Fabrication and Characterization of Sulfonated Carbon Materials and Chitosan-Derived Functioned Carbon via Schiff’s Base Process for Separation Purposes
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Fabrication of Functioned Carbon Materials via Schiff’s Base Process
2.3. Instrumentation
2.4. Capillary Coating
2.5. Chromatographic Evaluation
2.6. Thermodynamic Calculations
3. Results and Discussion
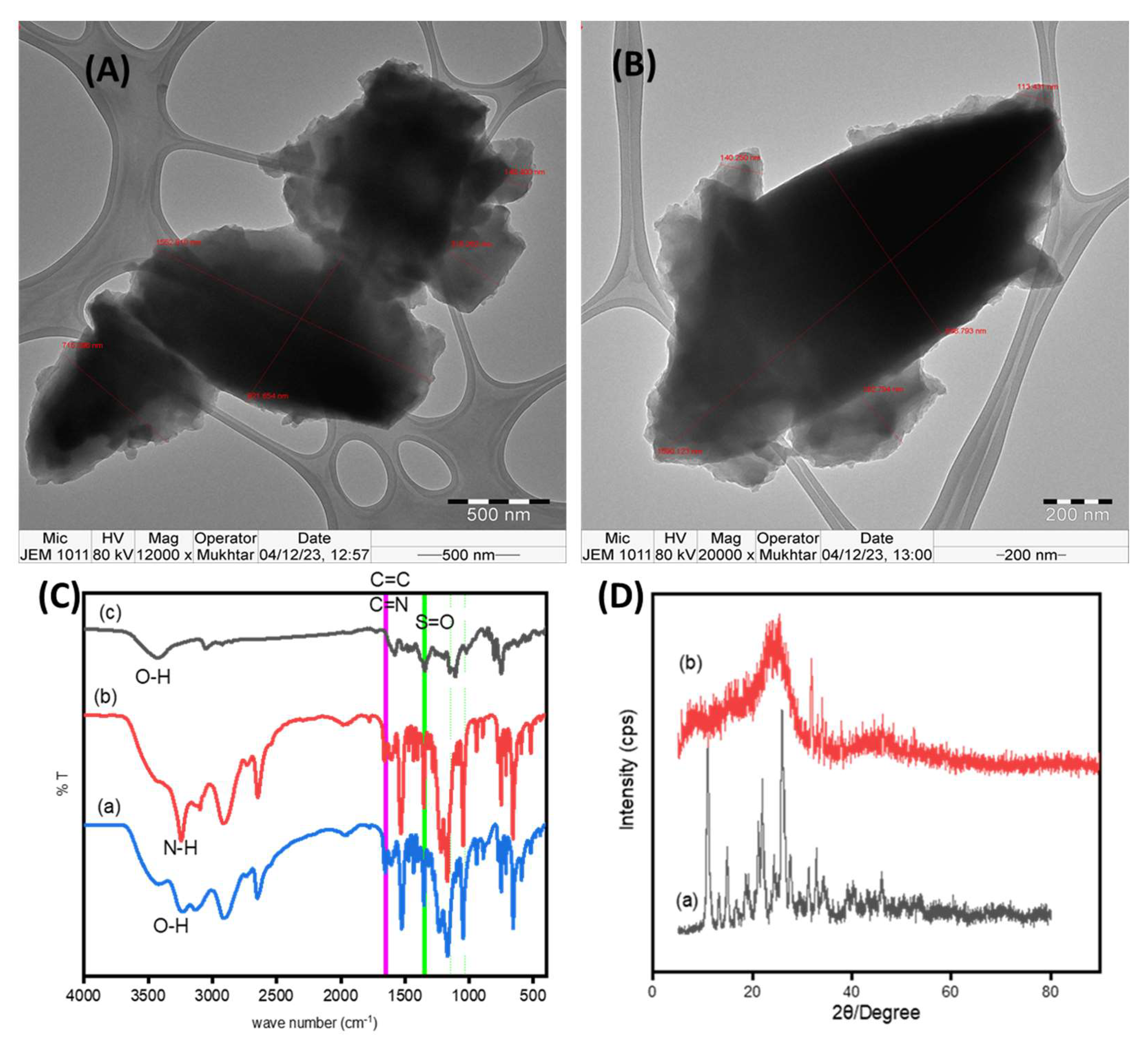
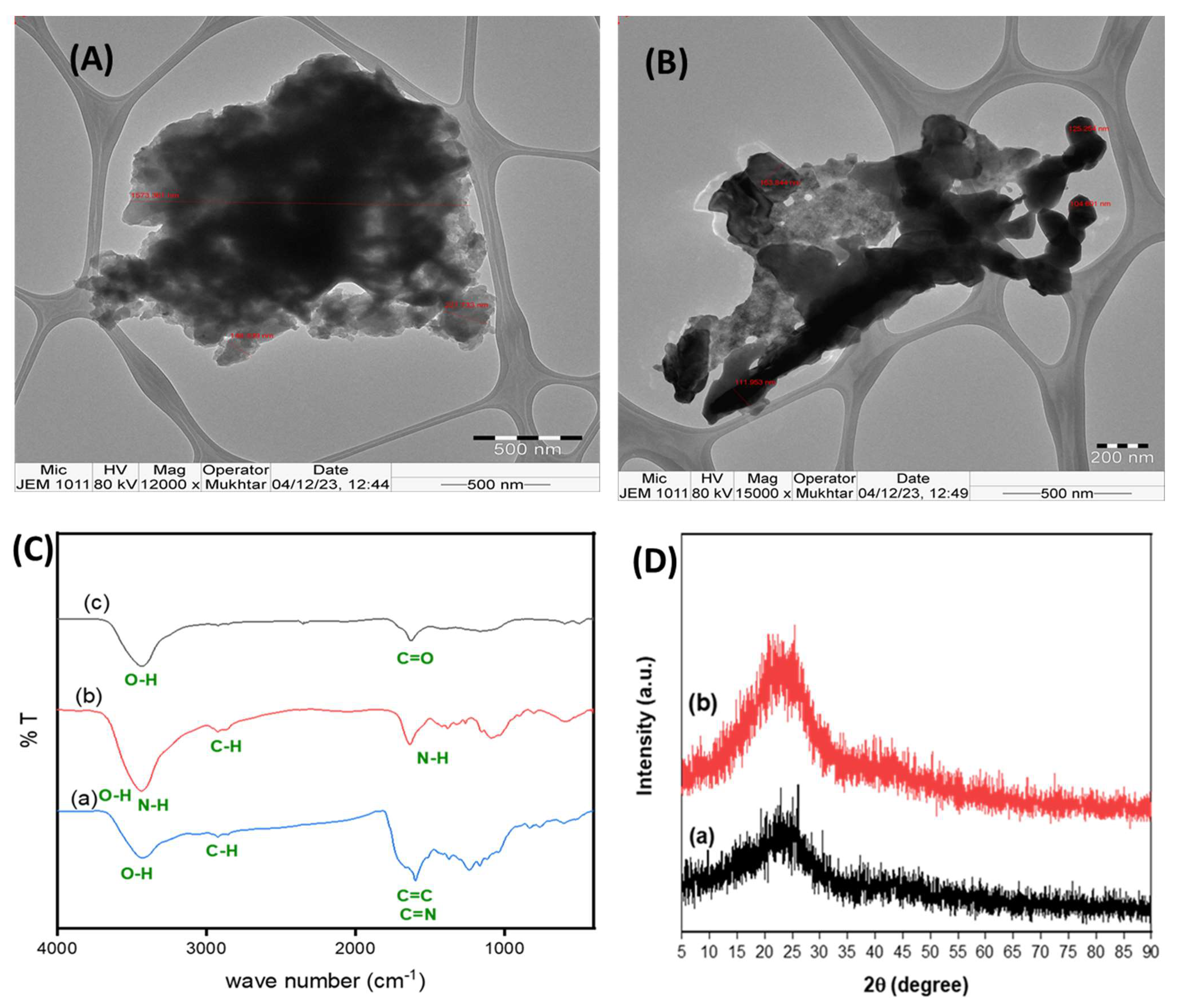
3.1. Characterization of the Fabricated Functioned Carbon Materials
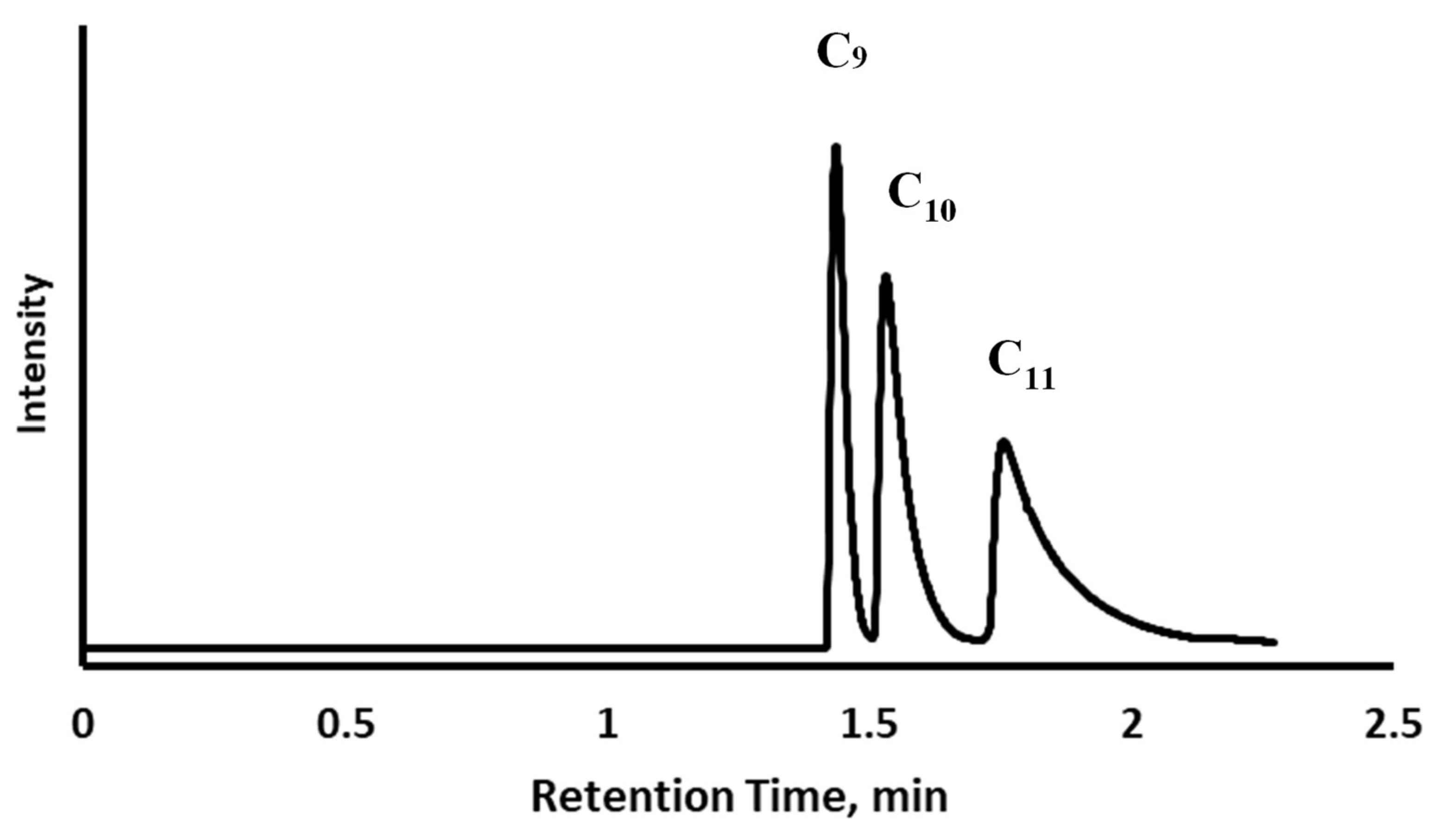
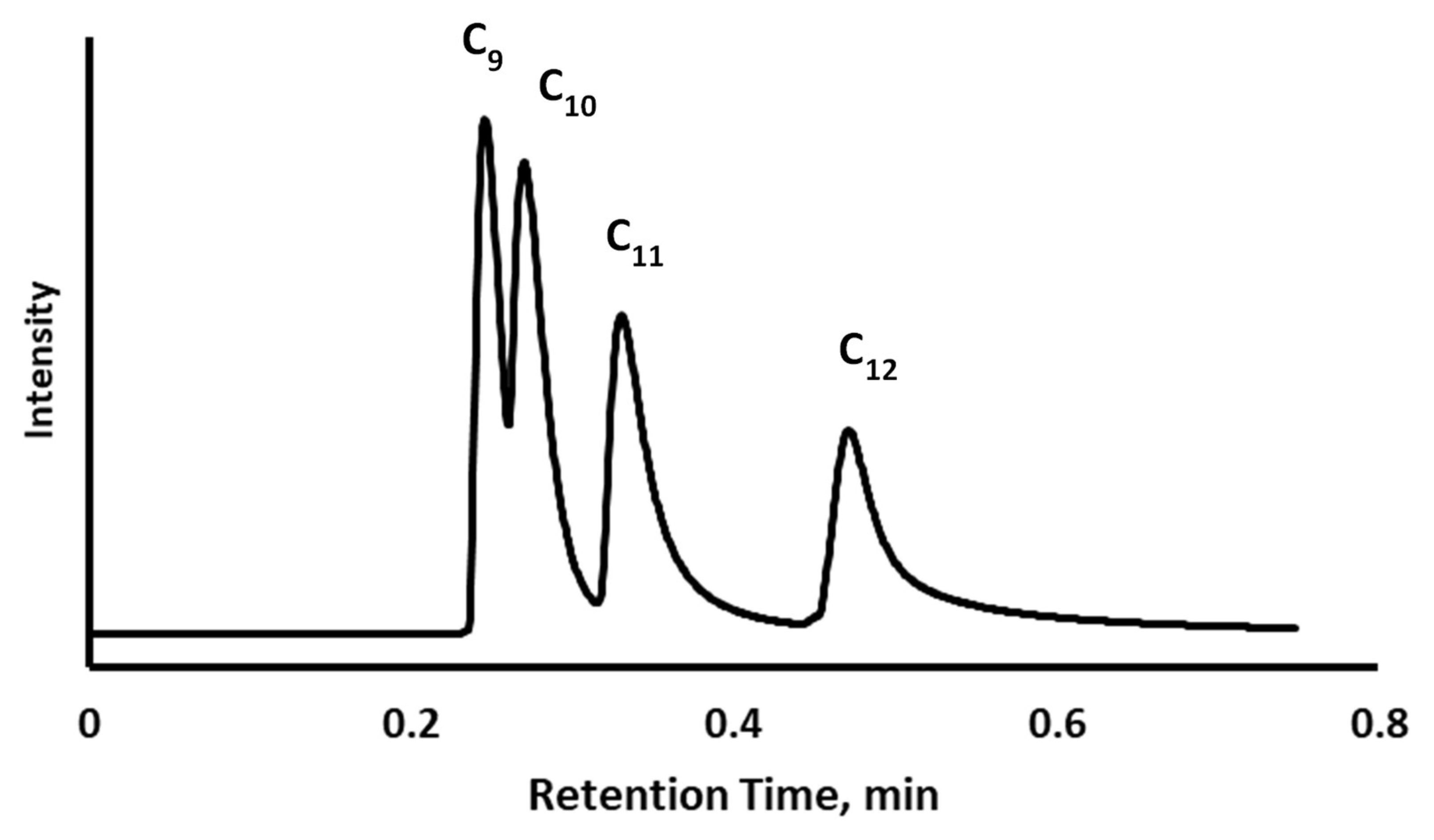
3.2. Chromatographic Evaluation
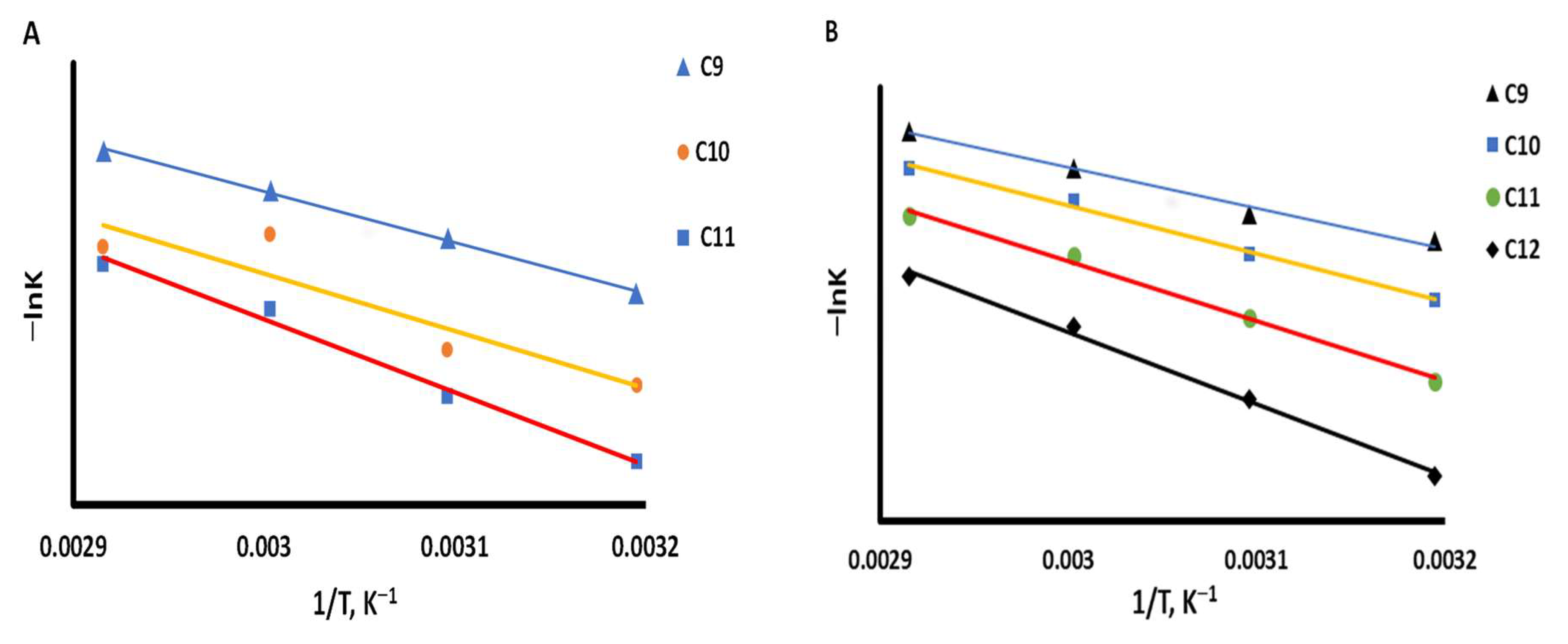
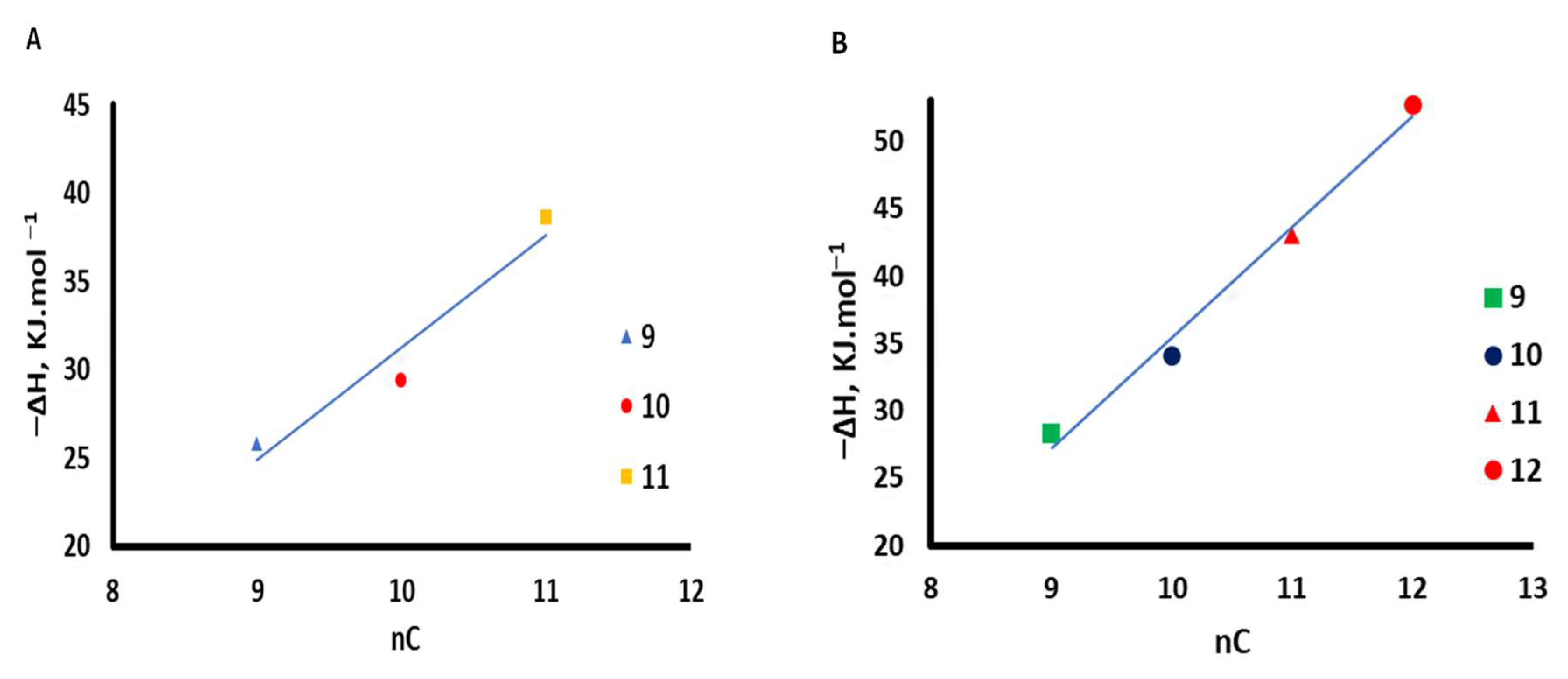
3.3. Thermodynamic Parameters Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, Y.X.; Lin, Y.F.; Xu, Z.L.; Mo, J.W.; Li, P.P. A Novel Clover-like COFs Membrane Fabricated via One-Step Interfacial Polymerization for Dye/Salt Separation. J. Membr. Sci. 2023, 673, 121470. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Y.; Gu, T.; Meng, X.; Wang, H.; Xu, K.; Cheng, L.; Kasher, R.; Zhang, R.; Jiang, Z. Covalent Organic Framework Membrane with Sub-Nano Pores for Efficient Desalination. J. Membr. Sci. 2023, 675, 121551. [Google Scholar] [CrossRef]
- Ma, D.; Ma, D.; Li, Z.; Zhu, J.; Zhou, Y.; Chen, L.; Mai, X.; Liufu, M.; Wu, Y.; Li, Y. Inverse and Highly Selective Separation of CO2/C2H2 on a Thulium–Organic Framework. J. Mater. Chem. A Mater. 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Atanes, E.; Cuesta-García, B.; Nieto-Márquez, A.; Fernández-Martínez, F. A Mixed Separation-Immobilization Method for Soluble Salts Removal and Stabilization of Heavy Metals in Municipal Solid Waste Incineration Fly Ash. J. Environ. Manag. 2019, 240, 359–367. [Google Scholar] [CrossRef]
- Kumar, P.; Guliants, V.V. Periodic Mesoporous Organic–Inorganic Hybrid Materials: Applications in Membrane Separations and Adsorption. Microporous Mesoporous Mater. 2010, 132, 1–14. [Google Scholar] [CrossRef]
- Krukonis, V.J. Supercritical Fluid Extraction in Flavor Applications; ACS: Washington, DC, USA, 1985; pp. 154–175. [Google Scholar] [CrossRef]
- Camel, V. Solid Phase Extraction of Trace Elements. Spectrochim. Acta 2003, 58, 1177–1233. [Google Scholar] [CrossRef]
- Shyam Sunder, G.S.; Adhikari, S.; Rohanifar, A.; Poudel, A.; Kirchhoff, J.R. Evolution of Environmentally Friendly Strategies for Metal Extraction. Separations 2020, 7, 4. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Wabaidur, S.M. Application of Carbon Nanotubes in Extraction and Chromatographic Analysis: A Review. Arab. J. Chem. 2019, 12, 633–651. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: Hoboken, NJ, USA, 1984; ISBN 0471866067. [Google Scholar]
- Cooney, D.O. Adsorption Design for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 1998; ISBN 1566703336. [Google Scholar]
- Antony, R.; Arun, T.; Manickam, S.T.D. A Review on Applications of Chitosan-Based Schiff Bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Monier, M.; Ayad, D.M.; Abdel-Latif, D.A. Adsorption of Cu(II), Cd(II) and Ni(II) Ions by Cross-Linked Magnetic Chitosan-2-Aminopyridine Glyoxal Schiff’s Base. Colloids Surf. B Biointerfaces 2012, 94, 250–258. [Google Scholar] [CrossRef]
- Tsaneva, D.; Nikolova, M.; Prokopov, T.; Petkova, N.; Stoyanova, A.; Denev, P. Evaluation of Adsorption Capacity of Chitosan-Cinnamaldehyde Schiff Base. J. Pharm. Sci. Res. 2017, 9, 1609. [Google Scholar]
- Wang, Y.; Liu, W.; Zhang, J.; Shan, Q. Preparation of Co-Schiff Base Complex and Its Adsorption Desulfurization. Fuel 2022, 324, 124696. [Google Scholar] [CrossRef]
- Xu, G.; Hou, L.; Li, B.; Wang, X.; Liu, L.; Li, N.; Wang, M.L.; Zhao, R.S. Facile Preparation of Hydroxyl Bearing Covalent Organic Frameworks for Analysis of Phenoxy Carboxylic Acid Pesticide Residue in Plant-Derived Food. Food Chem. 2021, 345, 128749. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent Organic Frameworks Based on Schiff-Base Chemistry: Synthesis, Properties and Potential Applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef]
- Qian, C.; Qi, Q.Y.; Jiang, G.F.; Cui, F.Z.; Tian, Y.; Zhao, X. Toward Covalent Organic Frameworks Bearing Three Different Kinds of Pores: The Strategy for Construction and COF-to-COF Transformation via Heterogeneous Linker Exchange. J. Am. Chem. Soc. 2017, 139, 6736–6743. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, P. Compare of Three Ways of Synthesis of Simple Schiff Base. Molbank 2006, 2006, M514. [Google Scholar] [CrossRef]
- Bhat, A.R.; Wagay, M.H. Synthesis of Schiff’s Base Derivatives Using Water as Solvent. (A Green Methodology). Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 971–982. [Google Scholar] [CrossRef]
- Li, W.; Wang, Q.; Cui, F.; Jiang, G. Covalent Organic Framework with Sulfonic Acid Functional Groups for Visible Light-Driven CO2 Reduction. RSC Adv. 2022, 12, 17984–17989. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, G.; Hu, Z.; Cheng, Y.; Chi, C.; Yuan, D.; Cheng, H.; Zhao, D. Mechanoassisted Synthesis of Sulfonated Covalent Organic Frameworks with High Intrinsic Proton Conductivity. ACS Appl. Mater. Interfaces 2016, 8, 18505–18512. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ding, L.G.; Yao, B.J.; Huang, N.; Li, J.T.; Fu, Q.J.; Dong, Y. Bin Pd Loaded and Covalent-Organic Framework Involved Chitosan Aerogels and Their Application for Continuous Flow-through Aqueous CB Decontamination. J. Mater. Chem. A Mater. 2018, 6, 11140–11146. [Google Scholar] [CrossRef]
- Fontana, R.; Marconi, P.C.R.; Caputo, A.; Gavalyan, V.B. Novel Chitosan-Based Schiff Base Compounds: Chemical Characterization and Antimicrobial Activity. Molecules 2022, 27, 2740. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-Based Smart Hybrid Materials: A Physico-Chemical Perspective. J. Mater. Chem. B 2021, 9, 594–611. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, H.; Wu, W.; Wei, S.; Xu, Y.; Kuang, Y. Studies of Heavy Metal Ion Adsorption on Chitosan/Sulfydryl-Functionalized Graphene Oxide Composites. J. Colloid Interface Sci. 2015, 448, 389–397. [Google Scholar] [CrossRef]
- Garcia Peña, L.V.; Petkova, P.; Margalef-Marti, R.; Vives, M.; Aguilar, L.; Gallegos, A.; Francesko, A.; Perelshtein, I.; Gedanken, A.; Mendoza, E.; et al. Hybrid Chitosan-Silver Nanoparticles Enzymatically Embedded on Cork Filter Material for Water Disinfection. Ind. Eng. Chem. Res. 2017, 56, 3599–3606. [Google Scholar] [CrossRef]
- Pratibha; Kapoor, A.; Rajput, J.K. Electroactive Core-Shell Chitosan-Coated Lanthanum Iron Oxide as a Food Freshness Level Indicator for Tyramine Content Determination. ACS Sustain. Chem. Eng. 2022, 10, 11666–11679. [Google Scholar] [CrossRef]
- Mohd Adnan, M.A.; Bao, L.P.; Muhd Julkapli, N. Mitigation of Pollutants by Chitosan/Metallic Oxide Photocatalyst: A Review. J. Clean Prod. 2020, 261, 121190. [Google Scholar] [CrossRef]
- Li, W.; Jiang, H.X.; Geng, Y.; Wang, X.H.; Gao, R.Z.; Tang, A.N.; Kong, D.M. Facile Removal of Phytochromes and Efficient Recovery of Pesticides Using Heteropore Covalent Organic Framework-Based Magnetic Nanospheres and Electrospun Films. ACS Appl. Mater. Interfaces 2020, 12, 20922–20932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, S.; Chen, Y.; Zhang, Z.; Ma, S. Covalent Organic Frameworks for Separation Applications. Chem. Soc. Rev. 2020, 49, 708–735. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van Der Bruggen, B. Covalent Organic Frameworks for Membrane Separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Xie, M.; Quan, K.; Li, H.; Liu, B.; Chen, J.; Yu, Y.; Wang, J.; Qiu, H. Non-Porous Silica Support Covalent Organic Frameworks as Stationary Phases for Liquid Chromatography. Chem. Commun. 2023, 59, 314–317. [Google Scholar] [CrossRef]
- Yusuf, K.; Natraj, A.; Li, K.; Ateia, M.; ALOthman, Z.A.; Dichtel, W.R. Inverse Gas Chromatography Demonstrates the Crystallinity-Dependent Physicochemical Properties of Two-Dimensional Covalent Organic Framework Stationary Phases. Chem. Mater. 2023, 35, 1691–1701. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, M.; Zhou, J.; Dai, X.; Yang, H.; Xia, Z.; Wang, L. One-Pot Method for the Synthesis of β-Cyclodextrin and Covalent Organic Framework Functionalized Chiral Stationary Phase with Mixed-Mode Retention Mechanism. J. Chromatogr. A 2022, 1662, 462731. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.L.; Zheng, S.R.; Tan, J.B.; Cai, S.L.; Fan, J.; Yan, X.; Zhang, W.G. Tubular Metal–Organic Framework-Based Capillary Gas Chromatography Column for Separation of Alkanes and Aromatic Positional Isomers. J. Chromatogr. A 2013, 1285, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal Organic Frameworks as Efficient Adsorbents for Drugs from Wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, Z.; Zhao, L.; Kushwaha, A.; Kumar, A.; Wang, J.; Pan, Y.; Muddassir, M.; Lan, Q. Porphyrin-Based Fe/La Metal-Organic Frameworks as Photocatalysts for Dye Photodegradation: Syntheses and Mechanism Investigation. Inorg. Chem. Commun. 2023, 154, 110920. [Google Scholar] [CrossRef]
- Qin, N.; Pan, A.; Yuan, J.; Ke, F.; Wu, X.; Zhu, J.; Liu, J.; Zhu, J. One-Step Construction of a Hollow Au@Bimetal-Organic Framework Core-Shell Catalytic Nanoreactor for Selective Alcohol Oxidation Reaction. ACS Appl. Mater. Interfaces 2021, 13, 12463–12471. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal-Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef]
- Zhou, Y.; Mao, Z.; Wang, W.; Yang, Z.; Liu, X. In-Situ Fabrication of Graphene Oxide Hybrid Ni-Based Metal-Organic Framework (Ni-MOFs@GO) with Ultrahigh Capacitance as Electrochemical Pseudocapacitor Materials. ACS Appl. Mater. Interfaces 2016, 8, 28904–28916. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Diao, Y.; He, Q.; Lu, C.; Singh, A.; Kumar, A.; Liu, J.; Lan, Q. Recent Advances in the Electrochemical Applications of Ni-Based Metal Organic Frameworks (Ni-MOFs) and Their Derivatives. Chemosphere 2022, 307, 135729. [Google Scholar] [CrossRef]
- Liu, T.; Tang, Q.; Lu, T.; Zhu, C.; Li, S.; Zhou, C.; Yang, H. Metal–Organic Frameworks-Based Membranes with Special Wettability for Oil–Water Separation: A Review. Coatings 2023, 13, 1241. [Google Scholar] [CrossRef]
- Liang, L.; Chen, J.; Chen, X.; Wang, J.; Qiu, H. In Situ Synthesis of a GO/COFs Composite with Enhanced Adsorption Performance for Organic Pollutants in Water. Environ. Sci. Nano 2022, 9, 554–567. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Amperometric Galectin-3 Immunosensor-Based Gold Nanoparticle-Functionalized Graphitic Carbon Nitride Nanosheets and Core–Shell Ti-MOF@COFs Composites. Nanoscale 2020, 12, 19824–19832. [Google Scholar] [CrossRef] [PubMed]
- Sesigur, F.; Sakar, D.; Yasa-Sahin, O.; Ocak, H.; Cankurtaran, O.; Bilgin-Eran, B.; Karaman, F. Thermodynamic and Surface Characterisation of (S)-5-Dodecycloxy-2-[[[4-(2-Methylbutoxy) Phenyl]Imino]Metheyl]Phenol Thermotropic Liquid Crystal by Inverse Gas Chromatography. Liq. Cryst. 2012, 39, 87–97. [Google Scholar] [CrossRef]
- Cui, W.G.; Hu, T.L.; Bu, X.H. Metal–Organic Framework Materials for the Separation and Purification of Light Hydrocarbons. Adv. Mater. 2020, 32, 1806445. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, H.; Liu, D.; Xiao, J.; Qian, Y.; Xi, H. Effective Ligand Functionalization of Zirconium-Based Metal-Organic Frameworks for the Adsorption and Separation of Benzene and Toluene: A Multiscale Computational Study. ACS Appl. Mater. Interfaces 2015, 7, 5775–5787. [Google Scholar] [CrossRef]
- González-Galán, C.; De Fez-Febré, M.; Giancola, S.; González-Cobos, J.; Vidal-Ferran, A.; Galán-Mascarós, J.R.; Balestra, S.R.G.; Calero, S. Separation of Volatile Organic Compounds in TAMOF-1. ACS Appl. Mater. Interfaces 2022, 14, 30772–30785. [Google Scholar] [CrossRef]
- Yu, H.; Zang, J.; Guo, C.; Li, B.; Li, B.; Zhang, X.; Chen, T. Research Progress on Adsorption and Separation of Petroleum Hydrocarbon Molecules by Porous Materials. Separations 2023, 10, 17. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Liu, M.; Zhou, Y.; Zhang, L.; Qiao, X. Core-Shell Metal-Organic Frameworks as the Mixed-Mode Stationary Phase for Hydrophilic Interaction/Reversed-Phase Chromatography. ACS Appl. Mater. Interfaces 2019, 11, 10320–10327. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, J.; Ma, J.; Jia, Q. Engineering Magnetic Guanidyl-Functionalized Supramolecular Organic Framework for Efficient Enrichment of Global Phosphopeptides. ACS Appl. Mater. Interfaces 2020, 12, 57468–57476. [Google Scholar] [CrossRef]
- Sinha Roy, K.; Goud, R.D.; Mazumder, A.; Chandra, B.; Purohit, A.K.; Palit, M.; Dubey, D.K. Triazine-Based Covalent Organic Framework: A Promising Sorbent for Efficient Elimination of the Hydrocarbon Backgrounds of Organic Sample for GC-MS and 1 H NMR Analysis of Chemical Weapons Convention Related Compounds. ACS Appl. Mater. Interfaces 2019, 11, 16027–16039. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, P.; Feng, J.; Sun, M. Nanostructured Silver Coating as a Stationary Phase for Capillary Gas Chromatography. Molecules 2019, 24, 4491. [Google Scholar] [CrossRef]
- Ban, S.; Van Laak, A.; De Jongh, P.E.; Van Der Eerden, J.P.J.M.; Vlugt, T.J.H. Adsorption Selectivity of Benzene/Propene Mixtures for Various Zeolites. J. Phys. Chem. C 2007, 111, 17241–17248. [Google Scholar] [CrossRef]
- Pasban, A.; Mostafavi, S.M.; Malekzadeh, H.; Nazari, B.M. Quantitative Determination of LPG Hydrocarbons by Modified Packed Column Adsorbent of Gas Chromatography Via Full Factorial Design. J. Nanoanal. 2017, 4, 31–40. [Google Scholar] [CrossRef]
- Speltini, A.; Merli, D.; Quartarone, E.; Profumo, A. Separation of Alkanes and Aromatic Compounds by Packed Column Gas Chromatography Using Functionalized Multi-Walled Carbon Nanotubes as Stationary Phases. J. Chromatogr. A 2010, 1217, 2918–2924. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.; Bruderreck, H.; Halász, I. Gas Chromatographic Separation of Hydrocarbons (C1 to C8) by Carbon Number Using Packed Capillary Columns. Anal. Chem. 1964, 36, 1533–1540. [Google Scholar] [CrossRef]
- Merli, D.; Speltini, A.; Ravelli, D.; Quartarone, E.; Costa, L.; Profumo, A. Multi-Walled Carbon Nanotubes as the Gas Chromatographic Stationary Phase: Role of Their Functionalization in the Analysis of Aliphatic Alcohols and Esters. J. Chromatogr. A 2010, 1217, 7275–7281. [Google Scholar] [CrossRef] [PubMed]
- Habila, M.A.; Alothman, Z.A.; Ali, R.; Ghafar, A.A.; Hassouna, M.S.E.-D. Removal of Tartrazine Dye onto Mixed-Waste Activated Carbon: Kinetic and Thermodynamic Studies. Clean 2014, 42, 1824–1831. [Google Scholar] [CrossRef]
- Ji, Z.; Majors, R.E.; Guthrie, E.J. Porous Layer Open-Tubular Capillary Columns: Preparations, Applications and Future Directions. J. Chromatogr. A 1999, 842, 115–142. [Google Scholar] [CrossRef]
- Haddad, P.R.; Taraji, M.; Szücs, R. Prediction of Analyte Retention Time in Liquid Chromatography. Anal. Chem. 2021, 93, 228–256. [Google Scholar] [CrossRef]
- Hinshaw, J.V. Practical Gas Chromatography. LCGC N. Am. 2013, 31, 932–937. [Google Scholar]
- Fidan, İ.; Daşdan, D.Ş.; Karaman, F.; Kaban, Ş. Surface Characterization of Thiazolidinone Derivatives by Inverse Gas Chromatography. Bulg. Chem. Commun. 2017, 49, 742–749. [Google Scholar]
- van de Kamer, J.H.; Gerritsma, K.W.; Wansink, E.J. Gas-Liquid Partition Chromatography: The Separation and Micro-Estimation of Volatile Fatty Acids from Formic Acid to Dodecanoic Acid. Biochem. J. 1955, 61, 174–176. [Google Scholar] [CrossRef]
- Gutmann, F.; Simmons, L.M. A Theoretical Basis for the Antoine Vapor Pressure Equation. J. Chem. Phys. 1950, 18, 696–697. [Google Scholar] [CrossRef]
- Yusuf, K.; Badjah-Hadj-Ahmed, A.Y.; Aqel, A.; Aouak, T.; ALOthman, Z.A. Zeolitic Imidazolate Framework-Methacrylate Composite Monolith Characterization by Inverse Gas Chromatography. J. Chromatogr. A 2016, 1443, 233–240. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Papaiconomou, N.; Baup, S.; Iojoiu, C.; Svecova, L.; Thivel, P.X. Surface Characterization of 1-Butyl-1-Ethylpiperidinium Bromide by Inverse Gas Chromatography. J. Mol. Liq. 2019, 287, 110945. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive Forces At Interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Schultz, J.; Martin, C. The Role of the Interface in Carbon Fibre-Epoxy Compositest. J. Adhes. 1987, 23, 45–60. [Google Scholar] [CrossRef]
- Dolatyari, L.; Yaftian, M.R.; Rostamnia, S. Removal of Uranium(VI) Ions from Aqueous Solutions Using Schiff Base Functionalized SBA-15 Mesoporous Silica Materials. J. Environ. Manag. 2016, 169, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Aslam, Z.; Shawabkeh, R.A.; Asghar, A.; Hussein, I.A. BET, FTIR, and RAMAN Characterizations of Activated Carbon from Waste Oil Fly Ash. Turk. J. Chem. 2020, 44, 279. [Google Scholar] [CrossRef]
- De Menezes, B.R.C.; Ferreira, F.V.; Silva, B.C.; Simonetti, E.A.N.; Bastos, T.M.; Cividanes, L.S.; Thim, G.P. Effects of Octadecylamine Functionalization of Carbon Nanotubes on Dispersion, Polarity, and Mechanical Properties of CNT/HDPE Nanocomposites. J. Mater. Sci. 2018, 53, 14311–14327. [Google Scholar] [CrossRef]
- Ostrowska-Czubenko, J.; Pieróg, M.; Gierszewska-Drużyńska, M. State of water in noncrosslinked and crosslinked hydrogel chitosan membranes—Dsc studies. Prog. Chem. Appl. Chitin Its Deriv. 2011, 16, 147–156. [Google Scholar]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An Overview of the Modification Methods of Activated Carbon for Its Water Treatment Applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Farma, R.; Wahyuni, F.; Awitdrus. Physical Properties Analysis of Activated Carbon from Oil Palm Empty Fruit Bunch Fiber on Methylene Blue Adsorption. J. Technomater. Phys. 2019, 1, 69–75. [Google Scholar] [CrossRef]
- Chester, T.L.; Coym, J.W. Effect of Phase Ratio on van’t Hoff Analysis in Reversed-Phase Liquid Chromatography, and Phase-Ratio-Independent Estimation of Transfer Enthalpy. J. Chromatogr. A 2003, 1003, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Yoon, P.R. Determination of Acid-Base Properties of Silicas by Inverse Gas Chromatography: Variation with Surface Treatment. Mater. Trans. 2007, 48, 1955–1960. [Google Scholar] [CrossRef]
- Denayer, J.F.; Baron, G.V.; Martens, J.A.; Jacobs, P.A. Chromatographic Study of Adsorption of N-Alkanes on Zeolites at High Temperatures. J. Phys. Chem. B 1998, 102, 3077–3081. [Google Scholar] [CrossRef]
- Luebbers, M.T.; Wu, T.; Shen, L.; Masel, R.I. Trends in the Adsorption of Volatile Organic Compounds in a Large-Pore Metal-Organic Framework, IRMOF-1. Langmuir 2010, 26, 11319–11329. [Google Scholar] [CrossRef]
- Gumrukcu, G.; Garikyan, S.; Keser Karaoglan, G.; Sakar, D. Structural and Surface Characterization of Newly Synthesized D-π-D Type Schiff Base Ligand: (1E,2E)-3-[4-(Dimethylamino)Phenyl]Prop-2-En-1-Ylidene) Phenylamine. J. Chem. 2013, 2013, 298205. [Google Scholar] [CrossRef]
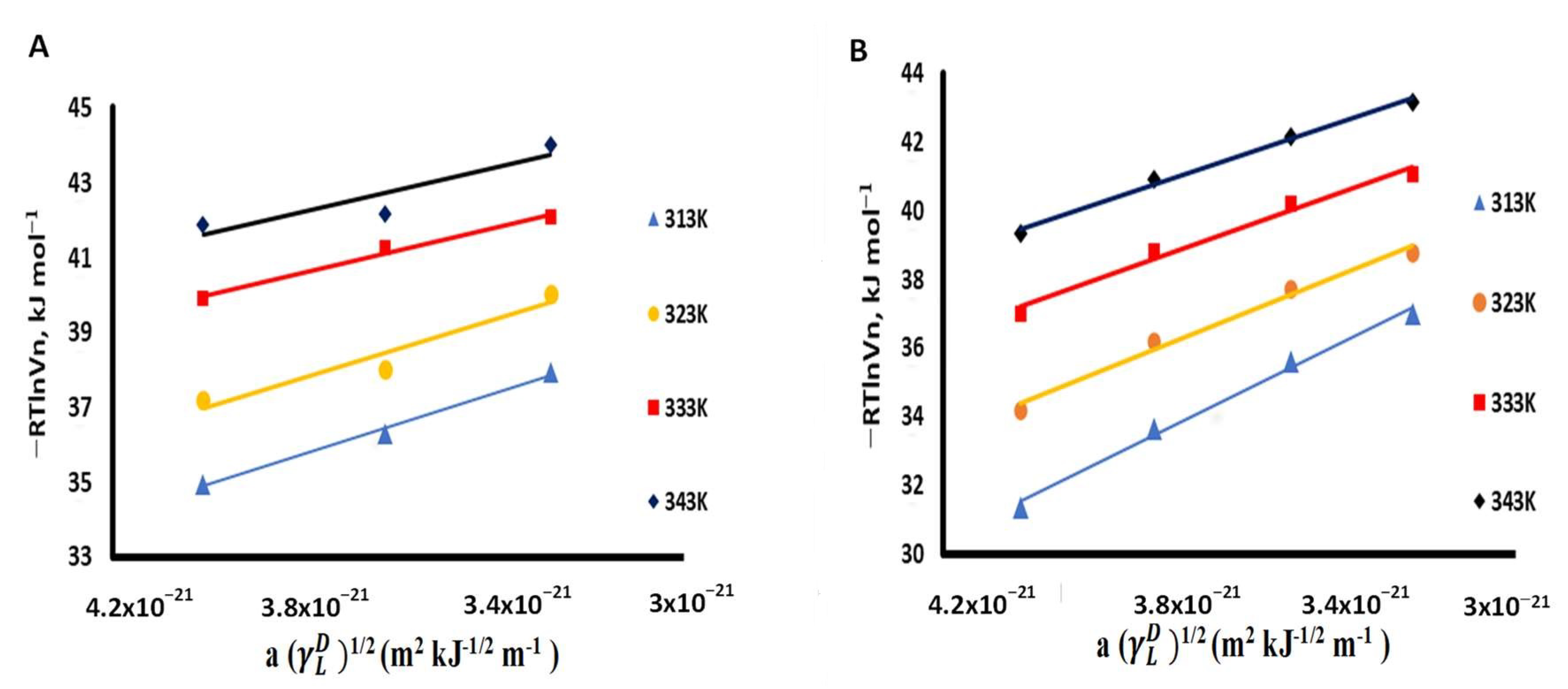
| Probe | a (Å2) | (mJ m−2) | 1/2 (m2 kJ−1/2 m−1) |
|---|---|---|---|
| n-nonane | 69 | 22.7 | 3.29 × 10−21 |
| n-decane | 75 | 23.4 | 3.63 × 10−21 |
| n-undecane | 81 | 24.6 | 4.01 × 10−21 |
| n-dodecane | 87 | 25.4 | 4.38 × 10−21 |
| Probe | −ΔGA (kJ mol−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| C@Chitosan-COL | Schiff’s-C-S | |||||||
| 313 K | 323 K | 333 K | 343 K | 313 K | 323 K | 333 K | 343 K | |
| n-Nonane | 6.49 | 7.20 | 7.94 | 8.78 | 9.68 | 10.80 | 11.38 | 12.09 |
| n-Decane | 5.42 | 6.59 | 6.18 | 8.09 | 8.40 | 9.20 | 9.65 | 10.57 |
| n-Undecane | 3.69 | 4.45 | 4.68 | 5.63 | 7.26 | 7.79 | 8.18 | 9.06 |
| n-Dodecane | - | - | - | - | 7.05 | 7.31 | 7.56 | 8.25 |
| Probe | −ΔHA (kJ mol−1) | |
|---|---|---|
| C@Chitosan-COL | Schiff’s-C-S | |
| 313–343 K | 313–343 K | |
| n-Nonane | 25.80 | 28.33 |
| n-Decane | 29.35 | 34.04 |
| n-Undecane | 38.58 | 42.99 |
| n-Dodecane | - | 52.73 |
| Probe | −ΔSA (J mol−1 K−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| C@Chitosan-COL | Schiff’s-C-S | |||||||
| 313 K | 323 K | 333 K | 343 K | 313 K | 323 K | 333 K | 343 K | |
| n-Nonane | 61.69 | 57.59 | 53.63 | 49.63 | 59.54 | 54.24 | 50.88 | 47.31 |
| n-Decane | 76.43 | 70.44 | 69.58 | 61.97 | 81.88 | 76.88 | 73.21 | 68.39 |
| n-Undecane | 111.47 | 105.68 | 101.79 | 96.07 | 114.14 | 108.97 | 133.79 | 98.92 |
| n-Dodecane | - | - | - | - | 145.94 | 140.61 | 135.65 | 129.68 |
| T (K) | (mJ m−2) | |
|---|---|---|
| C@Chitosan-COL | Schiff’s-C-S | |
| 313 | 11.41 | 18.206 |
| 323 | 10.41 | 12.071 |
| 333 | 6.26 | 9.410 |
| 343 | - | 8.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, A.S.; Yusuf, K.; Habila, M.A.; ALOthman, Z.A. Fabrication and Characterization of Sulfonated Carbon Materials and Chitosan-Derived Functioned Carbon via Schiff’s Base Process for Separation Purposes. Separations 2023, 10, 475. https://doi.org/10.3390/separations10090475
Alghamdi AS, Yusuf K, Habila MA, ALOthman ZA. Fabrication and Characterization of Sulfonated Carbon Materials and Chitosan-Derived Functioned Carbon via Schiff’s Base Process for Separation Purposes. Separations. 2023; 10(9):475. https://doi.org/10.3390/separations10090475
Chicago/Turabian StyleAlghamdi, Ali S., Kareem Yusuf, Mohamed A. Habila, and Zeid A. ALOthman. 2023. "Fabrication and Characterization of Sulfonated Carbon Materials and Chitosan-Derived Functioned Carbon via Schiff’s Base Process for Separation Purposes" Separations 10, no. 9: 475. https://doi.org/10.3390/separations10090475
APA StyleAlghamdi, A. S., Yusuf, K., Habila, M. A., & ALOthman, Z. A. (2023). Fabrication and Characterization of Sulfonated Carbon Materials and Chitosan-Derived Functioned Carbon via Schiff’s Base Process for Separation Purposes. Separations, 10(9), 475. https://doi.org/10.3390/separations10090475






