Purification of Rosmarinic Acid from Rosemary Extract and the Evaluation of the Antibacterial and Antioxidant Effects of Rosmarinic Acid and Its Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Purification of RA
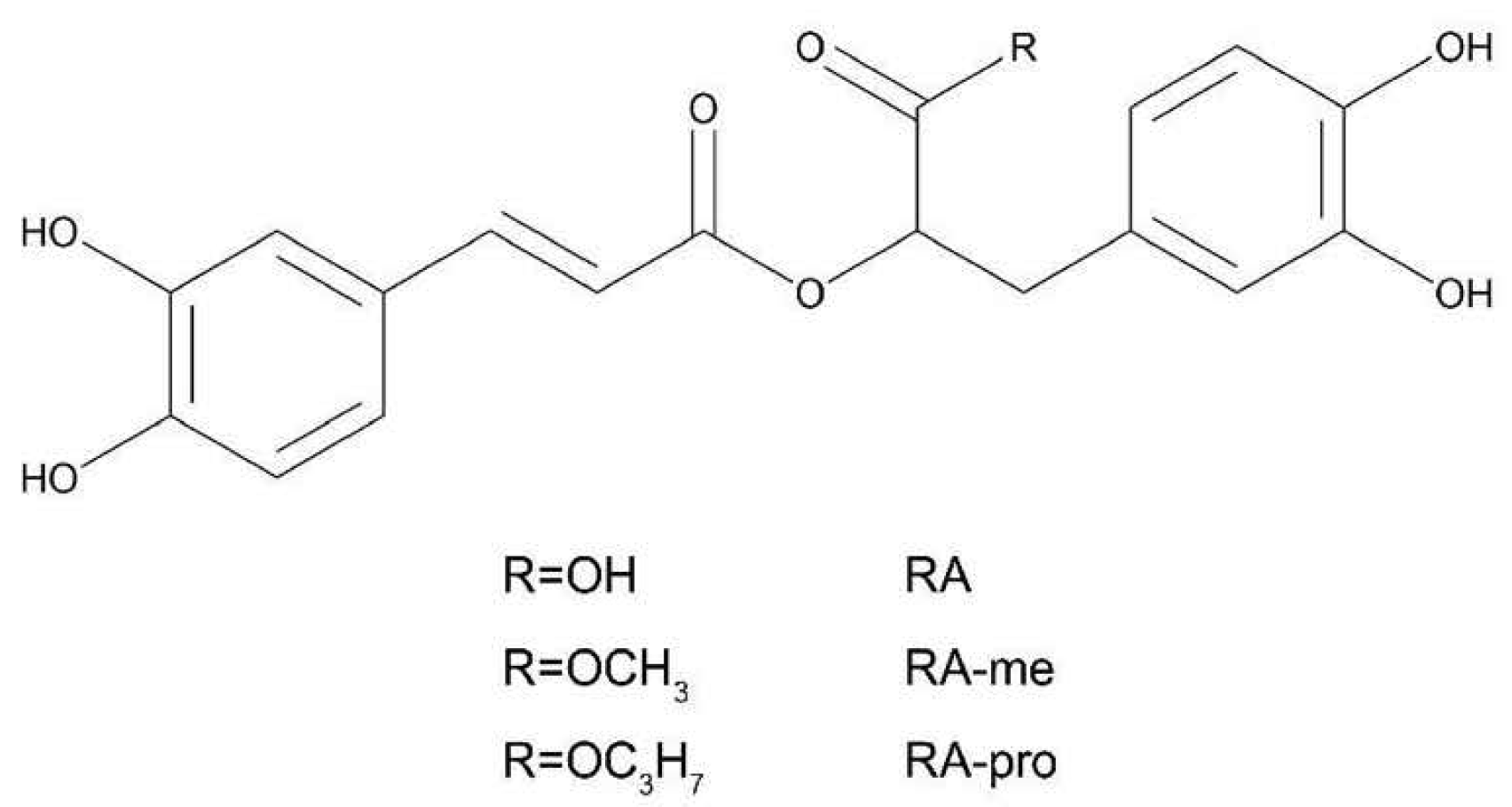
2.3. Esterification of RA
2.4. RA and Its Derivatives Analysis
2.4.1. HPLC Analysis
2.4.2. MS/MS Analysis
2.4.3. FT-IR Analysis
2.5. Antibacterial Activity Assay
2.5.1. Disk Diffusion Assay
2.5.2. Determination of Minimum Inhibitory Concentration (MIC)
2.6. Antioxidant Activity Assay
2.6.1. Radical Scavenging Assay
2.6.2. Ferric Reducing Antioxidant Power Assay
2.7. Statistical Analysis
3. Results and Discussion
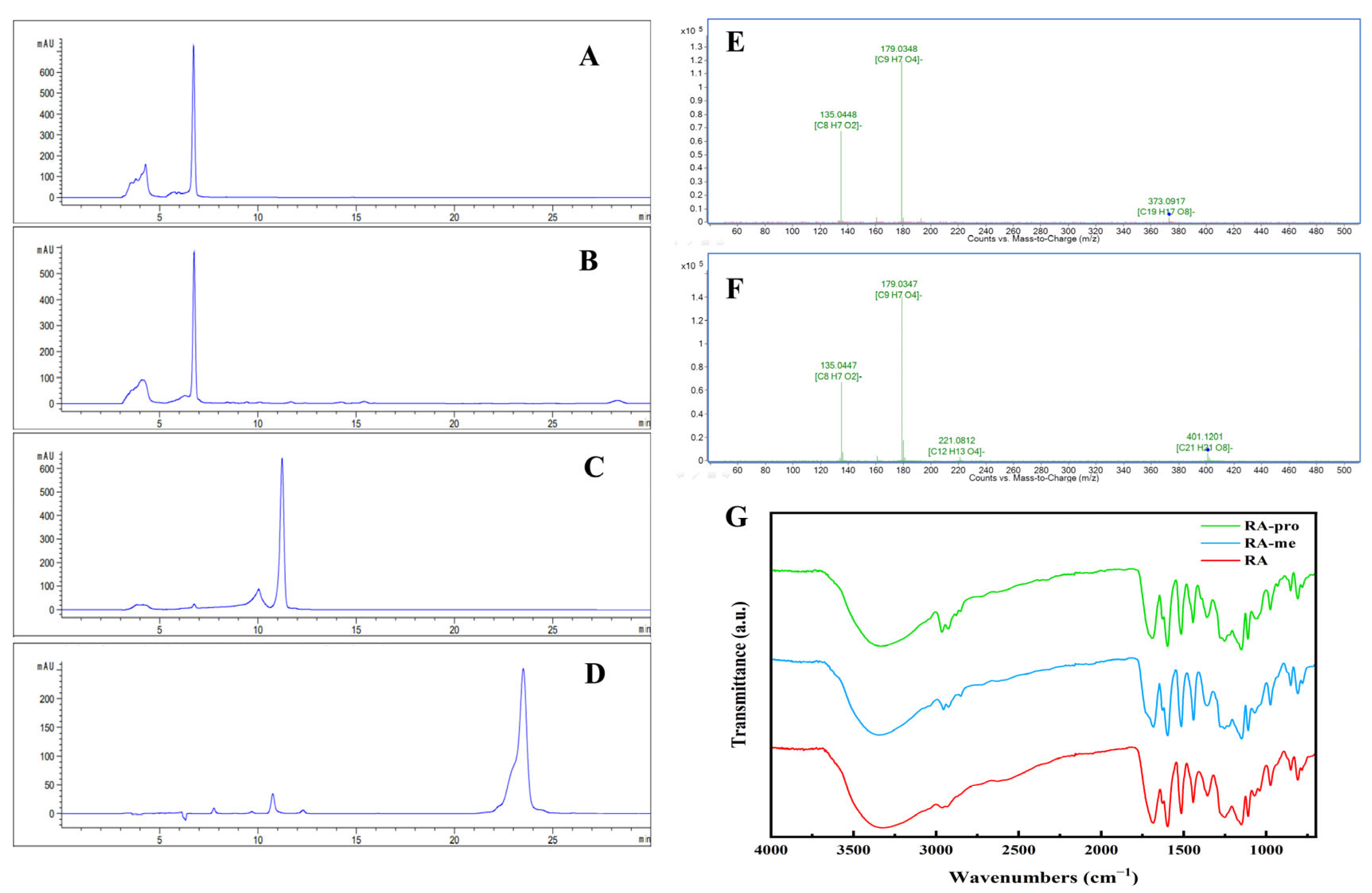
3.1. Rosmarinic Acid Purification
3.2. Preparation and Characterization of RA Derivatives
3.3. Antibacterial Activity Assay of RA and Its Derivatives
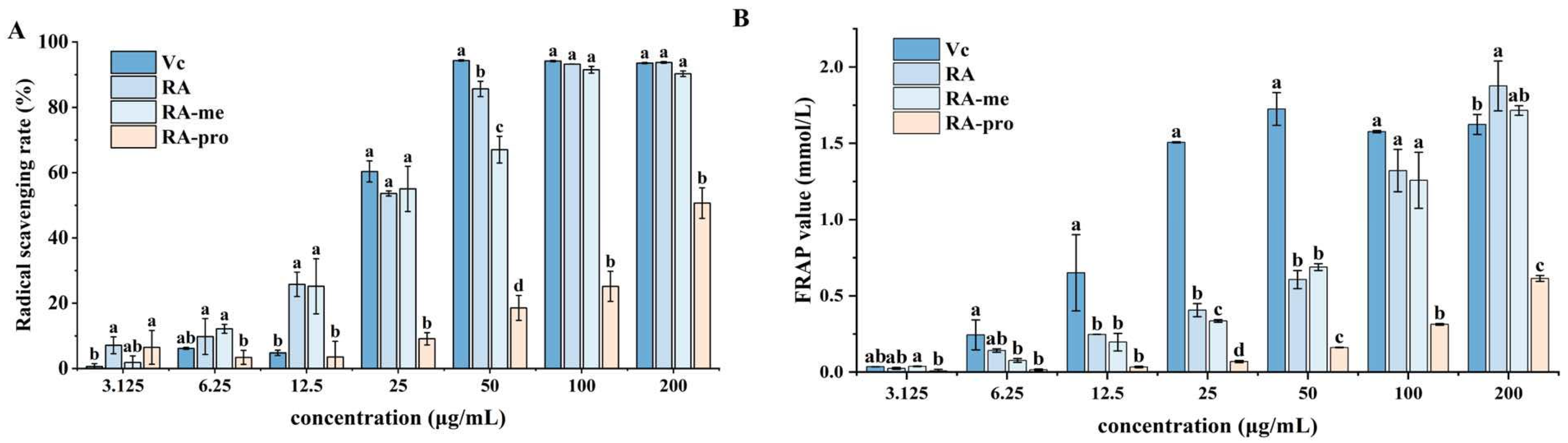
3.4. Antioxidant Activity Assay of RA and Its Derivatives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yarraguntla, S.R.; Kamala Kumari, P. Alternative to Artificial Preservatives. Syst. Rev. Pharm. 2019, 10, 99–102. [Google Scholar]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid—From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Mo, C.-J.; Xu, Y.-Q.; Feng, Y.; Chen, A.-J.; Yang, C.-W.; Ni, H. Simultaneous preparation of water- and lipid-soluble antioxidant and antibacterial activity of purified carnosic acid from Rosmarinus offoccnalis L. Ind. Crops Prod. 2022, 187, 115448. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, X.; Zhang, M.; Bai, B.; Yang, Y.; Fan, S. The antibacterial mechanism of perilla rosmarinic acid. Biotechnol. Appl. Biochem. 2022, 69, 1757–1764. [Google Scholar] [CrossRef]
- Kernou, O.N.; Azzouz, Z.; Madani, K.; Rijo, P. Application of Rosmarinic Acid with Its Derivatives in the Treatment of Microbial Pathogens. Molecules 2023, 28, 4243. [Google Scholar] [CrossRef] [PubMed]
- Makhathini, K.B.; Mabandla, M.V.; Daniels, W.M.U. Rosmarinic acid reverses the deleterious effects of repetitive stress and tat protein. Behav. Brain Res. 2018, 353, 203–209. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Ikeda, S.; Uwai, K.; Taguchi, R.; Chayama, K.; Sakaguchi, T.; Narita, R.; Yao, W.L.; Takeuchi, F.; Otakaki, Y.; et al. Rosmarinic acid is a novel inhibitor for Hepatitis B virus replication targeting viral epsilon RNA-polymerase interaction. PLoS ONE 2018, 13, e0197664. [Google Scholar] [CrossRef]
- Liu, T.; Sui, X.; Zhang, R.; Yang, L.; Zu, Y.; Zhang, L.; Zhang, Y.; Zhang, Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar] [CrossRef]
- Dahchour, A. Anxiolytic and antidepressive potentials of rosmarinic acid: A review with a focus on antioxidant and anti-inflammatory effects. Pharmacol. Res. 2022, 184, 106421. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef]
- Zhu, F.; Asada, T.; Sato, A.; Koi, Y.; Nishiwaki, H.; Tamura, H. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. Food Chem. 2014, 62, 885–892. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Effects of rosmarinic acid on nervous system disorders: An updated review. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1779–1795. [Google Scholar] [CrossRef]
- Mallegni, N.; Cicogna, F.; Passaglia, E.; Gigante, V.; Coltelli, M.; Coiai, S. Natural Antioxidants: Advancing Stability and Performance in Sustainable Biobased and Biodegradable Plastics. Compounds 2025, 5, 4. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Dziadek, M.; Sadowska, U.; Cholewa-Kowalska, K. The Changes in Bioactive Compounds and Antioxidant Activity of Chia (Salvia hispanica L.) Herb under Storage and Different Drying Conditions: A Comparison with Other Species of Sage. Molecules 2022, 27, 1569. [Google Scholar] [CrossRef]
- Saad, E.M.; El Gohary, N.A.; Abdel-Halim, M.; Handoussa, H.; El Nashar, R.M.; Mizaikoff, B. Molecularly imprinted polymers for selective extraction of rosmarinic acid from Rosmarinus officinalis L. Food Chem. 2021, 335, 127644. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, J.; Takano, H.; Xu, Z.; Nishiwaki, H.; Yonekura, L.; Yang, R.; Tamura, H. Rosmarinic acid and its ester derivatives for enhancing antibacterial, α-glucosidase inhibitory, and lipid accumulation suppression activities. J. Food Biochem. 2019, 43, e12719. [Google Scholar] [CrossRef]
- Rajitha, G.; Ravibabu, V.; Ramesh, G.; Rajitha, B.; Jobina, R.; Siddhardha, B.; Vijaya Laxmi, S. One-pot multicomponent synthesis of novel thiazolylhydrazone derivatives and their antimicrobial activity. Res. Chem. Intermed. 2015, 41, 9703–9713. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Zhang, M.; Bai, B.Q.; Jia, H.W.; Fan, S.H. Studies on Adsorption Kinetics and Thermodynamics of Macroporous Resin for Rosmarinic Acid. J. Oleo Sci. 2021, 70, 439–451. [Google Scholar] [CrossRef]
- Tang, W.; Sun, B.; Zhao, Y. Preparative separation and purification of rosmarinic acid from perilla seed meal via combined column chromatography. J. Chromatogr. B 2014, 947, 41–48. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Algieri, F.; Nogales, A.; Galvez, J.; Segura Carretero, A. Phytochemical profiling of anti-inflammatory Lavandula extracts via RP-HPLC-DAD-QTOF-MS and -MS/MS: Assessment of their qualitative and quantitative differences. Electrophoresis 2017, 39, 1284–1293. [Google Scholar] [CrossRef]
- Stehfest, K.; Boese, M.; Kerns, G.; Piry, A.; Wilhelm, C. Fourier transform infrared spectroscopy as a new tool to determine rosmarinic acid in situ. J. Plant Physiol. 2004, 161, 151–156. [Google Scholar] [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Li, X.Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef]
- Abedini, A.; Roumy, V.; Mahieux, S.; Biabiany, M.; Standaert-Vitse, A.; Rivière, C.; Sahpaz, S.; Bailleul, F.; Neut, C.; Hennebelle, T. Rosmarinic Acid and Its Methyl Ester as Antimicrobial Components of the Hydromethanolic Extract of Hyptis atrorubens Poit. (Lamiaceae). Evid. Based Complement. Altern. Med. 2013, 2013, 604536. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Al-Musayeib, N.; Perveen, S.; Fatima, I.; Nasir, M.; Hussain, A. Antioxidant, anti-glycation and anti-inflammatory activities of phenolic constituents from Cordia sinensis. Molecules 2011, 16, 10214–10226. [Google Scholar] [CrossRef]
- Lecomte, J.; Giraldo, L.J.L.; Laguerre, M.; Baréa, B.; Villeneuve, P. Synthesis, Characterization and Free Radical Scavenging Properties of Rosmarinic Acid Fatty Esters. J. Am. Oil Chem. Soc. 2010, 87, 615–620. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitr. 2017, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]


| Strain | S. aureus | E. coli | |
|---|---|---|---|
| MIC (mg/mL) | RA | 1 | 2 |
| RA-me | 0.5 | 1 | |
| RA-pro | 0.5 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.-J.; Lv, J.; Feng, Y.; Mo, C.-J.; Yang, C.-W.; Ni, H. Purification of Rosmarinic Acid from Rosemary Extract and the Evaluation of the Antibacterial and Antioxidant Effects of Rosmarinic Acid and Its Derivatives. Separations 2025, 12, 294. https://doi.org/10.3390/separations12110294
Chen A-J, Lv J, Feng Y, Mo C-J, Yang C-W, Ni H. Purification of Rosmarinic Acid from Rosemary Extract and the Evaluation of the Antibacterial and Antioxidant Effects of Rosmarinic Acid and Its Derivatives. Separations. 2025; 12(11):294. https://doi.org/10.3390/separations12110294
Chicago/Turabian StyleChen, Ai-Jing, Jin Lv, Yu Feng, Chang-Jia Mo, Cheng-Wei Yang, and He Ni. 2025. "Purification of Rosmarinic Acid from Rosemary Extract and the Evaluation of the Antibacterial and Antioxidant Effects of Rosmarinic Acid and Its Derivatives" Separations 12, no. 11: 294. https://doi.org/10.3390/separations12110294
APA StyleChen, A.-J., Lv, J., Feng, Y., Mo, C.-J., Yang, C.-W., & Ni, H. (2025). Purification of Rosmarinic Acid from Rosemary Extract and the Evaluation of the Antibacterial and Antioxidant Effects of Rosmarinic Acid and Its Derivatives. Separations, 12(11), 294. https://doi.org/10.3390/separations12110294





