h), respectively.
The results above were determined using a conductivity meter (DDS-11A, Shanghai Yueping Scientific Instrument Co., Ltd., Shanghai, China).
2.5. Salt Permeability, Solubility, and Diffusivity Performance Tests
To further evaluate the salt permeability of the MXene and STPP-MXene membranes, the solution–diffusion model was introduced to validate the experiments. As a comprehensive reflection of salt solubility and diffusivity into the membrane, salt permeability () can be calculated using Equation (2) as follows [33]:
In this equation, refers to the salt permeability (cm2/s), is the reaction time (s), is the salt concentration in the receptor at time t (mol/L), represents the initial donor chamber salt concentrations (mol/L), is the volume of the solution in the receiving compartment, l is the membrane thickness (nm), and refers to the effective area (cm2).
In addition, another experiment was conducted by immersing MXene membranes and modified STPP-MXene membranes in 30 mL of 0.1 M NaCl, MgCl2, Na2SO4, and MgSO4 aqueous solutions at 25 °C for at least 48 h to achieve saturation. Afterward, excess salt on the membrane surface was gently absorbed with a paper towel, and the membranes were then placed in 30 mL of deionized water for 24 h. Finally, the partition coefficient () and diffusion coefficient () were measured and calculated to assess and compare the transmembrane ion transport behavior from the entry to the exit of the membranes. The solubility () was determined using Equation (3) as follows [34]:
Here, represents the partition coefficient [(g salt/cm3 swollen polymer)/(g salt/cm3 solution)]. Additionally, and are the amount of salt extracted per unit volume of hydrated polymer and the salt concentration in the solution when the membrane was initially equilibrated, respectively. Finally, formula (4) is as follows:
The diffusivity () can be calculated using the division of salt permeability and solubility () using the formula above.
The results above were also determined using a conductivity meter.
2.6. Calculations of Activation Energy of Membranes
In addition, the transmembrane energy barriers of salts () were tested by stirring both a salt solution and deionized water 100 mL at 25, 35, 45, 55, and 65 °C to collect the results of energy barriers. The results were calculated using Equation (5) as follows [35,36]:
where refers to the salt flux (mol m−2 h−1), () is the difference in solution concentration across the membrane (mol/L), indicates the pre-exponential factor, and is the gas constant, which is equal to 8.3145 (J mol−1 K−1). (KJ mol−1) represents the activation energy, and (K) represents the test temperature. The temperatures, from low to high, are 25 °C, 35 °C, 45 °C, 55 °C, and 65 °C.
Obviously, Equation (5) can be transformed into a linear function (6) related to and 1000/T.
The results above were also determined using the same conductivity meter (DDS-11A, Shanghai Yueping Scientific Instrument Co., Ltd., Shanghai, China).
3. Results and Discussions
3.1. Characterizations of Membranes
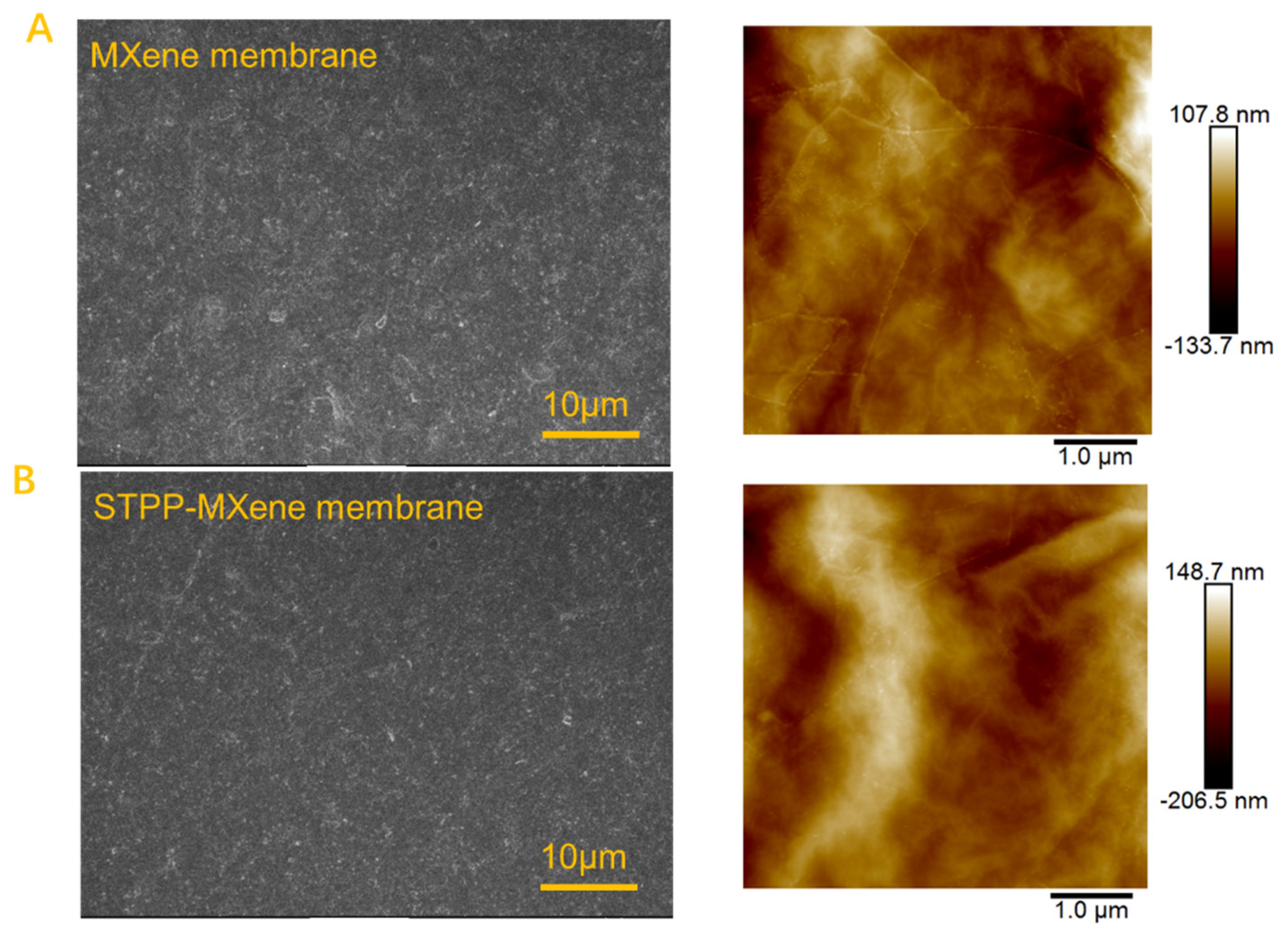
The morphology of the MXene and modified STPP-MXene membranes was characterized. The SEM and AFM images presented in Figure 2 demonstrate a smooth and continuous surface morphology of the membrane, confirming the homogeneous distribution of Ti3C2Tx nanosheets across the PVDF substrate. This uniform loading suggests an orderly arrangement of the nanosheets, which is critical for testing the membrane’s functional properties [37,38].
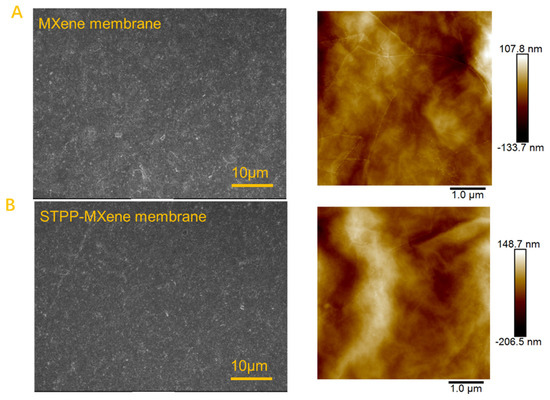
Figure 2.
SEM and AFM characterizations of (A) MXene membrane and (B) STPP-MXene membrane.
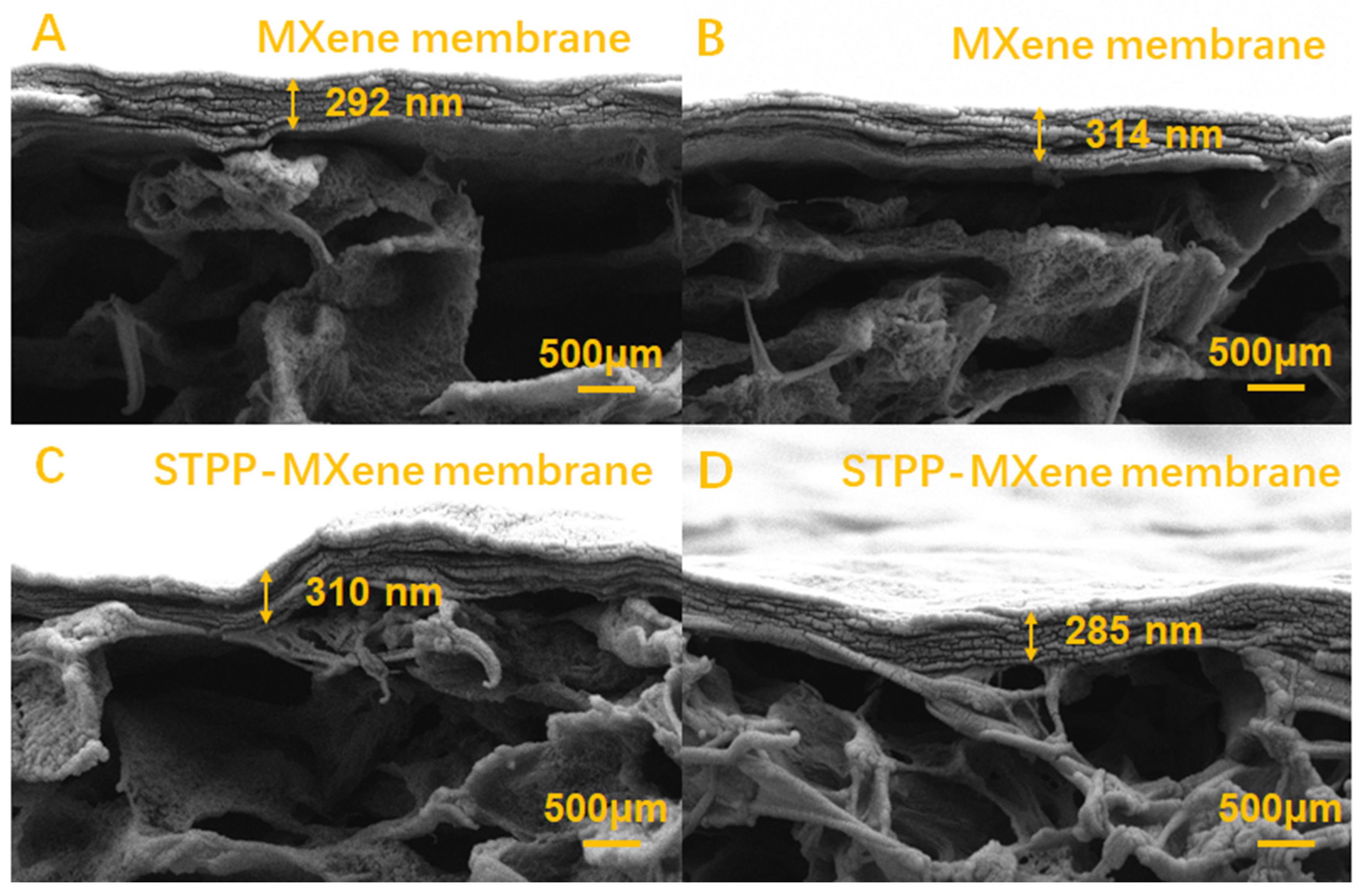
In addition, compared with the MXene membrane and the STPP-MXene membrane, the result of AFM arithmetic roughness (Ra) indicated a moderate increase from 18.3 nm to 32.7 nm. The rough surface was attributed to the disruption of the MXene membrane surface caused by the addition of STPP, a result that contributed to enhancing the membrane’s salt permeability [39,40]. The cross-sectional SEM images (Figure 3) of MXene and STPP-MXene membranes exhibited stacked and continuous two-dimensional channels, which were formed by Ti3C2Tx nanosheets. By comparing and measuring, the average loading thickness () of Ti3C2Tx nanosheets on the PVDF substrate was determined to be approximately 300 ± 0.25 nm. Therefore, we can confirm that the thickness of the membrane () in Equation (2) is 300 nm. Combined with the FTIR spectra results of the PVDF substrate, MXene, and STPP-MXene membranes (both the MXene and STPP-MXene membranes were fabricated via vacuum filtration using the same batch of MXene dispersion as in the previously reported study [25]), peaks were observed at 1172 cm−1 and 1405 cm−1, corresponding to the C-C bonds and C-F stretching vibrations of PVDF [41,42]. Additionally, MXene and STPP-MXene membranes exhibited new peaks at 3450 cm−1 and 1645 cm−1, which are attributed to the stretching vibrations of hydroxyl groups. These results further confirm the successful loading of two-dimensional MXene materials onto the PVDF substrate [43].
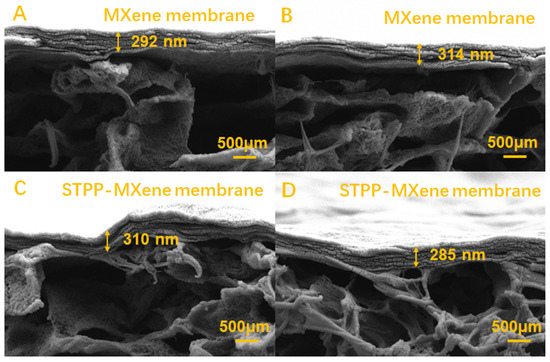
Figure 3.
Cross-section images of (A,B) MXene and (C,D) STPP-MXene membranes in different addition concentrations.
3.2. Salt Flux of MXene and Modified STPP-MXene Membranes
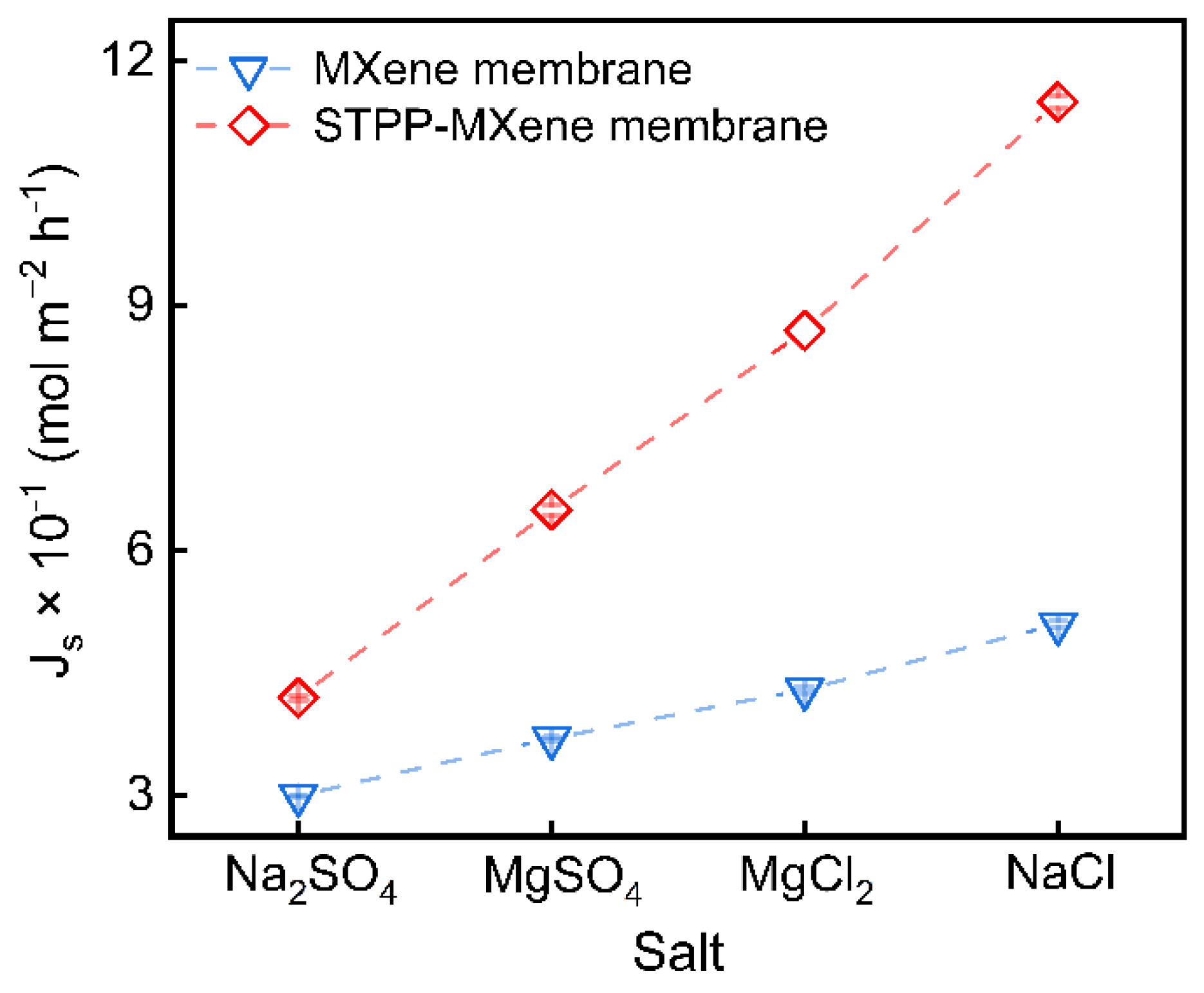
To preliminarily evaluate the difference in salt flux before and after modification, MXene and STPP-MXene membranes were placed in a diffusion cell at 25 °C for up to 0.5 h, and the continuous permeation of four salts (NaCl, Na2SO4, MgCl2, MgSO4) was carried out. The results are shown in Figure 4. Compared with the pristine MXene membrane, the salt flux () of the STPP-MXene membrane exhibited a significant increase. The NaCl flux of the STPP-MXene membrane increased to 11.5 × 10−1 mol m−2 h−1, which is approximately 2.25 times that of the MXene membrane. Similarly, the Na2SO4 flux increased from 3.0 × 10−1 mol m−2 h−1 to 4.2 × 10−1 mol m−2 h−1, the MgCl2 flux escalated from 3.7 × 10−1 mol m−2 h−1 to 6.5 × 10−1 mol m−2 h−1, and the MgSO4 flux increased from 4.3 × 10−1 mol m−2 h−1 to 8.7 × 10−1 mol m−2 h−1. Previous studies have shown that with the STPP inserted into the space of the MXene nanosheets, the interlayer spacing of STPP-MXene was moderately increased (from 1.44 to 1.63 nm) [25]. Obviously, similar to the increase in the water permeability of the two-dimensional membrane in the cross-flow device, the increase in salt permeability in the diffusion cell could be attributed to the rapid transfer of salts in the enlarged lateral membrane channels.
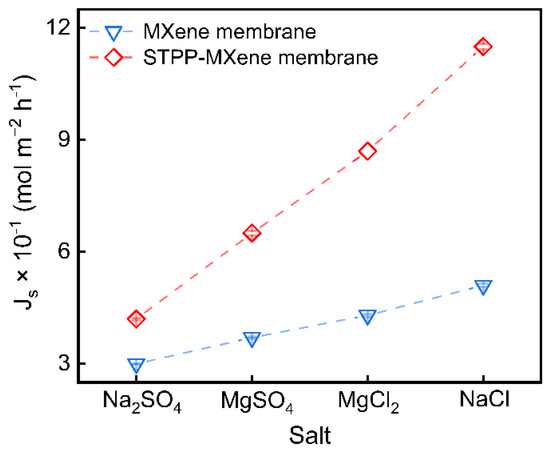
Figure 4.
NaCl, Na2SO4 MgCl2, and MgSO4 flux () of MXene and STPP-MXene membranes from low to high.
Nevertheless, the moderate increase in interlayer spacing can only be used to explain the overall improvement in the permeability of the STPP-MXene membrane from the perspective of steric hindrance. It is worth noting that the improvement in salt flux varies among the four different types of salt ions. Compared to the 2.25-fold increase in salt permeability observed for NaCl, the obviously lower enhancement factor for Na2SO4 (only 1.4-fold) represents the following mechanism: reduced electrostatic repulsion for Na+ transport and persistent negative surface charge in the interlayer channels. The adsorption of STPP on the Ti edges neutralized the charge at the ‘entrance’, thereby weakening the electrostatic repulsion against Na+ ions entering the interlayer channels. This allowed the sodium salts (NaCl and Na2SO4) to enter the membrane. However, STPP modification was unable to alter the intrinsically negatively charged functional groups (e.g., -O, -OH, -F) on the MXene surface. Consequently, the fully negatively charged interlayer channels continued to exert strong electrostatic repulsion against anions (Cl− and SO42−) and hindered their transport. Particularly for Na2SO4, SO42− has a higher charge density than Cl−, which inhibited the diffusion and mass transfer of Na2SO4 containing SO42−. In comparison, the positive charge intensity and hydrated radius of Mg2+ were larger than those of Na+, making the capping of the edges by STPP more effective in promoting the transport of Mg2+, resulting a relatively large increase in the magnesium salt permeability of the modified STPP-MXene membrane, with the difference in the results being relatively slight (MgCl2: 2.02-fold and MgSO4: 1.76-fold) [44].
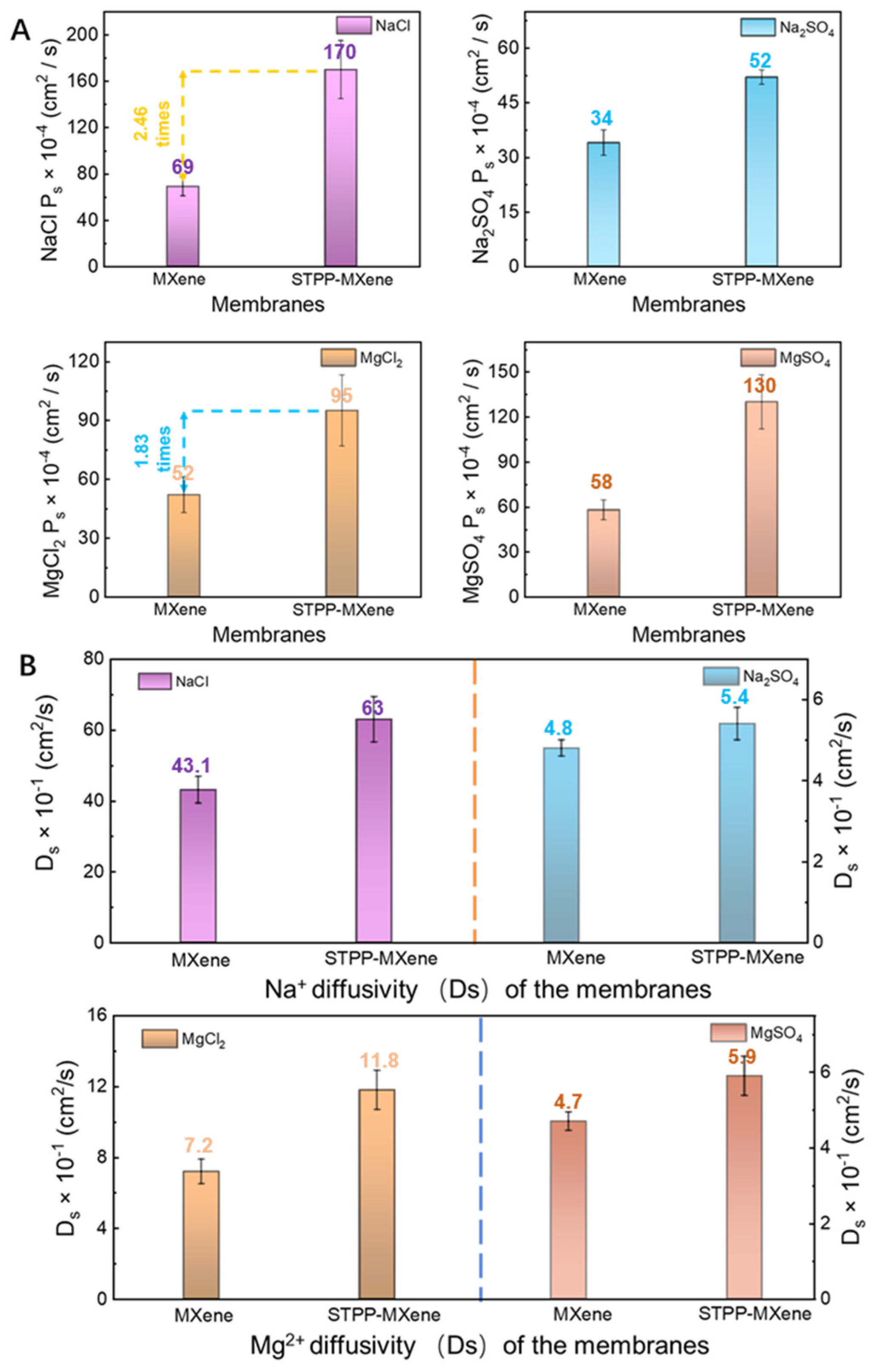
3.3. The Salt Permeability (Ps) and Diffusivity (Ds) of the Membranes
According to Equations (2)–(4), the salt permeability (), solubility (Ks), and diffusivity () of the MXene and STPP-MXene membranes for the four salts mentioned above was measured. The STPP-MXene membrane exhibited a marked enhancement in salt permeability (Ps). Specifically, its NaCl permeability surged to 170 cm2/s, representing a 2.46-fold increase compared to the pristine MXene membrane. This improvement was consistently observed across other salts: Na2SO4 permeability rose from 34 cm2/s to 52 cm2/s, MgCl2 permeability nearly doubled from 52 cm2/s to 95 cm2/s, and MgSO4 permeability showed the most pronounced growth, escalating from 58 cm2/s to 130 cm2/s (Figure 5A). In addition, to further distinguish the influence of edge capping on the separation ability of the MXene membrane, a kinetic desorption experiment was carried out to calculate the differences in salt solubility and diffusion capacity within the membranes. The of NaCl for the MXene membrane was determined to be 16 × 10−4 (g salt/cm3 swollen polymer)/(g salt/cm3 solution), while the STPP-MXene membrane was 27 × 10−4 (g salt/cm3 swollen polymer)/(g salt/cm3 solution), exhibiting a 1.69-fold improvement compared to the pristine membrane. In addition, the partition coefficient () values of the STPP-MXene membrane during the permeation of Na2SO4, MgCl2, and MgSO4 were all respectively larger than those of the MXene membrane. In terms of both and , the overall progress of STPP-MXene membrane in the transmembrane transport was consistent with the law obtained by observing . The salt diffusivity () result is shown in Figure 5B, which illustrates the diffusivility of NaCl, Na2SO4, MgCl2, and MgSO4 through the STPP-MXene and the pristine MXene membranes. As shown in Figure 5B, the of NaCl for the MXene membrane was 43 × 10−1 cm2/s, while the STPP-MXene membrane exhibited a higher of 63 × 10−1 cm2/s. The results indicate that the STPP-MXene membrane exhibited a relatively higher Ds than the pristine MXene membrane. Similarly, the of MgCl2 for the STPP-MXene membrane (11.8 × 10−1 cm2/s) was much higher than that of the MXene membrane (7.2 × 10−1 cm2/s). This result identified the positive effect of the addition of STPP on the diffusion of salts within the MXene membrane as a whole and was highly correlated with the results obtained from the measurement of : the ionic radius of Mg2+ in MgCl2 was larger than that of Na+, and there were more Cl− anions [45], which hindered the mass transfer of the salt in the interlayer channels, resulting in a lower salt diffusion () of MgCl2 within the membrane than that of NaCl. Moreover, due to the higher charge density of SO42− [46], the results for the MXene and STPP-MXene membranes regarding Na2SO4 and MgSO4 were far lower than the results of NaCl and MgCl2.
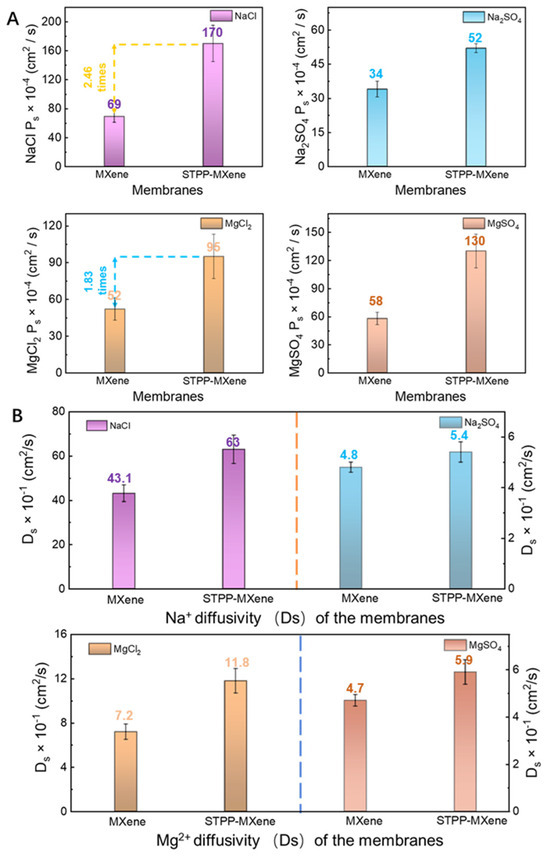
Figure 5.
Salt permeability () and diffusivity () of MXene and STPP-MXene membranes. (A) NaCl, Na2SO4, MgCl2, and MgSO4 permeability coefficient. (B) Comparison of NaCl, Na2SO4, MgCl2, and MgSO4 diffusivity.
In summary, compared to the pristine MXene membrane, the STPP-MXene membrane exhibited higher partition coefficients () and diffusion coefficients (). The elevated indicated an easier way to drive cations such as Na+ and Mg2+ to absorb onto the chemical groups (e.g., -OH, -O) of the MXene surface within the interlayer channels [34]. This enhancement could be attributed to the addition of the STPP. With the STPP capping the edges of Ti between adjacent nanosheets, the shoulder spacing pathway acted as a newly created pathway to reduce the repulsion of the cations, allowing them to enter the membrane more easily [25]. Concurrently, the higher result suggested a reduction in diffusion resistance within the membrane. Therefore, the expanded interlayer spacing of the STPP-MXene membrane allowed salts such as NaCl and MgSO4 to permeate efficiently through the interlamellar channels. The synergistic optimization of and enhanced the dual advantages of the STPP-MXene membrane in ion transporting, ultimately leading to an increase in the salt flux of the MXene-based membrane.
However, the aforementioned analyses of experimental results treated the performance of the pristine and modified MXene membranes as holistic systems, attributing the observed changes to pre-existing intrinsic properties such as the expansion of interlayer spacing and the charge distribution between interlayer channels and functional groups. These discussions failed to specifically evaluate the contribution of edge-capping to salt permeation and transportation. Notably, the research revealed that the enhancement in the diffusion coefficient () for Mg-containing salts in the modified STPP-MXene membrane exceeded that of Na-containing salts correspondingly. This phenomenon is worth further investigation using transition state theory (TST) [36,47] because the energy barrier was very suitable for measuring the ‘degree of difficulty’ for salts to enter the spaces between adjacent lateral nanosheets (shoulder spacing) of the MXene membrane.
3.4. Calculation and Mechanism of Energy Barriers Around the Membranes
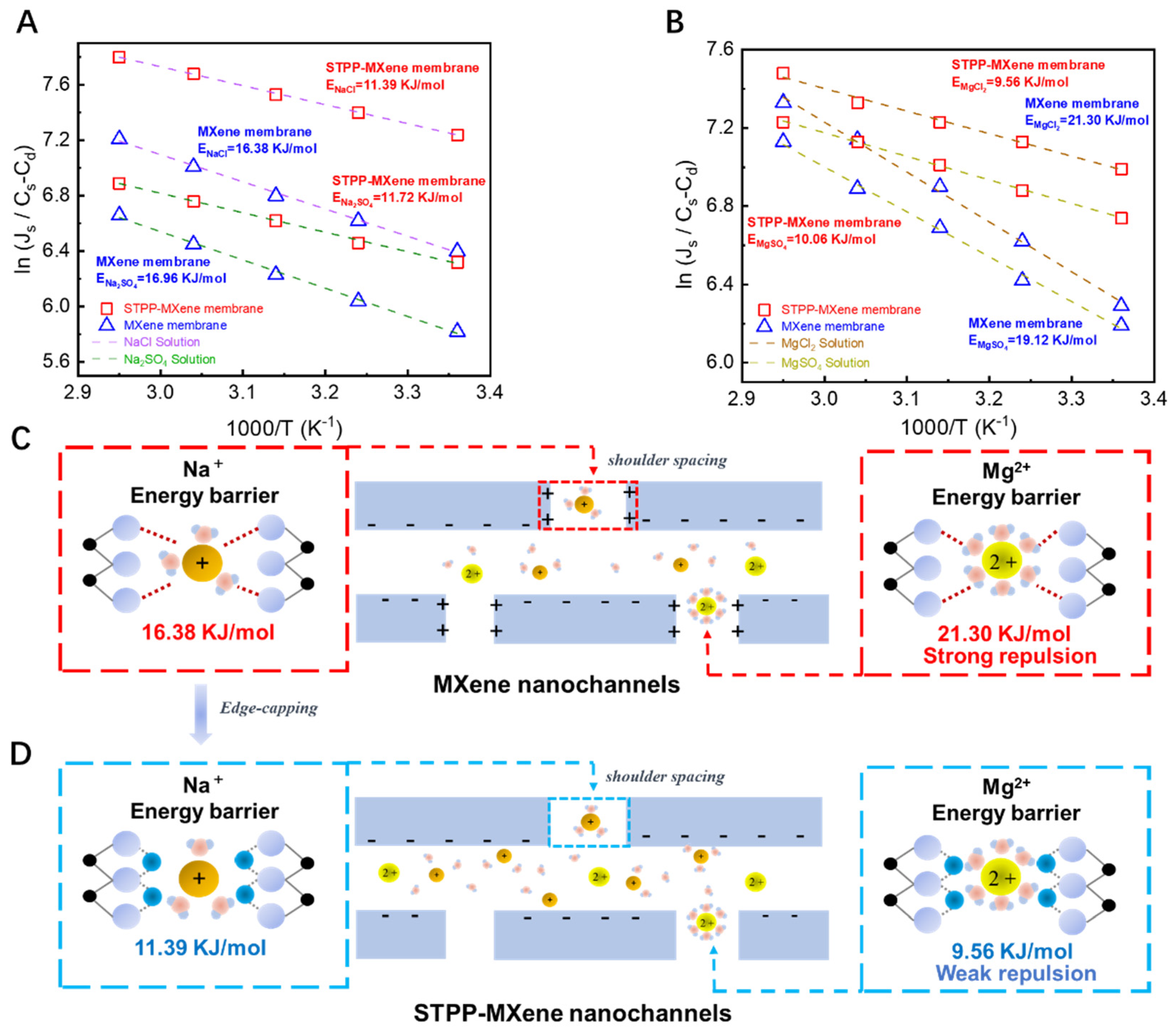
To verify the influence of edge capping on MXene membrane separation performance and to elucidate the mechanism of Ti-edge modification in the membrane separation performance, we conducted concentration-driven diffusion experiments to measure the transmembrane energy barrier of the salts . The key factors that affected the salt permeation were investigated by placing modified and pristine MXene membranes in neutral pH diffusion cells with 0.1 M solutions of four salts (NaCl, Na2SO4, MgCl2, MgSO4). The experimental results, exhibited in Figure 6A,B, according to the Arrhenius Equation (6), demonstrated that when salts permeated through the pristine MXene membrane, both Mg salts exhibited higher transmembrane energy barriers than Na salts that contained the same acid radical ion in the diffusion cell (EMgCl2 21.30 kJ/mol > ENaCl 16.38 kJ/mol; EMgSO4 19.12 kJ/mol > ENa2SO4 16.96 kJ/mol). This phenomenon can be attributed to the influence exerted by the unsaturated Lewis acidic Ti sites (shown in Figure 6C) that were contained in the edges of the unmodified MXene membrane [3]. The positively charged Ti sites generated stronger electrostatic repulsion toward Mg2+ ions, which have a higher charge density compared to Na+ ions. This greater electrostatic repulsive force would make it more challenging for Mg2+ to enter the interlayer channels of MXene membranes along the Ti edges under initial conditions [48]. Notably, the transmembrane energy barrier differences between MgCl2 and MgSO4 were minimal, whether through MXene or STPP-MXene membranes, and the same trend applied to NaCl and Na2SO4. This implies that during the first stage of membrane transport, the entrance of ions into the 2D MXene membrane, the Donnan effect between cations and MXene nanosheets dominated the magnitude of the transmembrane energy barrier. In contrast, the relationship between acid radical ions (Cl−, SO42−) and the functional groups on MXene surfaces (along with the interlayer spacing formed by upper/lower functional groups) primarily influenced mass transfer efficiency after ions entered the interlayer channels.
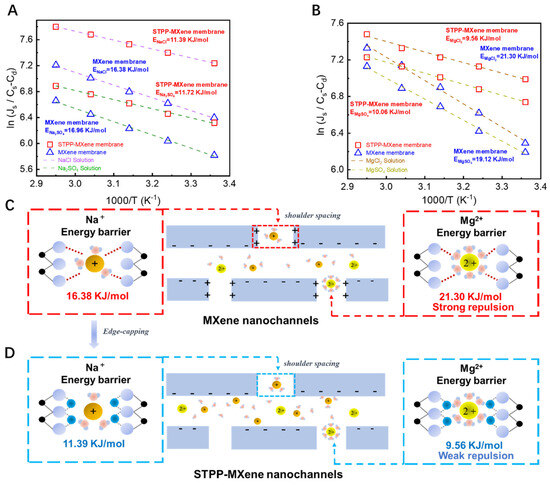
Figure 6.
Arrhenius-type plots for salts during the diffusion and transmembrane processes through MXene and STPP-MXene membranes. The natural logarithm of the salt flux at four different temperatures was plotted as a function of 1/T. (A) The transmembrane energy barrier for the diffusion of NaCl and Na2SO4 and (B) for the diffusion of MgCl2 and MgSO4. Mechanism of salt transmembrane energy barrier in (C) MXene membrane and (D) STPP-MXene membrane.
With the successful introduction of STPP, the flux of the four salts () permeating through the STPP-MXene membrane are all higher than those through the MXene membrane. At the same time, the transmembrane reaction energy barriers of these four salts through the STPP-MXene membrane have been significantly reduced (ENaCl decreased from 16.38 KJ/mol to 11.39 KJ/mol, ENa2SO4 decreased from 16.96 KJ/mol to 11.72 KJ/mol, EMgCl2 decreased from 21.30 KJ/mol to 9.56 KJ/mol, and EMgSO4 decreased from 19.12 KJ/mol to 10.06 KJ/mol). A reduction in activation energy () indicated a decrease in the energy barrier for ion transport within the membrane. Among these, the decreases for MgSO4 (55.1%) and MgCl2 (47.4%) are much higher than those for NaCl (30.5%) and Na2SO4 (30.9%), showing that the STPP modification has a more significant optimization effect on the energy barrier of high-valence cations (Mg2+) and further highlighting the influence of edge capping on salt permeation (shown in Figure 6D). The differences in the activation energy of the four salts originated from the synergistic effect of ion properties and membrane–ion interactions [49]. With the phosphate groups (-PO43−) of STPP modifying the Ti edges of the membrane, the electrostatic repulsion between the adjacent nanosheets and the high-valence cations (Mg2+) was greatly reduced, thus allowing the cations to enter the channels conveniently. In addition, the reduction in the repulsive force that the cations required to enter the membrane eventually weakened the initial energy barrier for Mg2+ to enter the interlayer of the membraneand drove its transmembrane transport more efficiently. However, the charge density of Na+ (+1) was relatively low; thus, the reduction of electrostatic repulsion was relatively weak, and the decrease in the energy barrier was smaller. In addition, previous studies have also shown that the introduction of STPP was beneficial for improving the hydrophilicity of the MXene membrane. Although the hydration energy of Mg2+ (~1920 kJ/mol) was significantly higher than that of Na+ (~405 kJ/mol), Mg2+ was more likely to undergo partial dehydration under the negatively charged surface of the STPP-MXene membrane [36,50]. However, the hydration shell of Na+ was looser compared with that of Mg2+ (hydrated radius ~0.36 nm vs. Mg2+ ~0.43 nm), and the demand for dehydration was lower. Therefore, the optimization effect of STPP modification on its transport energy barrier was limited [45].
The abovementioned experimental results and analysis demonstrate that the Ti atoms at the edges of MXene, after STPP capping, and the phosphate groups of STPP can form a stable relationship based on non-covalent bonds. The capping of STPP increased the hydrophilicity of the membrane surface and resulted in the lateral edges losing their charge, thereby creating new shoulder spacing channels and reducing the transition state energy required for cations to enter the membrane phase from the solution phase and resulting in the reduction of the transmembrane energy barrier for salts. Notably, for Mg2+, due to the weakened electrostatic repulsion, the decrease in its energy barrier was more obvious. Generally, the STPP modification systematically reduced the transport activation energy of Na+ and Mg2+ by expanding the interlayer spacing, optimizing the surface charge, and enhancing hydrophilicity. This result clearly demonstrates the unique and significant contribution of the edge-capping method to the mass transport efficiency of MXene membranes.
4. Conclusions
This research systematically investigated the performance optimization mechanism of STPP-modified MXene membranes in terms of salt permeability (NaCl, Na2SO4, MgCl2, and MgSO4) and transmembrane energy barriers () by employing an edge capping method to functionalize Ti atoms at the MXene membrane edges via non-covalent bonds. The experimental results demonstrated that the salt flux () of the modified STPP-MXene membrane increased by approximately 1.86 times compared with the pristine membrane. In addition, the solution–diffusion model was introduced to evaluate the partition coefficient () and diffusion coefficient () during permeation, thereby dividing the salt transport process in MXene membranes. Furthermore, analysis of transmembrane energy barriers revealed that STPP modification induced larger reductions in activation energy for Mg2+ compared to Na+. This disparity arose from the weakened electrostatic interactions between high-valent Mg2+ and the modified membrane edges, as well as the partial dehydration effect. In contrast, the limited charge density of Na+ resulted in relatively modest optimization. In summary, the prominent contribution of shoulder spacing channels from capping STPP on the Ti edges of adjacent MXene nanosheets was observed. When salts (such as NaCl) entered the MXene membrane, the energy barrier required for cation transmembrane transport was significantly reduced (the ENaCl decreased from 16.38 kJ/mol to 11.39 kJ/mol). As a result, the salt flux of NaCl increased by 2.25 times, from 5.1 × 10−1 mol m−2 h−1 to 11.5 × 10−1 mol m−2 h−1. This result further confirmed that the STPP-MXene membrane could achieve the selective enhancement of high-valent cation transport through interlayer spacing regulation, surface charge optimization, and hydrophilicity improvement, thereby designing high-efficiency membranes for ion permeation. This research elucidates the mechanism by which edge-capped MXene membranes balance permeability and molecule/ion selectivity, thereby advancing the application of two-dimensional MXene membranes in resource recovery.
Author Contributions
Conceptualization, Y.L., X.X. and F.L.; methodology, Y.L. and X.X.; experiments and characterizations, Y.L.; original draft, Y.L.; reviewing and editing, Y.L., X.X., X.F. and F.L.; data curation, Y.L. and F.L.; funding acquisition, F.L., Y.L. and X.X. contributed equally to this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 5240071) and the Fundamental Research Funds for the Central Universities (No. 2232024G-11).
Data Availability Statement
Data are available upon request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tian, S.; Pan, Q.; Li, H.; Sui, X.; Chen, Y. Two-dimensional material membrane fabrication: Progress and challenges. Curr. Opin. Chem. Eng. 2023, 39, 100900. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 13384–13397. [Google Scholar] [CrossRef]
- Xu, R.; Kang, Y.; Zhang, W.; Pan, B.; Zhang, X. Two-dimensional MXene membranes with biomimetic sub-nanochannels for enhanced cation sieving. Nat. Commun. 2023, 14, 4907. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Mao, Y.; Li, G.; Croes, T.; Zhu, J.; You, X.; Volodin, A.; Zheng, J.; Van der Bruggen, B. Recycling the High-Salinity Textile Wastewater by Quercetin-Based Nanofiltration Membranes with Minimal Water and Energy Consumption. Environ. Sci. Technol. 2022, 56, 17998–18007. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y.; et al. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef]
- Huang, Z.; Ling Zhao, D.; Shen, L.; Lin, H.; Chen, C.; Xu, Y.; Li, B.; Teng, J.; Han, L.; Chung, T.-S. Mxenes for membrane separation: From fabrication strategies to advanced applications. Sci. Bull. 2024, 69, 125–140. [Google Scholar] [CrossRef]
- Meng, B.; Liu, G.; Mao, Y.; Liang, F.; Liu, G.; Jin, W. Fabrication of surface-charged MXene membrane and its application for water desalination. J. Membr. Sci. 2021, 623, 119076. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Wang, Y.; Chen, H.; Caro, J.; Wang, H. A Two-Dimensional Lamellar Membrane: MXene Nanosheet Stacks. Angew. Chem. Int. Ed. 2017, 56, 1825–1829. [Google Scholar] [CrossRef]
- Pan, J.; Xia, F. Membrane separation assisted by subnanometer channels. Matter 2022, 5, 2526–2528. [Google Scholar] [CrossRef]
- Zhou, S.; Guan, K.; Fang, S.; Wang, Z.; Li, Z.; Xu, P.; Nakagawa, K.; Takagi, R.; Matsuyama, H. Nanochannel characteristics contributing to ion/ion selectivity in two-dimensional graphene oxide membranes. J. Membr. Sci. 2024, 689, 122185. [Google Scholar] [CrossRef]
- Ghanbari, R.; Wu, D.; Heynderickx, P.M. Fabrication of MXene-based membranes and their application in per- and polyfluorinated substances removal: Comparison with commercial membranes, challenges, and future improvements. Coord. Chem. Rev. 2025, 523, 216253. [Google Scholar] [CrossRef]
- Xiang, B.; Gong, J.; Sun, Y.; Yan, W.; Jin, R.; Li, J. High permeability PEG/MXene@MOF membrane with stable interlayer spacing and efficient fouling resistance for continuous oily wastewater purification. J. Membr. Sci. 2024, 691, 122247. [Google Scholar] [CrossRef]
- Ding, L.; Li, L.; Liu, Y.; Wu, Y.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain. 2020, 3, 296–302. [Google Scholar] [CrossRef]
- Yan, Y.; Han, H.; Dai, Y.; Zhu, H.; Liu, W.; Tang, X.; Gan, W.; Li, H. Nb2CTx MXene Nanosheets for Dye Adsorption. ACS Appl. Nano Mater. 2021, 4, 11763–11769. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, D.; Li, S.; Cui, L.; Zhuang, Y.; Xing, W.; Jing, W. Assembly of multidimensional MXene-carbon nanotube ultrathin membranes with an enhanced anti-swelling property for water purification. J. Membr. Sci. 2021, 623, 119075. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, F.; Meng, L.; Gao, Q.; Wang, X.; Lou, M.; Xu, X.; Zhang, W.; Li, F.; Van der Bruggen, B. MXene Membranes Inserted with Tannic Acid Etched MOF Nanocrystals for Ultrafast Water Permeation: Elucidating the Water Transport Mechanism in Nanoconfined Interlaminar Channels. Nano Lett. 2025, 25, 2810–2819. [Google Scholar] [CrossRef]
- Jang, J.; Kang, Y.; Jang, K.; Kim, S.; Chee, S.-S.; Kim, I.S. Ti3C2TX-Ethylenediamine nanofiltration membrane for high rejection of heavy metals. Chem. Eng. J. 2022, 437, 135297. [Google Scholar] [CrossRef]
- Yu, C.; Nghiem, L.D.; Zou, L. Catalytic chitosan/MXene/GO nanocomposite membrane for removing dye and heavy metals. Desalination 2025, 594, 118313. [Google Scholar] [CrossRef]
- Qiu, M.; Shen, Z.; Xia, Q.; Li, X.; Huang, H.; Wang, Y.; Liu, Y.; Wang, Y. Metal-polyphenol cross-linked titanium carbide membranes with stable interlayer spacing for efficient wastewater treatment. J. Colloid Interface Sci. 2022, 628, 649–659. [Google Scholar] [CrossRef]
- Qu, K.; Dai, L.; Xia, Y.; Wang, Y.; Zhang, D.; Wu, Y.; Yao, Z.; Huang, K.; Guo, X.; Xu, Z. Self-crosslinked MXene hollow fiber membranes for H2/CO2 separation. J. Membr. Sci. 2021, 638, 119669. [Google Scholar] [CrossRef]
- Helal, M.I.; Sinopoli, A.; Gladich, I.; Tong, Y.; Alfahel, R.; Gomez, T.; Mahmoud, K.A. Understanding the swelling behavior of Ti3C2Tx MXene membranes in aqueous media. J. Mater. Chem. A 2024, 12, 30729–30742. [Google Scholar] [CrossRef]
- Jiang, J.; Bai, S.; Zou, J.; Liu, S.; Hsu, J.-P.; Li, N.; Zhu, G.; Zhuang, Z.; Kang, Q.; Zhang, Y. Improving stability of MXenes. Nano Res. 2022, 15, 6551–6567. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, Y.; Deng, J.; Ding, L.; Li, Z.-K.; Wang, H. Self-Crosslinked MXene (Ti3C2Tx) Membranes with Good Antiswelling Property for Monovalent Metal Ion Exclusion. ACS Nano 2019, 13, 10535–10544. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Jing, X.; Li, X.; Wang, J.; Kang, M.; Zhao, Y.; Li, Q. Construction and effect of intramolecular hydrogen bond on solvent resistance of polymeric membranes and their application in impermeable membranes. J. Ind. Eng. Chem. 2022, 107, 302–312. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, X.; Gao, Q.; Bai, Y.; Lou, M.; Huang, S.; Li, F.; Van der Bruggen, B. Regulating molecules/ions sieving channels of MXene-based membranes by edge-capping strategy. J. Membr. Sci. 2024, 712, 123236. [Google Scholar] [CrossRef]
- Huang, S.; Natu, V.; Tao, J.; Xia, Y.; Mochalin, V.N.; Barsoum, M.W. Understanding the effect of sodium polyphosphate on improving the chemical stability of Ti3C2Tz MXene in water. J. Mater. Chem. A 2022, 10, 22016–22024. [Google Scholar] [CrossRef]
- Natu, V.; Hart, J.L.; Sokol, M.; Chiang, H.; Taheri, M.L.; Barsoum, M.W. Edge Capping of 2D-MXene Sheets with Polyanionic Salts to Mitigate Oxidation in Aqueous Colloidal Suspensions. Angew. Chem. Int. Ed. 2019, 58, 12655–12660. [Google Scholar] [CrossRef]
- Azam, R.S.; Almasri, D.A.; Alfahel, R.; Hawari, A.H.; Hassan, M.K.; Elzatahry, A.A.; Mahmoud, K.A. MXene (Ti3C2Tx)/Cellulose Acetate Mixed-Matrix Membrane Enhances Fouling Resistance and Rejection in the Crossflow Filtration Process. Membranes 2022, 12, 406. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, L.; Lu, Z.; Deng, J.; Wei, Y. Two-dimensional MXene membrane for ethanol dehydration. J. Membr. Sci. 2019, 590, 117300. [Google Scholar] [CrossRef]
- Xia, H.; Zhou, W.; Qu, X.; Wang, W.; Wang, X.; Qiao, R.; Zhang, Y.; Wu, X.; Yang, C.; Ding, B.; et al. Electricity generated by upstream proton diffusion in two-dimensional nanochannels. Nat. Nanotechnol. 2024, 19, 1316–1322. [Google Scholar] [CrossRef]
- DuChanois, R.M.; Heiranian, M.; Yang, J.; Porter, C.J.; Li, Q.; Zhang, X.; Verduzco, R.; Elimelech, M. Designing polymeric membranes with coordination chemistry for high-precision ion separations. Sci. Adv. 2022, 8, eabm9436. [Google Scholar] [CrossRef]
- Fu, J.; Xiao, S.; Cao, J.; Liang, Z.; Chen, J.; Jiang, Y.; Xing, M. Mass Transfer-Enhanced Photothermal Membranes with Synergistic Light Utilization for High-Turbidity Wastewater Purification. Angew. Chem. Int. Ed. 2024, 64, e202421800. [Google Scholar] [CrossRef]
- Xu, T.; Wu, B.; Li, W.; Li, Y.; Zhu, Y.; Sheng, F.; Li, Q.; Ge, L.; Li, X.; Wang, H.; et al. Perfect confinement of crown ethers in MOF membrane for complete dehydration and fast transport of monovalent ions. Sci. Adv. 2024, 10, eadn0944. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, X.; Chen, J.; Zhang, X.; Wang, Z.; Li, F. Lithium complexing strategy based on host-guest recognition for efficient Mg2+/Li+ separation. Water Res. 2025, 274, 123100. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Wang, Y.-L.; Dai, R.; Li, X.; Wang, Z. Roles of Anion–Cation Coupling Transport and Dehydration-Induced Ion–Membrane Interaction in Precise Separation of Ions by Nanofiltration Membranes. Environ. Sci. Technol. 2022, 56, 14069–14079. [Google Scholar] [CrossRef]
- Shefer, I.; Peer-Haim, O.; Leifman, O.; Epsztein, R. Enthalpic and Entropic Selectivity of Water and Small Ions in Polyamide Membranes. Environ. Sci. Technol. 2021, 55, 14863–14875. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, S.; Wei, C.; Wu, C.; Mochalin, V.N. Adhesion of two-dimensional titanium carbides (MXenes) and graphene to silicon. Nat. Commun. 2019, 10, 3014. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Zhang, M.; Wu, B.; Zhuang, Y.; Ramachandran, R.; Zhao, C.; Wang, F. Nanocomposites of pre-oxidized Ti3C2Tx MXene and SnO2 nanosheets for highly sensitive and stable formaldehyde gas sensor. Ceram. Int. 2023, 49, 2583–2590. [Google Scholar] [CrossRef]
- Shamaei, L.; Khorshidi, B.; Islam, M.A.; Sadrzadeh, M. Development of antifouling membranes using agro-industrial waste lignin for the treatment of Canada’s oil sands produced water. J. Membr. Sci. 2020, 611, 118326. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, M.; Chang, J.; Sikdar, A.; Wang, N.; An, Q.-F.; Yuan, J. Heterostructure membranes of high permeability and stability assembled from MXene and modified layered double hydroxide nanosheets. J. Membr. Sci. 2023, 688, 122100. [Google Scholar] [CrossRef]
- Xu, S.; Liu, C.; Jiang, X.; Wang, X.; Zhang, S.; Zhang, Y.; Wang, Q.; Xiong, W.; Zhang, J. Ti3C2 MXene promoted Fe3+/H2O2 fenton oxidation: Comparison of mechanisms under dark and visible light conditions. J. Hazard. Mater. 2023, 444, 130450. [Google Scholar] [CrossRef] [PubMed]
- Sadidi, M.; Hajilary, N.; Abbasi, F. Optimization of biofuel dehydration performance of PES/PEI/MXene membrane by response surface method. J. Environ. Chem. Eng. 2023, 11, 110946. [Google Scholar] [CrossRef]
- Parker, T.; Zhang, D.; Bugallo, D.; Shevchuk, K.; Downes, M.; Valurouthu, G.; Inman, A.; Chacon, B.; Zhang, T.; Shuck, C.E.; et al. Fourier-Transform Infrared Spectral Library of MXenes. Chem. Mater. 2024, 36, 8437–8446. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Liu, M.; Guo, H.; Luo, J.; Gui, X.; Xing, Y.; Cao, Y. Investigation on the effect of metal cation radius on montmorillonite hydration: Combining experiments with molecular dynamics simulation. Sep. Purif. Technol. 2025, 353, 128474. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Kang, Y.; Zhu, Y.; Simon, G.P.; Wang, H. Voltage-Gated Ion Transport in Two-Dimensional Sub-1 nm Nanofluidic Channels. ACS Nano 2019, 13, 11793–11799. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, C.; Zhang, M.; Zhao, C.; Chao, D.; Zhou, G.; Zhang, C. MXene Triggered Free Radical Polymerization in Minutes Toward All-Printed Zn-Ion Hybrid Capacitors and Beyond. Angew. Chem. Int. Ed. 2025, 64, e202413728. [Google Scholar] [CrossRef]
- Natu, V.; Sokol, M.; Verger, L.; Barsoum, M.W. Effect of Edge Charges on Stability and Aggregation of Ti3C2Tz MXene Colloidal Suspensions. J. Phys. Chem. C 2018, 122, 27745–27753. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving Through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Ding, S.; Dai, X.; Tian, Y.; Chen, J.; Wang, L.; Li, G.; Li, S.; Meng, A.; Li, Z. Study on synergy effect of hydrated ionic radius and oxidation state for pre-intercalated cations towards highly reversible magnesium ions batteries. Chem. Eng. J. 2023, 471, 144615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).