Screening Biogenic Volatile Organic Compounds from Common Portuguese Shrubs Using Headspace–Bar Adsorptive Microextraction (HS-BAµE)
Abstract
1. Introduction
2. Materials and Methods
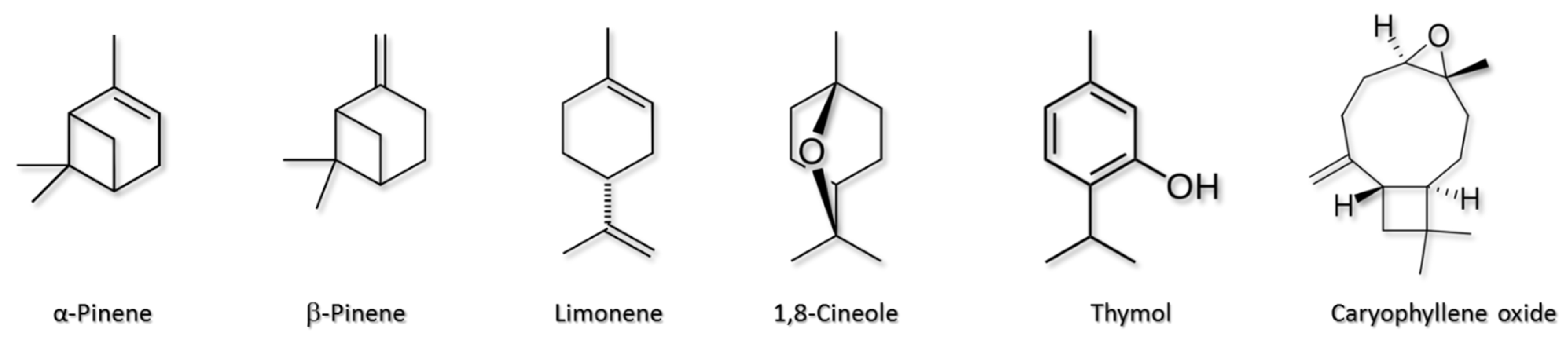
2.1. Chemical Standards, Materials, and Samples
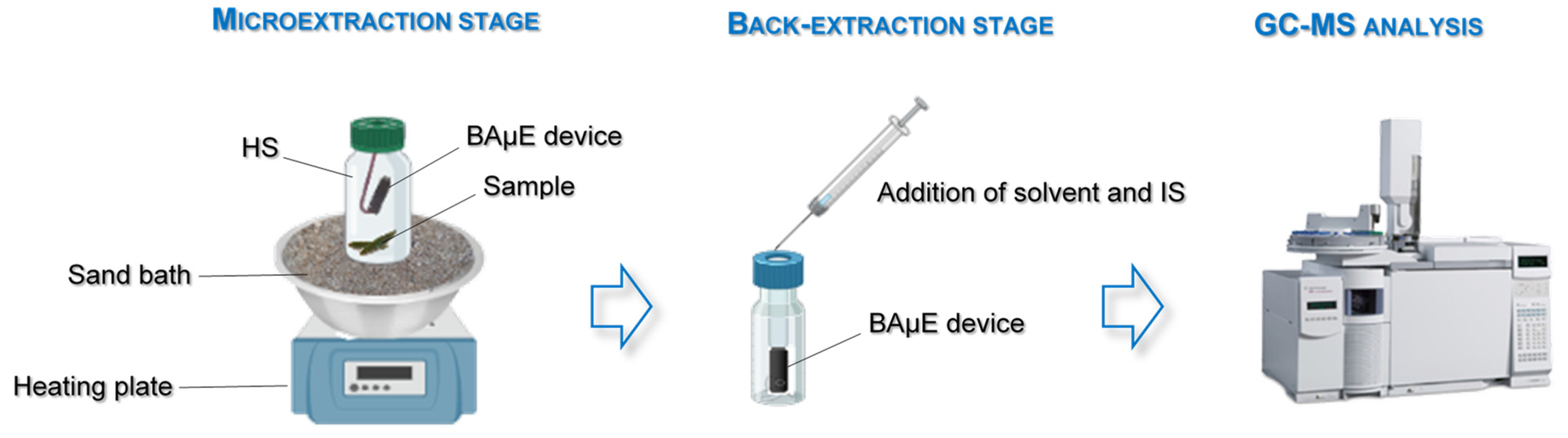
2.2. Experimental Set-Up
2.2.1. Standard Solutions and Real Sample Preparation
2.2.2. Preparation and Conditioning of BAμE Devices
2.2.3. HS-BAμE Optimization and Validation Assays
2.2.4. Application to Shrub Leaves
2.3. Instrumental Set-Up
3. Results and Discussion
3.1. GC-MS Conditions
3.2. Evaluation of the HS-BAμE/GC-MS Methodology
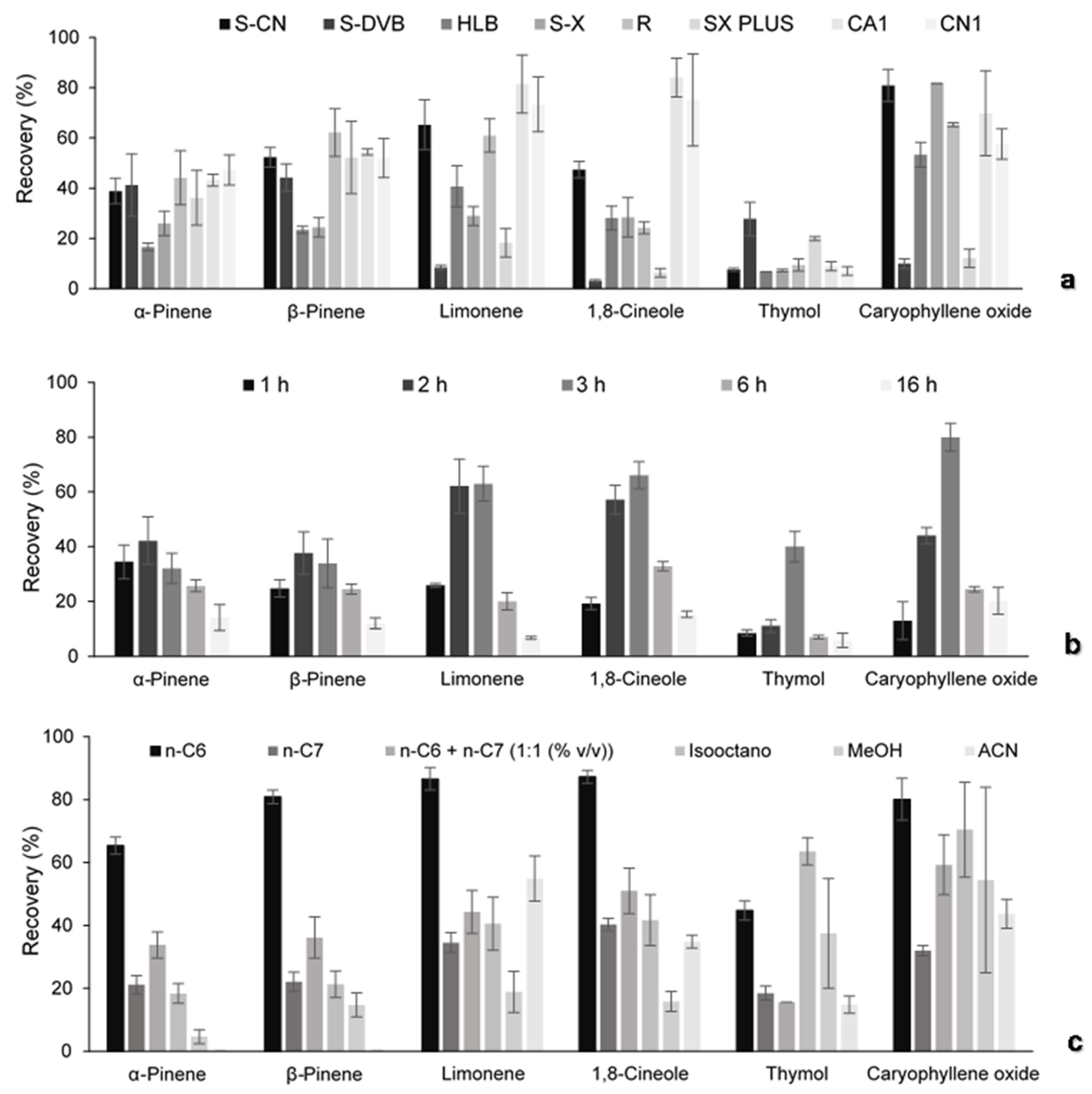
3.2.1. Optimization Assays
3.2.2. Validation Parameters
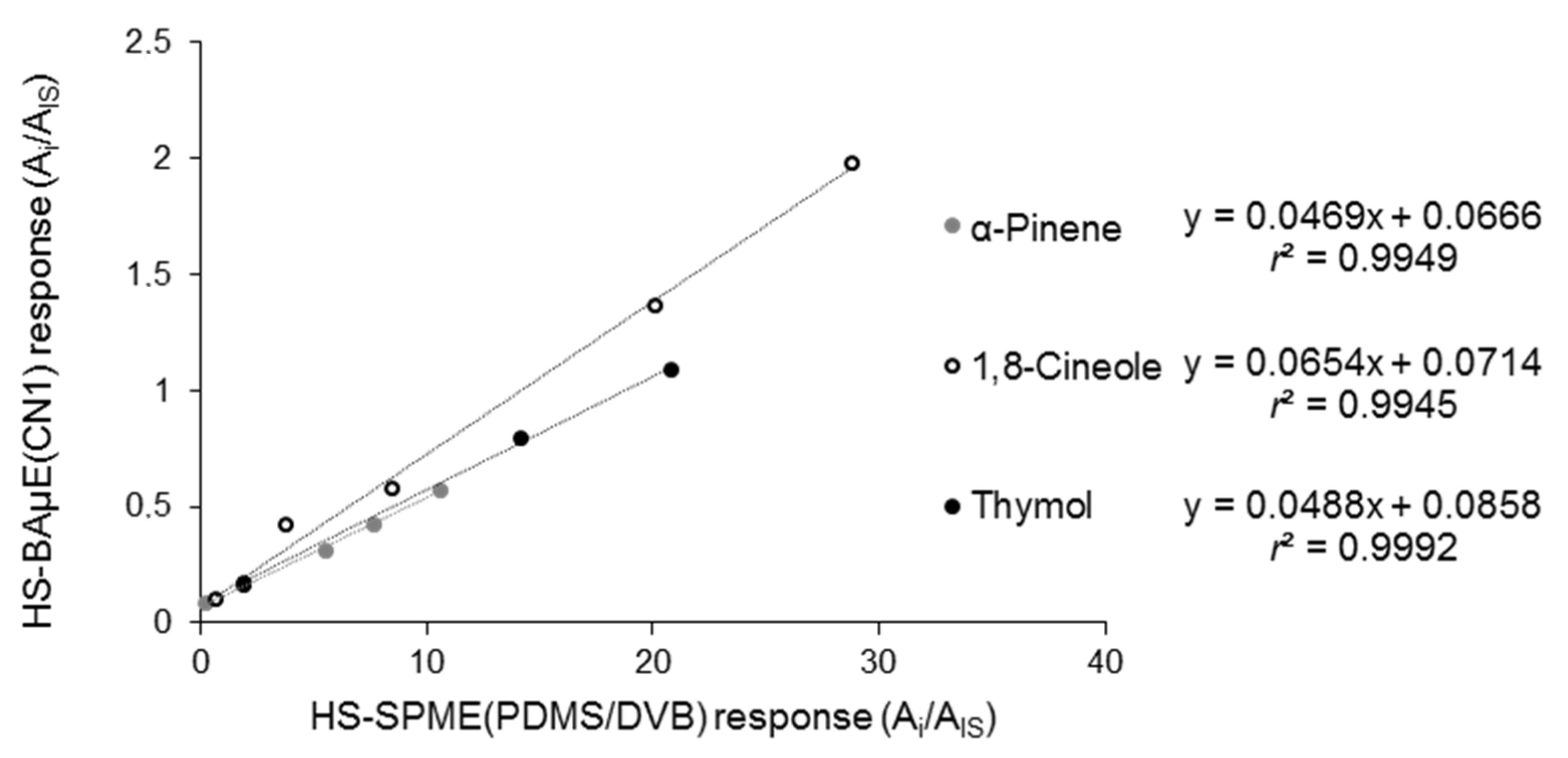
3.3. Comparison with Reference Methodologies
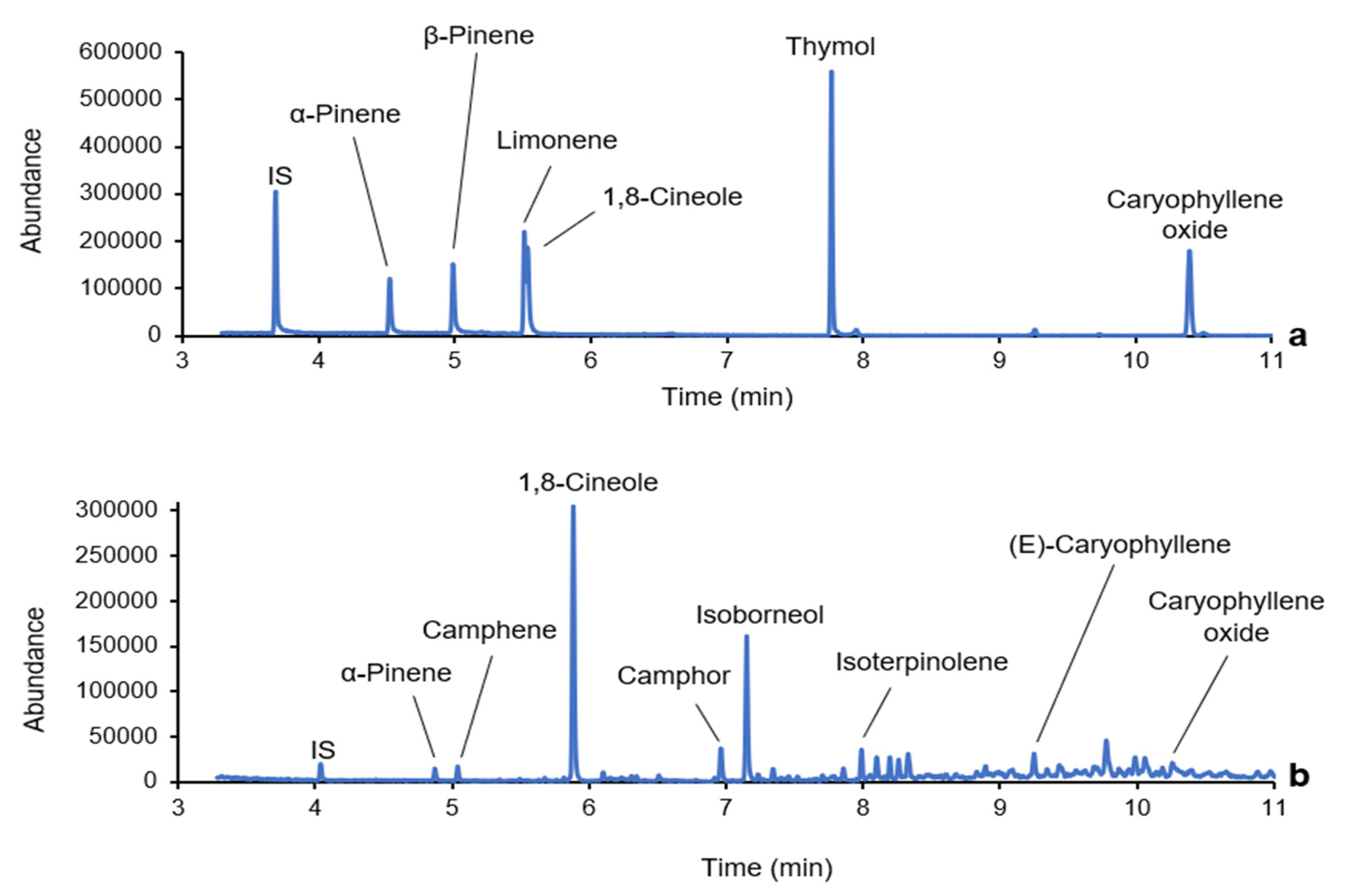
3.4. Application to Shrub Leaves
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koppmann, R. Volatile Organic Compounds in the Atmosphere, 1st ed.; John Wiley & Sons: Oxford, UK, 2007. [Google Scholar] [CrossRef]
- Ciccioli, P.; Centritto, M.; Loreto, F. Biogenic volatile organic compound emissions from vegetation fires. Plant Cell Environ. 2014, 37, 1810–1825. [Google Scholar] [CrossRef] [PubMed]
- Plana, E.; Front, M.; Serra, M.; Borràs, M.; Vilalta, O. Fire and Forest Fires in the Mediterranean; A Relationship Story between Forests and Society; Forest Sciences Centre of Catalonia: Lleida, Spain, 2016. [Google Scholar]
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile profile of Spanish Cistus plants as sources of antimicrobials for industrial applications. Ind. Crops Prod. 2015, 74, 425–433. [Google Scholar] [CrossRef]
- Łyczko, J.; Jałoszyński, K.; Surma, M.; Masztalerz, K.; Szumny, A. HS-SPME analysis of true lavender (lavandula angustifolia mill.) leaves treated by various drying methods. Molecules 2019, 24, 764. [Google Scholar] [CrossRef]
- Paolini, J.; Leandri, C.; Desjobert, J.M.; Barboni, T.; Costa, J. Comparison of liquid-liquid extraction with headspace methods for the characterization of volatile fractions of commercial hydrolats from typically Mediterranean species. J. Chromatogr. A 2008, 1193, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, O.C.; Cerqueira, J.S.R.F.; Mestre, A.S.; Neng, N.R.; Nogueira, J.M.F. HS-BAμE: A New Alternative Approach for VOCs Analysis—Application for Monitoring Biogenic Emissions from Tree Species. Molecules 2023, 28, 1179. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Daryasari, A.P.; Ghorbani, A.; Hejri, O.M.; Mazaheri, R. Analysis of the Volatile Compounds in Thymus vulgaris L. Using Improved HS-SPME-GC-MS and Comparison with Conventional Methods. J. Essent. Oil-Bear. Plants 2015, 17, 1233–1240. [Google Scholar] [CrossRef]
- Rodríguez, I.; Carpinteiro, J.; Quintana, J.B.; Carro, A.M.; Lorenzo, R.A.; Cela, R. Solid-phase microextraction with on-fiber derivatization for the analysis of anti-inflammatory drugs in water samples. J. Chromatogr. A 2004, 1024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Panigrahi, S. Solid-Phase Microextraction (SPME) Techniques for Quality Characterization of Food Products: A Review. Food Bioprocess Technol. 2011, 4, 1–26. [Google Scholar] [CrossRef]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Elsevier: North York, ON, Canada, 2012. [Google Scholar] [CrossRef]
- Neng, N.R.; Silva, A.R.M.; Nogueira, J.M.F. Adsorptive micro-extraction techniques-Novel analytical tools for trace levels of polar solutes in aqueous media. J. Chromatogr. A 2010, 1217, 7303–7310. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Nogueira, J.M.F. Determination of steroid sex hormones in real matrices by bar adsorptive microextraction (BAμE). Talanta 2015, 136, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ide, A.H.; Nogueira, J.M.F. New-generation bar adsorptive microextraction (BAμE) devices for a better eco-user-friendly analytical approach–Application for the determination of antidepressant pharmaceuticals in biological fluids. J. Pharm. Biomed. Anal. 2018, 153, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Abujaber, F.; Ahmad, S.M.; Neng, N.R.; Martín-Doimeadios, R.C.R.; Bernardo, F.J.G.; Nogueira, J.M.F. Bar adsorptive microextraction coated with multi-walled carbon nanotube phases—Application for trace analysis of pharmaceuticals in environmental waters. J. Chromatogr. A 2019, 1600, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Qu, D.; Hardy, G.E.S.J.; Trengove, R.; Agarwal, M.; Ren, Y. Optimization of headspace solid-phase microextraction conditions for the identification of Phytophthora cinnamomi rands. Plant Dis. 2014, 98, 1088–1098. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, H.; Zhu, J.; Wang, L.; Li, Z. Development of a SPME-GC-MS method for the determination of volatile compounds in Shanxi aged vinegar and its analytical characterization by aroma wheel. J. Food Sci. Technol. 2016, 53, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Owen, S.M.; Harley, P.; Guenther, A.; Hewitt, C.N. Light dependency of VOC emissions from selected Mediterranean plant species. Atmos. Environ. 2002, 36, 3147–3159. [Google Scholar] [CrossRef]
- Cheong, M.W.; Lee, J.Y.K.; Liu, S.Q.; Zhou, W.; Nie, Y.; Kleine-Benne, E.; Curran, P.; Yu, B. Simultaneous quantitation of volatile compounds in citrus beverage through stir bar sorptive extraction coupled with thermal desorption-programmed temperature vaporization. Talanta 2013, 107, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Benet, I.; Ibañez, C.; Guàrdia, M.D.; Solà, J.; Arnau, J.; Roura, E. Optimisation of stir-bar sorptive extraction (SBSE), targeting medium and long-chain free fatty acids in cooked ham exudates. Food Chem. 2015, 185, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.P.; Emídio, E.S.; De Marchi, M.R.R. Method validation using weighted linear regression models for quantification of UV filters in water samples. Talanta 2015, 131, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Maroto, M.C.; Perez-Coello, M.S.; Cabezucto, M.D. Headspace Solid-Phase Microextraction Analysis of Volatile Components of Spices. Chromatographia 2002, 55, 729–735. [Google Scholar] [CrossRef]
| BVOCs | LOD (μg/L) | LOQ (μg/L) | Linear Range (μg/L) | a | b | r2 | Intra-Day Precision (n = 5; RSD,%) | Inter-Day Precision (n = 3; RSD,%) |
|---|---|---|---|---|---|---|---|---|
| α-Pinene | 5.0 | 15.0 | 20.0–100.0 | 0.0090 | 0.0614 | 0.9951 | 6.3–22.8 | 10.4–28.7 |
| β-Pinene | 0.0123 | −0.0563 | 0.9912 | 8.2–22.1 | 12.9–26.9 | |||
| Limonene | 20.0–120.0 | 0.0199 | 0.0846 | 0.9879 | 13.8–18.5 | 13.4–26.7 | ||
| 1,8-Cineole | 0.0250 | 0.0557 | 0.9895 | 4.0–11.3 | 13.4–15.8 | |||
| Thymol | 15.0 | 49.5 | 50.0–100.0 | 0.0102 | 0.0195 | 0.9872 | 17.5–28.4 | 18.0–26.4 |
| Caryophyllene oxide | 0.0347 | 0.1738 | 0.9947 | 10.2–16.5 | 18.8–30.1 |
| Shrub Species | ||||||
|---|---|---|---|---|---|---|
| Lavandula stoechas L. | Cistus ladanifer L. | Cistus monspeliensis L. | Thymus villosus L. | Thymus camphoratus | Erica scoparia L. | |
| BVOCs | Concentration (µg/g) | |||||
| α-Pinene | 29.6 ± 0.2 | 325.1 ± 0.2 | 22.6 ± 0.1 | 69.1 ± 0.1 | 763.9 ± 0.5 | d |
| β-Pinene | - | 148.5 ± 0.5 | 27.5 ± 0.2 | - | 259.3 ± 0.5 | - |
| Limonene | 23.6 ± 0.5 | d | d | 138.8 ± 0.3 | - | d |
| 1,8-Cineole | - | d | - | 104.1 ± 0.3 | 4136.9 ± 6.3 | - |
| Thymol | - | - | - | - | - | - |
| Caryophyllene oxide | - | - | - | - | 411.4 ± 0.3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerqueira, J.S.R.F.; Nogueira, J.M.F. Screening Biogenic Volatile Organic Compounds from Common Portuguese Shrubs Using Headspace–Bar Adsorptive Microextraction (HS-BAµE). Separations 2024, 11, 264. https://doi.org/10.3390/separations11090264
Cerqueira JSRF, Nogueira JMF. Screening Biogenic Volatile Organic Compounds from Common Portuguese Shrubs Using Headspace–Bar Adsorptive Microextraction (HS-BAµE). Separations. 2024; 11(9):264. https://doi.org/10.3390/separations11090264
Chicago/Turabian StyleCerqueira, Jéssica S. R. F., and José M. F. Nogueira. 2024. "Screening Biogenic Volatile Organic Compounds from Common Portuguese Shrubs Using Headspace–Bar Adsorptive Microextraction (HS-BAµE)" Separations 11, no. 9: 264. https://doi.org/10.3390/separations11090264
APA StyleCerqueira, J. S. R. F., & Nogueira, J. M. F. (2024). Screening Biogenic Volatile Organic Compounds from Common Portuguese Shrubs Using Headspace–Bar Adsorptive Microextraction (HS-BAµE). Separations, 11(9), 264. https://doi.org/10.3390/separations11090264







