Abstract
Podostroma cornu-damae, one of the lethal toxic mushrooms, is known to contain macrocyclic trichothecene mycotoxins exhibiting potent cytotoxic effects, attracting attention as an important research subject for scientists interested in natural product chemistry and toxicity research. To investigate the mycotoxins from the toxic mushroom P. cornu-damae and evaluate their cytotoxic activities, the fungus was large-cultured on solid plates and successively extracted to acquire a crude methanol (MeOH) extract. After performing successive separation and purification processes, a total of eight macrocyclic trichothecenes were isolated from the MeOH extract of plate cultures of P. cornu-damae using the liquid chromatography/mass spectrometry (LC/MS)-guided isolation technique. Extensive interpretation of nuclear magnetic resonance (NMR) spectroscopic and high-resolution (HR)-electrospray ionization (ESI)-MS data allowed for the structural identification of all isolated macrocyclic trichothecenes, including satratoxin I (1), satratoxin H (2), roridin E (3), miophytocen D (4), roridin L-2 (5), trichoverritone (6), 12′-episatratoxin H (7), and roridin F (8). We conducted a cytotoxicity evaluation of compounds 1–8 against 4T1 breast cancer cells and fibroblast cell lines (L929 cells) using the Counting Kit-8 (CCK-8) cell viability assay to validate their cytotoxic potential. Our results indicated that compounds 1–6 lack anti-cancer effects on 4T1 cells and have minimal impact on the viability of the fibroblast cell line, L929 cells. In contrast, compounds 7 and 8 exhibited no cytotoxicity in normal cells (L929) and demonstrated specific cytotoxicity in breast cancer cell lines. Notably, the cytotoxic effects of compounds 7 and 8 in 4T1 cells were significantly stronger than those observed with free doxorubicin. These findings suggest that compounds 7 and 8 may possess targeted anti-cancer effects, specifically against breast cancer cells, emphasizing their efficient and selective toxicity towards breast cancer cells.
1. Introduction
For centuries, mushrooms have been utilized in traditional medicine. They house bioactive compounds believed to offer diverse health advantages [1,2,3]. Some mushrooms are thought to enhance the immune system, possess anti-inflammatory properties, and even show potential for anti-cancer effects [1,2,3]. While many mushrooms are safe and even beneficial, certain species contain harmful compounds, some of which can be fatal if consumed [4]. The severity of symptoms depends on the specific toxins present in the mushroom and the amount ingested. Mild symptoms include nausea, vomiting, abdominal pain, and diarrhea, resembling common food poisoning. However, specific toxic mushrooms contain compounds that can harm the liver, kidneys, and other organs, leading to more severe and potentially life-threatening consequences [5]. Accurate identification is crucial due to the potential dangers, as some toxic mushrooms closely resemble edible varieties. In the identification process, exploring the chemical metabolites of toxic mushrooms is vital to differentiate their harmful or beneficial properties.
By isolating and identifying the specific mycotoxins found in poisonous mushrooms, researchers can enhance their understanding of how these compounds interact with the human body and cause harmful effects. The process involves separating and purifying the toxic compounds, followed by detailed chemical analysis, such as nuclear magnetic resonance (NMR) spectroscopy, column chromatography, and mass spectrometry, to determine their structures and properties [6]. Developing reliable analytical methods for mycotoxin detection is crucial for promptly and accurately diagnosing cases of mushroom poisoning. It also contributes to establishing safety guidelines for the consumption of wild mushrooms [6,7,8]. Overall, the isolation and characterization of fungal compounds from toxic mushrooms play a vital role in advancing our understanding of mushroom toxicity and improving public health measures related to mushroom foraging and consumption.
Previous studies exploring the pharmacological properties of toxic mushrooms have unveiled potential anti-cancer, antimicrobial, and immunomodulatory activities [9,10,11]. The same compounds that render certain mushrooms toxic to humans can showcase diverse and often beneficial effects when studied for other applications. However, the reported compounds derived from poisonous mushrooms remain limited. This scarcity may be attributed to the intricate chemical profiles of mushrooms and the potential risks associated with handling and studying toxic compounds [12]. Additionally, safety concerns have understandably overshadowed a more extensive exploration of the beneficial properties of poisonous mushrooms.
The presence of trichothecenes in Podostroma cornu-damae, one of the lethal toxic mushrooms from the Hypocreaceae family, makes it particularly hazardous, as these mycotoxins can cause a range of adverse health effects, including nausea, vomiting, and, in severe cases, organ damage or even death [13]. The mushroom’s toxic properties highlight the importance of caution and awareness when dealing with wild mushrooms, emphasizing the need for accurate identification to avoid accidental ingestion of poisonous species. Ganoderma lucidum, known for its medicinal properties in traditional Chinese medicine, has distinct characteristics that differ from potentially toxic mushrooms. However, during their early growth phases, G. lucidum and P. cornu-damae share visual similarities with other less benign species, leading to misidentification and accidental poisonings [13]. Early symptoms of P. cornu-damae poisoning are reported to include dehydration, vomiting, and diarrhea. Subsequently, within approximately three days of exposure, affected individuals may experience anuria, thrombocytopenia, hypotension, leukopenia, polypnea, and altered consciousness.
In our ongoing effort to find bioactive compounds from intriguing natural sources [14,15,16,17,18], we have directed our focus toward investigating the presence of mycotoxins in the methanol (MeOH) extract of plate cultures of the fungus P. cornu-damae. In a recent study on the mycotoxins of P. cornu-damae, we isolated several macrocyclic trichothecenes, along with novel compounds (roridin F, satratoxin I, and miophytocen D) [19]. In their evaluation for cytotoxic activity, most of the macrocyclic trichothecenes displayed significant effects against human cancer cell lines, including ovary cancer, skin cancer, lung cancer, colon cancer, and breast cancer cells, with IC50 values ranging from 0.02 to 80 nM. Some of these compounds exhibited even greater potency than doxorubicin, the positive control. In the current subsequent investigation of P. cornu-damae aimed at the efficient isolation of macrocyclic trichothecenes and validation of their cytotoxic activity, we explored macrocyclic trichothecenes from the scale-up cultivation of the fungus P. cornu-damae in the process of extensive chromatographic purifications using liquid chromatography/mass spectrometry (LC/MS)-guided isolation techniques, coupled with 3D plot visualization of LC/MS data and reference to an in-house library of ultraviolet (UV) spectra. As a result, this successfully led to the efficient isolation of eight macrocyclic trichothecenes. The chemical structures were determined by comparing their NMR data with previously reported NMR spectra and LC/MS analysis data. The isolated macrocyclic trichothecenes were validated for their cytotoxic effects on cancer and normal cell lines using the D-PlusTM CCK cell viability assay kit, CCK-8 (Dongin LS, Seoul, Republic of Korea) method. In this study, we describe the isolation and structural characterization of the macrocyclic trichothecenes from P. cornu-damae, along with their validation of cytotoxicity.
2. Results and Discussion
2.1. Isolation and Structural Characterization of the Macrocyclic Trichothecenes 1–8
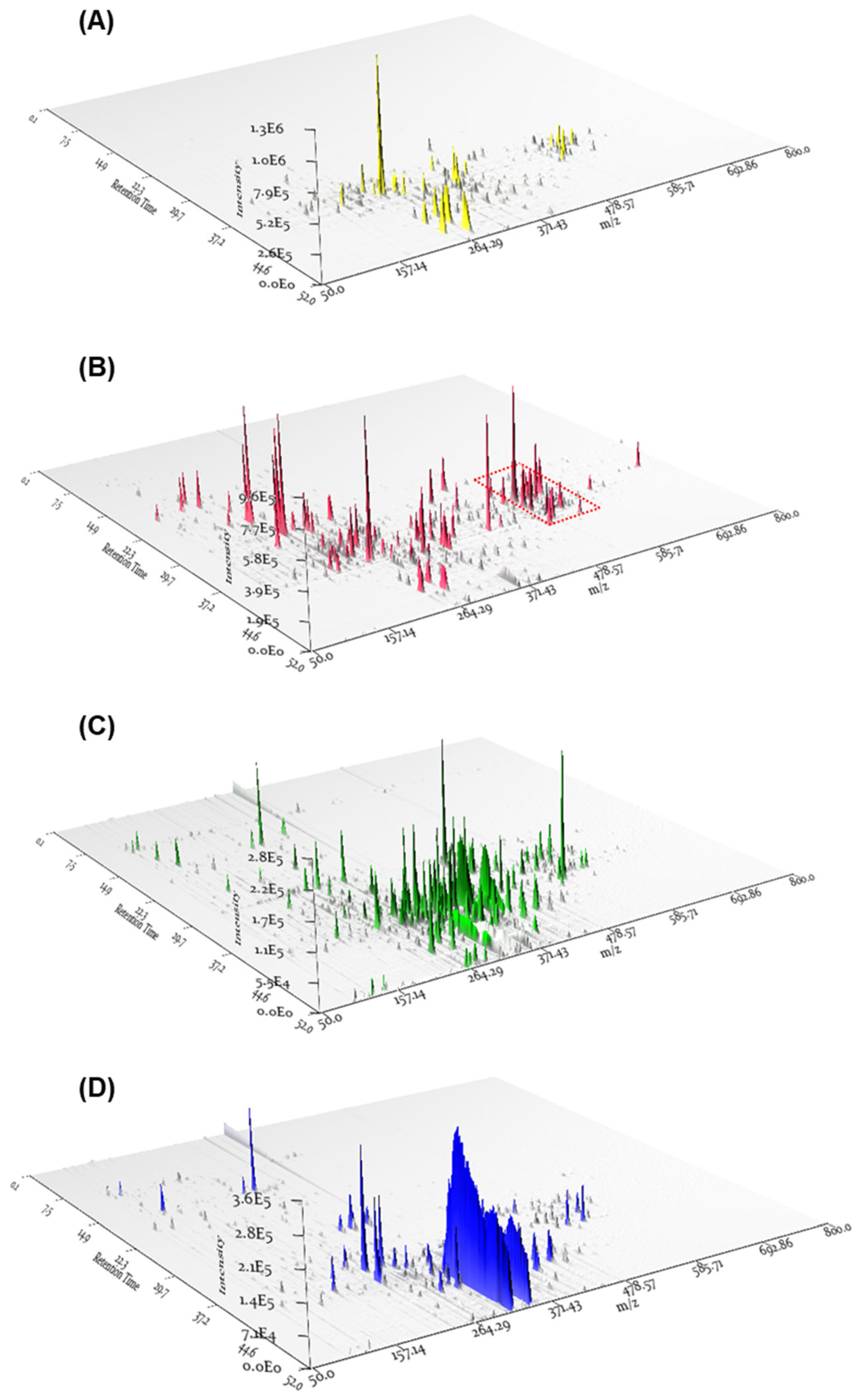
The MeOH extract obtained from the cultivation of P. cornu-damae on potato dextrose agar (PDA) plates underwent solvent partitioning using four organic solvents (hexane, dichloromethane (CH2Cl2), ethyl acetate (EtOAc), and n-butanol), which afforded four main fractions, including hexane, CH2Cl2, EtOAc, and n-butanol soluble fractions. Based on previous research associating P. cornu-damae with macrocyclic trichothecenes, each fraction was analyzed by LC/MS, and the 3D plot visualization of the LC/MS data was processed using the application MZmine 3.2.8 (Figure 1). The 3D plot visualization showed that the CH2Cl2-soluble fraction has many peaks with the polarity of macrocyclic trichothecenes and promising molecular formula at m/z 500–550 (Figure 1). This allowed the CH2Cl2-soluble fraction to be considered a promising fraction for investigating macrocyclic trichothecenes.
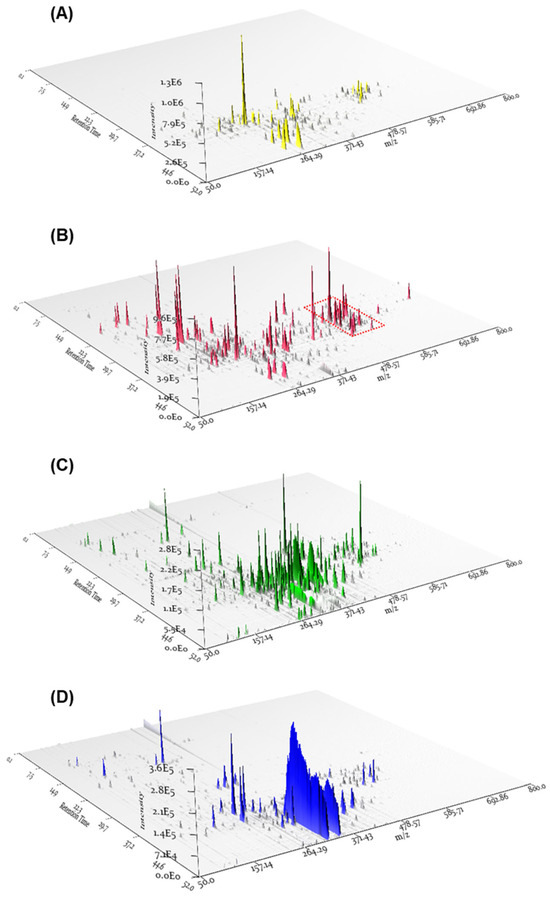
Figure 1.
The 3D plot visualizations of the LC/MS data from the four main fractions, including hexane (A), CH2Cl2 (B), EtOAc (C), and n-butanol (D) soluble fractions. The red-dotted boxplot indicates the expected locations of peaks corresponding to macrocyclic trichothecenes.
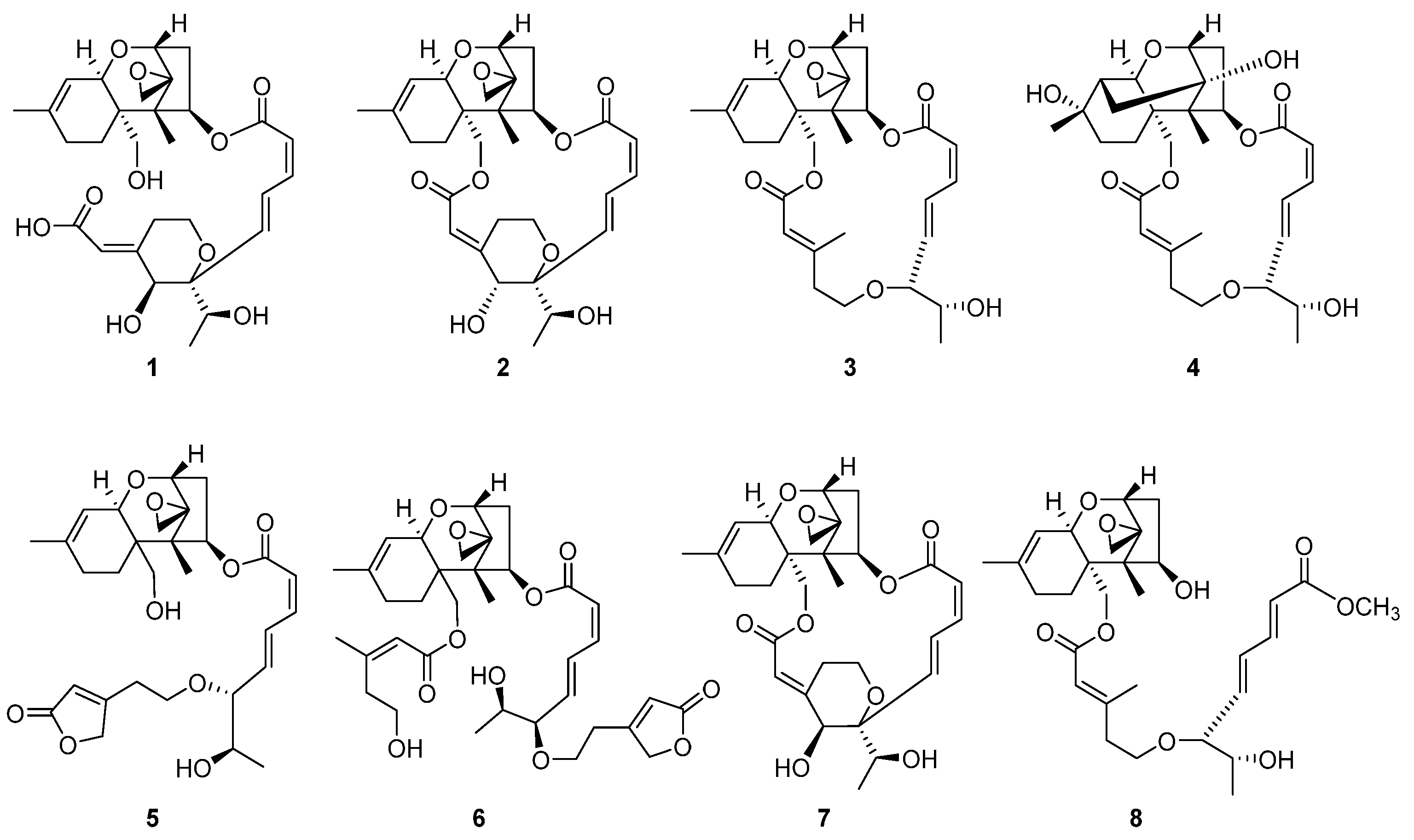
To specifically isolate the macrocyclic trichothecenes from the CH2Cl2-soluble fraction, we employed an LC/MS-guided isolation approach. The CH2Cl2-soluble fraction underwent fractionation by preparative reversed-phase high-performance liquid chromatography (HPLC), yielding six fractions, each of which was monitored by LC/MS using the Agilent G6545B quadrupole time-of-flight (Q-TOF) mass spectrometer in conjunction with an in-house library of UV spectra for the early detection of macrocyclic trichothecenes. The LC/MS-guided isolation and semi-preparative reversed-phase HPLC purification led to the efficient isolation of a total of eight macrocyclic trichothecene-type compounds (1–8). In the MeOH extract of P. cornu-damae, the total percentage of the eight isolated macrocyclic trichothecenes was 0.28%. Table S1 presents the percentage ratio of each isolated compound within the total macrocyclic trichothecenes. The NMR data of these eight compounds were compared with previously reported NMR spectra and LC/MS analysis data confirmed their structures as satratoxin I (1) [19], satratoxin H (2) [20], roridin E (3) [20], miophytocen D (4) [19], roridin L-2 (5) [21], trichoverritone (6) [22], 12’-episatratoxin H (7) [23], and roridin F (8) [19] (Figure 2).
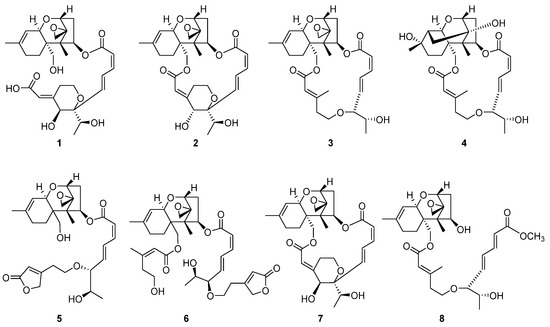
Figure 2.
The chemical structures of the isolated macrocyclic trichothecenes 1–8.
2.2. Evaluation of Cytotoxicity of Compounds 1–8 on Cancer and Normal Cell Lines
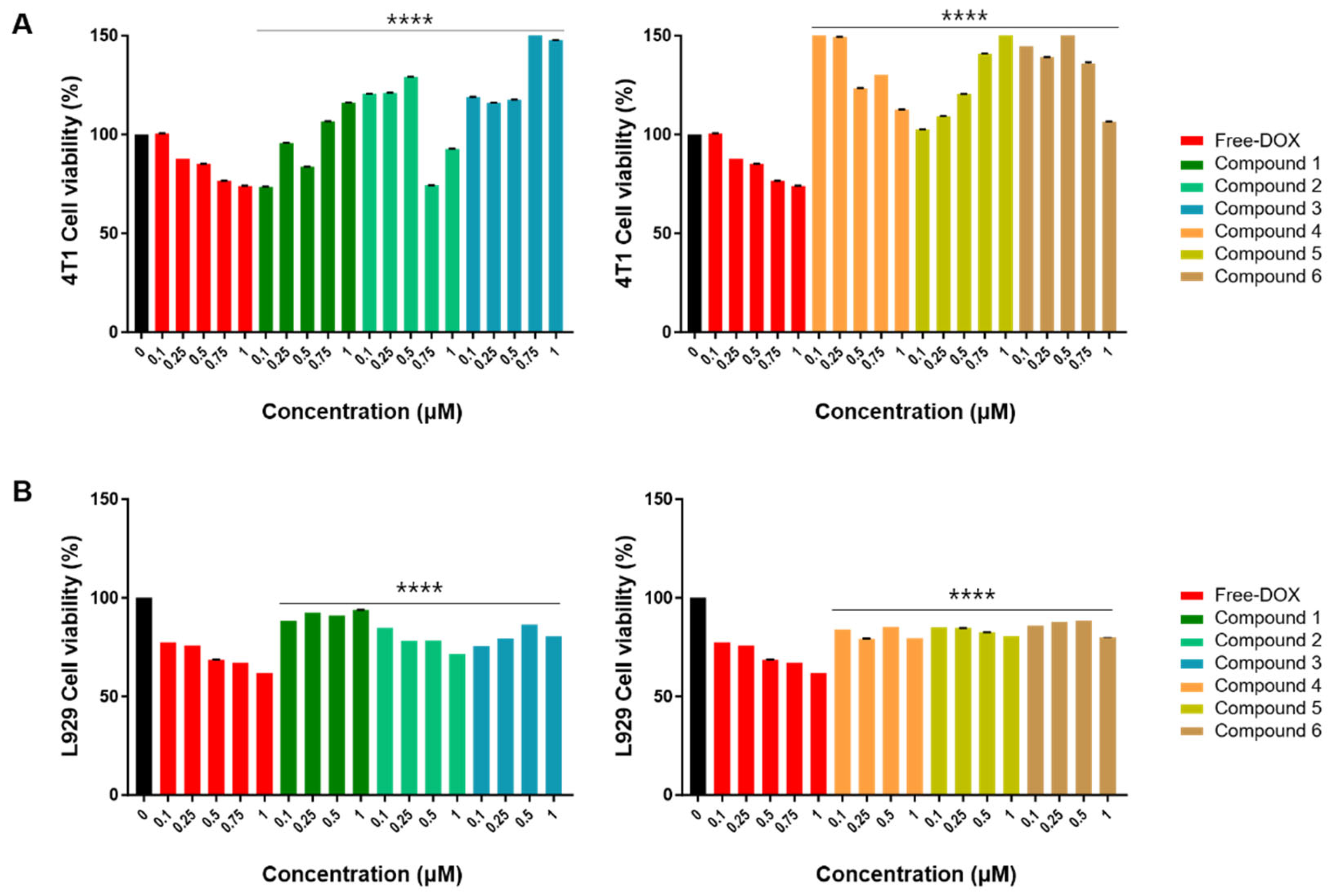
To assess the cytotoxicity of compounds 1–8, we incubated these compounds and free doxorubicin (DOX) at concentrations of 0.1, 0.25, 0.5, 0.75, and 1 μM with 4T1 breast cancer cells. After 24 h incubation, the cytotoxicity of compounds 1–8 was tested using the CCK-8 cell viability assay kit, following the manufacturer’s protocols. Vehicle controls received treatment with 0.5% dimethyl sulfoxide in cell culture media, and 0.5% DMSO is known to have a non-cytotoxic effect on breast cancer cells [24]. The results demonstrated that 4T1 cell viability gradually decreased as the concentration of free DOX increased; however, 4T1 cell viability showed an increasing pattern when treated with compounds 1–6 (Figure 3). This suggests that compounds 1–6 do not have an anti-cancer effect on 4T1 cells but rather increase their activity. As demonstrated in Figure 3B, with the increasing concentration of compounds 1–6, there was a negligible influence on the fibroblast cell line, L929 cell viability. Even when the concentration was increased to 1 µM, the cell viability remained over 80%, indicating good biocompatibility.
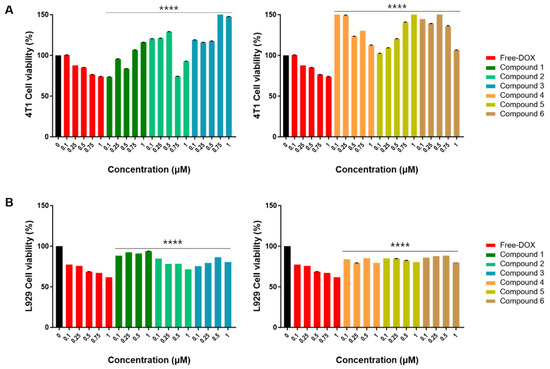
Figure 3.
The CCK-8 assay of compounds 1–6 on 4T1 and L929 cells at various concentrations. (A) The effects of compounds (1–6) on mouse breast cancer (4T1) cell viability. 4T1 cells were treated with free DOX and compounds 1–6 (0.1, 0.25, 0.5, 0.75, and 1 μM) for 24 h. Vehicle controls received treatment with 0.5% dimethyl sulfoxide in cell culture media. (B) The effects of compounds (1–6) on mouse fibroblast (L929) cell viability. L929 cells were treated with free DOX (0.1, 0.25, 0.5, 0.75, and 1 μM) and compounds 1–6 (0.1, 0.25, 0.5, and 1 μM) for 24 h. Vehicle controls were treated with 0.5% dimethyl sulfoxide in cell culture media. Cell viability was assessed using the CCK-8 cell viability assay. The data are presented as the mean ± standard error of the mean (SEM), with n = 3. Statistical significance is denoted as **** p < 0.0001 compared with the control (0 µM).
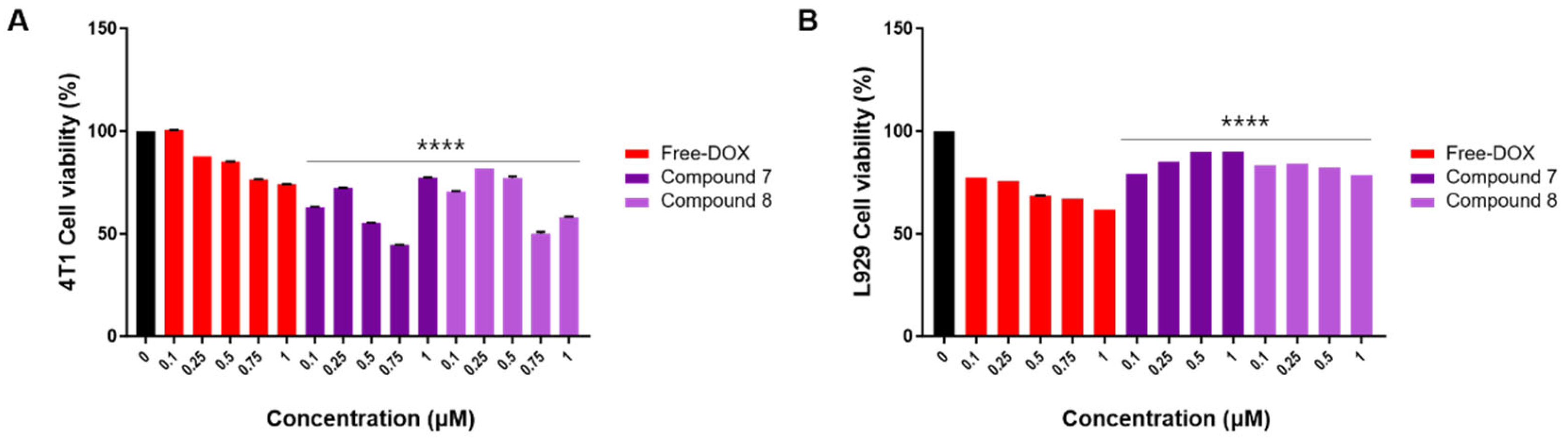
On the other hand, the viability of 4T1 cells significantly decreased when treated with compounds 7 and 8 compared to the normal fibroblast cell line, L929 (Figure 4). Moreover, the cytotoxic effects of compounds 7 and 8 in 4T1 cells were notably stronger than those of free DOX (Figure 4). As these compounds demonstrated no cytotoxicity in normal cells (L929) and exhibited specific cytotoxicity in breast cancer cell lines, it suggests that compounds 7 and 8 may possess anti-cancer effects specifically targeting breast cancer cells. These results highlight the efficient targeted toxicity of compounds 7 and 8 toward breast cancer cells. In our recent study [25], we evaluated the cytotoxicity of satratoxin H, 12′-episatratoxin H, and roridin F against four human-derived cancer cell lines: SK-OV-3 (ovary malignant ascites), A549 (non-small cell lung carcinoma), SK-MEL-2 (skin melanoma), and HCT (colon adenocarcinoma). The assessment was conducted using the sulforhodamine B (SRB) bioassay [25], with doxorubicin serving as the positive control. Results showed that 12′-episatratoxin H demonstrated potent cytotoxic effects against all four tested cell lines, with IC50 values ranging from 0.7 to 2.8 nM, surpassing those of doxorubicin. Satratoxin H exhibited moderate cytotoxic potency against all four cell lines, with IC50 values ranging from 1.93 to 4.22 μM. In contrast, roridin F did not show significant cytotoxicity (IC50, >10.0) [25]. The observed potent cytotoxicity of 12′-episatratoxin H compared to satratoxin H aligns well with previous data despite differences in the tested cancer cell lines. For roridin F, although it did not exhibit cytotoxicity against SK-OV-3, A549, SK-MEL-2, and HCT, it displayed selective cytotoxic effects against the breast cancer cell line 4T1 with no impact on normal cells (L929) in this study. In another recent study focusing on breast cancer cell lines (Bt549, HCC70, MDA-MB-231, and MDA-MB-468) [19], 12′-episatratoxin H again demonstrated potent cytotoxic effects, with IC50 values ranging from 0.3 to 3.0 nM, surpassing those of doxorubicin. Satratoxin H exhibited moderate cytotoxic activity, while roridin F did not show cytotoxicity [19]. The consistency in results between satratoxin H and 12′-episatratoxin H across different cell lines, albeit with differences in breast cancer cells, suggests a potential role for stereochemistry, particularly the 12′-OH configuration, in their relative cytotoxicity. This emphasizes the potential for optimizing trichothecene mycotoxins to achieve higher efficacy and lower toxicity, making them promising candidates for chemotherapeutic agents in various cancer treatments.
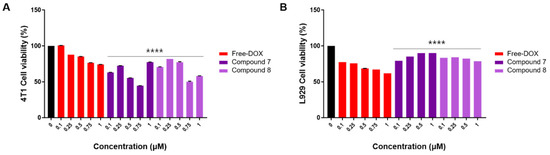
Figure 4.
The CCK-8 assay of compounds 7 and 8 on 4T1 and L929 cells at different concentrations. (A) Effects of compounds (7 and 8) on mouse breast cancer (4T1) cell viability. 4T1 cells were treated with free DOX and compounds 7 and 8 (0.1, 0.25, 0.5, 0.75, and 1 μM) for 24 h. Vehicle controls received treatment with 0.5% dimethyl sulfoxide in cell culture media. (B) Effects of compounds (7 and 8) on mouse fibroblast (L929) cell viability. L929 cells were treated with free DOX (0.1, 0.25, 0.5, 0.75, and 1 μM) and compounds 7 and 8 (0.1, 0.25, 0.5, and 1 μM) for 24 h. Vehicle controls were treated with 0.5% dimethyl sulfoxide in cell culture media. Cell viability was assessed using the CCK-8 cell viability assay. The data are presented as the mean ± standard error of the mean (SEM), with n = 3. Statistical significance is denoted as **** p < 0.0001 compared with the control (0 µM).
The findings in this study delve into the potential advantages of natural products in cancer treatment when compared to traditional drugs like cisplatin, doxorubicin, and fluorouracil [26]. The isolated natural products are notable for their low toxicity in non-cancer cells while exhibiting specific toxicity in breast cancer cells. However, the discussion also acknowledges certain limitations and areas for future exploration. Although natural products show promise in reducing toxicity, the precise pathways through which they exert their effects remain incompletely understood. The suggestion is to integrate the study of natural products with classical anti-cancer prescriptions to obtain a more comprehensive understanding of their anti-cancer effects. Furthermore, the passage underscores the potential synergies between natural products and traditional anti-tumor therapies, indicating that combining them with existing treatments could amplify overall effectiveness. The development and progress of science and technology are deemed crucial for discovering new natural products, advancing anti-tumor drug research, and instilling hope in cancer patients.
There are significant challenges in cancer drug development, encompassing the need to effectively target cancer cells, achieve water solubility, and minimize cytotoxicity to normal cells [27]. The importance of testing potential anti-tumor agents extends beyond tumor cells to encompass normal cells for a comprehensive understanding of their effects. Current agents for treating solid tumors are potent in killing cancer cells, including resistant ones, through various mechanisms targeting mitosis, DNA repair, or protein synthesis. However, these treatments also affect normal cells, tissues, and organ systems, leading to debilitating side effects for patients. Without the ability to selectively target cancer cells, the entire body becomes susceptible [28,29].
In a prior study, Gollahon et al. introduced the potential of a new “Green” anti-cancer compound derived from Arctium lappa known as Greater Burdock. The compound, identified as NeoImmune®-07 or NI-07, was found to selectively destroy various breast cancer cells while exhibiting minimal cytotoxicity to normal cells. According to the data, NI-07 performed comparably or even better than anti-tumor agents like Taxol™ in killing cancer cells [30]. Our results demonstrated significant decreases in cell viability in cancer cells treated with compounds 7 and 8 after 24 h, in contrast to the more rapid effect of free DOX. Additionally, compounds 7 and 8 showed reduced cytotoxicity in normal cells. The absence of cytotoxicity, coupled with their efficacy in killing cancer cells, suggests that compounds 7 and 8 could potentially serve as robust anti-cancer compounds or neo-adjuvants to current chemotherapy-based treatments. Overall, we propose that compounds 7 and 8 derived from P. cornu-damae could be promising options for anti-cancer therapy.
Early diagnosis and prompt intervention are pivotal for effectively managing cancer. While treatments like radiation therapy and surgical resection can be highly effective, particularly in the early stages of the disease, they may not always suffice for advanced or metastatic cancers. In such cases, chemotherapy becomes indispensable. Chemotherapy targets rapidly dividing cells, including cancer cells, and is utilized to shrink tumors, control cancer spread, or alleviate symptoms in metastatic disease. Despite its side effects resulting from its impact on normal rapidly dividing cells, chemotherapy remains a cornerstone of cancer treatment, especially for malignancies that have metastasized. Combination chemotherapy is a widely used strategy in cancer treatment. By employing multiple drugs with diverse mechanisms of action, combination chemotherapy can target cancer cells more effectively and mitigate the risk of resistance development. These combinations often include drugs derived from both natural and synthetic sources. Natural compounds have been a valuable source of anti-cancer agents, often serving as the foundation for synthetic analogs optimized for efficacy and safety. Combination chemotherapy with natural compounds can produce synergistic effects, enhancing overall treatment outcomes. In essence, combination chemotherapy represents a robust approach to cancer treatment, leveraging the strengths of both natural and synthetic compounds to improve patient outcomes.
3. Materials and Methods
3.1. General Experimental Procedures
The equipment and devices used in the analyses and experimental procedures are listed in Table S2.
3.2. Fungus Material
Fresh fruiting bodies of P. cornu-damae were collected from a forest in Pocheon, Korea, in 2020, and the mycelium was isolated from its fruiting body tissue. The amplified internal transcribed spacer (ITS) region sequence was then compared to the available sequences in the NCBI Gene Bank. Upon analysis of the highest score and homology, ITS sequence was determined as a match to P. cornu-damae.
3.3. Extraction and Fractionation
P. cornu-damae was cultivated on 200 potato dextrose agar (PDA) plates (90.0 × 15.0 mm) at 25 °C for 30 days. Following the fungal growth covering the PDA plates, they were chopped into small pieces and combined, and an overnight extraction was conducted with 90% MeOH three times. The MeOH phase was filtered and then evaporated under vacuum conditions to obtain a MeOH extract. Dissolving the MeOH extract (12.0 g) in distilled water (700 mL) and partitioning with four different organic solvents (each 700 mL), namely hexane, CH2Cl2, EtOAc, and n-BuOH three times resulted in four main fractions, including hexane, CH2Cl2, EtOAc, and n-butanol soluble fractions for 0.2, 0.6, 0.4, and 0.8 g, respectively. Based on the LC/MS analysis of each fraction obtained, the CH2Cl2-soluble fraction was selected as a promising fraction for investigating macrocyclic trichothecenes.
3.4. LC/MS Analysis for Fractions and 3D Plot Visualization
The LC/MS analysis was carried out using an Agilent 1200 series HPLC system equipped with a diode array detector and a 6130 Series ESI mass spectrometer. For the analysis, an analytical Kinetex C18 100 Å column (100 × 2.1 mm i.d., 5 μm particle size) from Phenomenex (Torrance, CA, USA) was employed, with a flow rate maintained at 0.3 mL/min. The fraction samples were analyzed using a gradient elution program from 10% MeOH-H2O to 100% MeOH for 52 min [10% MeOH-H2O → 100% MeOH (0–30 min), 100% MeOH (30–41 min), 100% MeOH → 10% MeOH-H2O (41–42 min), 10% MeOH-H2O (42–52 min), and a flow rate of 0.3 mL/min]. For the LC/MS data analysis, the application MZmine 3.2.8 was used to process the 3D plot visualization of the LC/MS data. The parameters were set as follows: MS mass range 100–1000 Da, retention time resolution 500, m/z resolution 500, MS level 1, Polarity +, Spectrum type Centroided.
3.5. Isolation of Compounds 1–8
The CH2Cl2-soluble fraction (600 mg) underwent fractionation by preparative reversed-phase HPLC (Column: Phenomenex Luna C18, 5 μm, 250 × 21.2 mm i.d.) using solvent of CH3CN-H2O (2:8–1:0, v/v, gradient system) with a flow rate maintained at 5 mL/min, yielding six fractions (A−F). Based on the LC/MS analysis for each fraction using the Agilent G6545B Q-TOF mass spectrometer in conjunction with an in-house library of UV spectra, compounds 1 (5.0 mg, tR = 24.0 min) and 4 (3.4 mg, tR = 35.0 min) were purified from fraction C through semi-preparative reversed-phase HPLC (Column: Phenomenex Luna C18, 5 μm, 250 × 21.2 mm i.d.) with a 52% MeOH−H2O isocratic system with a flow rate maintained at 2 mL/min. Utilizing semi-preparative reversed-phase HPLC (Column: Phenomenex Luna C18, 5 μm, 250 × 21.2 mm i.d.) using a 60% MeOH−H2O isocratic system with a flow rate maintained at 2 mL/min afforded the isolation of compounds 2 (2.7 mg, tR = 34.5 min), 5 (3.5 mg, tR = 18.0 min), 6 (3.7 mg, tR = 57.0 min), and 8 (4.3 mg, tR = 32.0 min) from fraction D (30 mg). Fraction E (25 mg) was separated by semi-preparative reversed-phase HPLC with a 62% MeOH-H2O isocratic system (flow rate: 2 mL/min) to yield compound 7 (5.5 mg, tR = 42.0 min). Finally, compound 3 (6.0 mg, tR = 36.0 min) was obtained from fraction F (26 mg) by using semi-preparative reversed-phase HPLC with a solvent system of 70% MeOH-H2O with a flow rate maintained at 2 mL/min.
3.6. Cell Culture
Mouse breast cancer 4T1-Luc2 cells, which were stably tagged with the luciferase-2 gene, were procured from ATCC (Manassas, VA, USA). Additionally, the mouse fibroblast cell line L929 was obtained from ATCC. Both cell lines were cultured in RPMI-1640 media (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin–streptomycin (both from Gibco), and they were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
3.7. Cell Viability Assay
4T1 cells or L929 cells were seeded in a 96-well plate at a density of 10,000 cells per well. After 24 h of incubation at 37 °C, 4T1 cells were treated with free-doxorubicin (DOX) or compounds 1–8 (100 μL per well) at different DOX and compounds 1–8 concentrations (0.1, 0.25, 0.5, 0.75, and 1 μM) and then further incubated for 24 h at 37 °C. L929 cells were incubated with free DOX or compounds 1–8 at concentrations of 0.1, 0.25, 0.5, and 1 μM for 24 h. Following the incubation, 10 μL of CCK-8 stain was added to each well. Two hours later, the quantification of CCK-8 was conducted by measuring the optical densities at a wavelength of 450 nm using a microplate reader (Model 550; Bio-Rad, Hercules, CA, USA) [31]. The percentage viability of the cells was calculated by comparing cells treated with the compounds and free DOX with the untreated group. CCK-8 assay was performed in triplicate from each reagent.
3.8. Statistical Analysis
After the normality test, data that exhibited normal distribution were analyzed using one-way ANOVA followed by multiple comparisons between groups using Tukey’s post-hoc test. For flow cytometry data analysis, a Mann–Whitney U test was employed. All statistical analyses were conducted using GraphPad Prism 10.0 software, and a p-value of <0.05 was considered significant.
4. Conclusions
In conclusion, our study focused on exploring mycotoxins from the toxic mushroom P. cornu-damae, specifically macrocyclic trichothecenes, known for their potent cytotoxic effects. Through a comprehensive extraction, separation, and purification process by LC/MS-guided isolation, we successfully identified eight macrocyclic trichothecenes from the MeOH extract of P. cornu-damae, including satratoxin I (1), satratoxin H (2), roridin E (3), miophytocen D (4), roridin L-2 (5), trichoverritone (6), 12’-episatratoxin H (7), and roridin F (8). Subsequent cytotoxicity evaluations on 4T1 breast cancer cells and fibroblast cell lines (L929 cells) revealed that compounds 1–6 did not show a killing effect on 4T1 breast cancer cell lines but rather induced cell proliferation. It was also confirmed that compounds 1–6 were less cytotoxic against L929 normal cells than doxorubicin but did not induce cell proliferation of L929. In contrast, compounds 7 and 8 showed higher toxicity than doxorubicin in 4T1 breast cancer cells but lower toxicity than doxorubicin in L929 normal cell lines. These results suggest the possibility of a tumor-targeting effect, as compounds 7 and 8 demonstrated higher apoptosis effects than doxorubicin in 4T1 tumor cells while displaying distinct patterns in normal cells. These findings underscore the potential of compounds 7 and 8 as targeted anti-cancer agents, specifically effective against breast cancer cells, showcasing their efficient and selective toxicity. However, there are also certain limitations that must be addressed to enhance the understanding and significance of these compounds. Therefore, further studies are required to investigate whether treatment with these compounds affects other types of cancer cells (e.g., Mc38, B16-f10, etc.), and toxicity assessments should also be conducted on various normal cell lines such as 3T3 cells. Additionally, it is essential to validate the effectiveness of these compounds in killing cancer cells through clonogenic assays, cell cycle progression analysis, and apoptotic assays. These additional studies could significantly enhance the clinical relevance of the findings in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations11030065/s1, Table S1: The percentage ratio of each isolated compound within the total macrocyclic trichothecenes; Table S2: Equipment used for analyses.
Author Contributions
Conceptualization, S.L., C.G.P. and K.H.K.; investigation, B.S.L. and Y.Y.L.; methodology, B.S.L., Y.Y.L., S.R.L., Y.S.J., R.R., W.P. and S.-N.K.; formal analysis, B.S.L., Y.Y.L., S.R.L., Y.S.J., R.R., W.P. and S.-N.K.; writing—original draft preparation, B.S.L., Y.Y.L., S.R.L. and K.H.K.; writing—review and editing, S.L., C.G.P. and K.H.K.; supervision, S.L., C.G.P. and K.H.K.; funding acquisition, C.G.P. and K.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; grant numbers 2023-00208913, 2019R1A5A2027340, and 2021R1A2C2007937).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
Author Se-Na Kim was employed by the company MediArk Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef] [PubMed]
- Vivek-Ananth, R.; Sahoo, A.K.; Baskaran, S.P.; Samal, A. Scaffold and structural diversity of the secondary metabolite space of medicinal fungi. ACS Omega 2023, 8, 3102–3113. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.-S.; Hossain, M.A.; Park, S.-C. Toxicological profiles of poisonous, edible, and medicinal mushrooms. Mycobiology 2014, 42, 215–220. [Google Scholar] [CrossRef]
- Govorushko, S.; Rezaee, R.; Dumanov, J.; Tsatsakis, A. Poisoning associated with the use of mushrooms: A review of the global pattern and main characteristics. Food Chem. Toxicol. 2019, 128, 267–279. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Gorniak, L.; Stela, M.; Bijak, M. The existing methods and novel approaches in mycotoxins’ detection. Molecules 2021, 26, 3981. [Google Scholar] [CrossRef]
- Singh, J.; Mehta, A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: A review. Food Sci. Nutr. 2020, 8, 2183–2204. [Google Scholar] [CrossRef]
- Ahmed, W.H.A.; Gonmori, K.; Suzuki, M.; Watanabe, K.; Suzuki, O. Simultaneous analysis of α-amanitin, β-amanitin, and phalloidin in toxic mushrooms by liquid chromatography coupled to time-of-flight mass spectrometry. Forensic Toxicol. 2010, 28, 69–76. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal mushrooms: Bioactive compounds, use, and clinical trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef]
- Chugh, R.M.; Mittal, P.; Mp, N.; Arora, T.; Bhattacharya, T.; Chopra, H.; Cavalu, S.; Gautam, R.K. Fungal mushrooms: A natural compound with therapeutic applications. Front. Pharmacol. 2022, 13, 925387. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yu, J.S.; Lee, S.R.; Kim, K.H. Non-peptide secondary metabolites from poisonous mushrooms: Overview of chemistry, bioactivity, and biosynthesis. Nat. Prod. Rep. 2022, 39, 512–559. [Google Scholar] [CrossRef]
- Nieminen, P.; Mustonen, A.-M. Toxic potential of traditionally consumed mushroom species—A controversial continuum with many unanswered questions. Toxins 2020, 12, 639. [Google Scholar] [CrossRef]
- Kim, H.N.; Do, H.H.; Seo, J.S.; Kim, H.Y. Two cases of incidental Podostroma cornu-damae poisoning. Clin. Exp. Emerg. Med. 2016, 3, 186. [Google Scholar] [CrossRef]
- Lee, B.S.; So, H.M.; Kim, S.; Kim, J.K.; Kim, J.-C.; Kang, D.-M.; Ahn, M.-J.; Ko, Y.-J.; Kim, K.H. Comparative evaluation of bioactive phytochemicals in Spinacia oleracea cultivated under greenhouse and open field conditions. Arch. Pharm. Res. 2022, 45, 795–805. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.H.; Han, S.H.; Kim, H.-J.; Cho, I.-H.; Lee, S. Structure determination of heishuixiecaoline A from Valeriana fauriei and its content from different cultivated regions by HPLC/PDA Analysis. Nat. Prod. Sci. 2022, 28, 181–186. [Google Scholar] [CrossRef]
- Yu, J.S.; Jeong, S.Y.; Li, C.; Oh, T.; Kwon, M.; Ahn, J.S.; Ko, S.-K.; Ko, Y.-J.; Cao, S.; Kim, K.H. New phenalenone derivatives from the Hawaiian volcanic soil-associated fungus Penicillium herquei FT729 and their inhibitory effects on indoleamine 2, 3-dioxygenase 1 (IDO1). Arch. Pharm. Res. 2022, 45, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Lee, B.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Yi, S.A.; Han, J.-W.; Kim, S.; Kim, J.K.; Kim, J.-C. Identification of anti-adipogenic withanolides from the roots of Indian ginseng (Withania somnifera). J. Ginseng Res. 2022, 46, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Park, K.H.; Hong, J.H.; Kim, S.H.; Park, K.M.; Kim, K.H. Anti-osteoporosis effects of triterpenoids from the fruit of sea buckthorn (Hippophae rhamnoides) through the promotion of osteoblast differentiation in mesenchymal stem cells, C3H10T1/2. Arch. Pharm. Res. 2023, 46, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Seok, S.; Ryoo, R.; Choi, S.U.; Kim, K.H. Macrocyclic trichothecene mycotoxins from a deadly poisonous mushroom, Podostroma cornu-damae. J. Nat. Prod. 2018, 82, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Saikawa, Y.; Okamoto, H.; Inui, T.; Makabe, M.; Okuno, T.; Suda, T.; Hashimoto, K.; Nakata, M. Toxic principles of a poisonous mushroom Podostroma cornu-damae. Tetrahedron 2001, 57, 8277–8281. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Cheng, Z.; Proksch, P.; Lin, W. Cytotoxic trichothecene-type sesquiterpenes from the sponge-derived fungus Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv. 2017, 7, 7259–7267. [Google Scholar] [CrossRef]
- Mondol, M.A.M.; Surovy, M.Z.; Islam, M.T.; Schüffler, A.; Laatsch, H. Macrocyclic trichothecenes from Myrothecium roridum strain M10 with motility inhibitory and zoosporicidal activities against Phytophthora nicotianae. J. Agric. Food Chem. 2015, 63, 8777–8786. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.-Z.; Shen, L.; Wang, F.-W. A new trichothecene from Myrothecium roridum QDFE005, a symbiotic fungus isolated from Mactra chinensis. J. Asian Nat. Prod. Res. 2013, 15, 1284–1289. [Google Scholar] [CrossRef]
- Deng, R.; Wang, S.-M.; Yin, T.; Ye, T.-H.; Shen, G.-B.; Li, L.; Zhao, J.-Y.; Sang, Y.-X.; Duan, X.-G.; Wei, Y.-Q. Dimethyl Sulfoxide Suppresses Mouse 4T1 Breast Cancer Growth by Modulating Tumor-Associated Macrophage Differentiation. J. Breast Cancer 2014, 17, 25–32. [Google Scholar] [CrossRef]
- Lee, B.S.; Jung, S.M.; Ryoo, R.; Choi, S.U.; An, S.; Kim, K.H. I-Hydroxy-Phe-Phe, a new dipeptide, and cytotoxic macrocyclic trichothecenes from the lethal toxic mushroom Podostroma cornu-damae. Org. Biomol. Chem. 2023, 21, 8521–8527. [Google Scholar] [CrossRef]
- Razavi-Azarkhiavi, K.; Iranshahy, M.; Sahebkar, A.; Shirani, K.; Karimi, G. The protective role of phenolic compounds against doxorubicin-induced cardiotoxicity: A comprehensive review. Nutr. Cancer 2016, 68, 892–917. [Google Scholar] [CrossRef] [PubMed]
- Sherbenou, D.W.; Druker, B.J. Applying the discovery of the Philadelphia chromosome. J. Clin. Investig. 2007, 117, 2067–2074. [Google Scholar] [CrossRef] [PubMed]
- Defossez, G.; Mathoulin-Pelissier, S.; Ingrand, I.; Gasquet, I.; Sifer-Riviere, L.; Ingrand, P.; Salamon, R.; Migeot, V.; The REPERES Research Network. Satisfaction with care among patients with non-metastatic breast cancer: Development and first steps of validation of the REPERES-60 questionnaire. BMC Cancer 2007, 7, 129. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Colleoni, M.; Domenighetti, G.; Gelber, R. Systemic treatments for women with breast cancer: Outcome with relation to screening for the disease. Ann. Oncol. 2003, 14, 1212–1214. [Google Scholar] [CrossRef]
- Gollahon, L.S.; Jeong, Y.; Finckbone, V.; Lee, K.; Park, J.-S. The Natural Product NI-07, is effective against breast cancer cells while showing no cytotoxicity to normal cells. Open Breast Cancer J. 2011, 3, 31–44. [Google Scholar] [CrossRef]
- Elisia, I.; Popovich, D.G.; Hu, C.; Kitts, D.D. Evaluation of viability assays for anthocyanins in cultured cells. Phytochem. Anal. 2008, 19, 479–486. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).