Abstract
This paper presents a process for efficiently recovering palladium (Pd) from spent Pd/Al2O3 catalysts used for hydrogenation reactions, using ultrasound-assisted leaching (UAL). A system composed of H2SO4 and NaCl was investigated under ultrasound-enhanced conditions and compared to regular leaching methods to demonstrate the superiority of UAL. Single-factor experiments were conducted to determine the optimal conditions for leaching, which included an ultrasound power of 200 W, a liquid–solid ratio of 5:1, a leaching time of 1 h, a leaching temperature of 60 °C, H2SO4 concentration of 60%, and 0.1 mol of NaCl. The leaching rate under these conditions was found to be 99%. Additionally, kinetic analysis of the UAL process showed that the apparent activation energy of the Pd leaching reaction was 28.7 kJ/mol, and it was found that Pd leaching from spent catalysts was controlled by diffusion. The tailings were analyzed by SEM, revealing that during ultrasonic leaching, the specific surface area of the spent catalyst increased, the mass transfer rate of the solution was accelerated, the passivation film on the surface of the spent catalyst was peeled off, and a new reaction interface was formed. This improved the leaching rate of Pd and provided a new approach to efficiently leach precious metals such as Pd from spent catalysts.
1. Introduction
Palladium (Pd) is a type of platinum group metal known for its exceptional catalytic activity, making it a valuable material for catalyst preparation across various fields [1,2,3,4,5]. As industrial demand for Pd continues to rise, it has become a critical component of many industrial applications [6,7,8,9,10,11]. However, after deactivation due to poisoning, pollution, or sintering in practical use, the catalyst becomes solid waste and is listed as a hazardous material on the National Hazardous Waste List [12,13]. In recent years, the volume of deactivated spent catalysts containing Pd has increased, and there is growing interest in the potential recovery of Pd from these materials [14,15,16,17]. Therefore, there is a need to explore efficient technologies for recovering precious metals such as Pd from spent catalysts.
There are currently two main methods used to recover Pd from failed catalysts: the fire method and the wet method. The fire method involves reduction melting, ash blowing, and refining. While this method is adaptable and has a straightforward process, it consumes a significant amount of energy, has a low recovery rate, and contributes to substantial pollution. Additionally, there is a considerable loss of Pd in the slag, and the purity of the final product falls short of reaching 99.99%. Therefore, the wet recovery process is often used to treat failed palladium-containing catalysts in industry, which encompasses various wet processes such as carrier dissolution, active component dissolution, and total dissolution methods. However, traditional hydrometallurgy suffers from low leaching efficiency, extended leaching time, and low metal recovery rates, leading to a significant waste of Pd resources. Therefore, exploring alternative methods such as ultrasonic-assisted leaching (UAL) is of great significance for improving the efficient recovery and utilization of Pd resources.
Ultrasonic waves can produce strong impact and cavitation at interfaces, capable of reaching temperatures up to 5000 K and pressure up to 101.3 MPa. The rate of temperature change can reach 10 K/s, and the intensity of ultrasonic waves surpasses that of ordinary sound waves. As a result, these waves exert a significant sound pressure effect on the medium particles. The macroscopic turbulence and high-speed impact caused by ultrasonic shock waves can reduce the thickness of the boundary layer, increase the mass transfer rate, and improve the leaching of metals. Furthermore, the energy characteristics of ultrasonic waves can influence the morphology, composition, structure, and chemical reactivity of the solid surface when interacting with solid–liquid interfaces. This property has undergone extensive exploration in the field of material preparation. Therefore, ultrasonic waves have the potential to enhance leaching performance in hydrometallurgy processes. Previous studies have investigated the use of UAL for extracting various metals from different sources. Chen et al. [18] showed that UAL could extract more than 90% of V from coke in a short time, owing to the significant reduction in contact angle and surface tension between the lixivium and coke. Zhang et al. [19] found that the Cu leaching rate of tailings was significantly increased by UAL with acids. According to prior research by Chang et al. [20], the application of UAL led to a notable increase in Ag removal efficiency and a significant reduction in desilverization time. Jiang and Li et al. [21,22] reported that ultrasound-assisted leaching improved the recovery of Co and Li from spent LIBs due to the unique cavitation effect of ultrasonic waves. Ultrasound-enhanced processes are not only efficient but also environmentally friendly. Yin et al. [23] reported that ultrasonic-enhanced leaching increased the total recovery of rare earth from 53.6% to 92.2% from rare earth ore. Meanwhile, Zhang et al. [24] studied the ultrasonic-assisted leaching of lead-rich antimony oxide slag with hydrochloric acid/sodium chloride and found that the leaching rate of ultrasonic-assisted antimony leaching for 15 min was similar to that of conventional leaching for 45 min, and the reaction time was shortened by 2/3. M. Marafi and Xue et al. [25,26] also showed that ultrasonic assistance could extract Mo, V, Ni, and other precious metals from spent hydrogenation catalyst and nickel sulfide concentrate, and the leaching rate was increased while the reaction time was shortened compared to conventional leaching. However, there is a lack of research on using ultrasonic waves to enhance the leaching of precious metals from spent catalysts, which is the focus of this study.
The aim of this study is to investigate the efficiency of UAL compared to conventional leaching and to determine the effects of ultrasonic power, leaching time, leaching temperature, H2SO4 concentration, and liquid–solid ratio on the leaching efficiency of Pd from spent catalysts. Furthermore, XRD and SEM-EDS were used to explain the interfacial reaction mechanism of UAL. Additionally, the leaching kinetics of Pd in sulfuric acid solution was analyzed based on the shrinkage kernel model, and the activation energy was calculated. These results offer a novel approach for efficiently leaching Pd from spent catalysts and a new idea for recycling solid waste.
2. Materials and Methods
2.1. Materials
The catalyst used in this study was obtained from a company in Xuzhou and is composed primarily of alumina and palladium, with palladium serving as the active catalytic component. The chemical composition of the catalyst is presented in Table 1, which shows that it consists of 99.23% alumina and 0.16% Pd, with few other impurities. All reagents used in the study were p.a. grade. Before commencing the leaching study, it is essential to subject the waste catalyst to pretreatment. The waste catalyst is placed quantitatively into a ceramic crucible to undergo grinding treatment. Following this, the waste catalyst, having a particle size of 80 mesh, is meticulously gathered and stored separately in a designated medicine bag for the purpose of conducting the leaching study.

Table 1.
The chemical composition of spent catalyst obtained from XRF data.
2.2. Experimental Procedure for Ultrasound-Assisted Leaching (UAL)
The experimental setup used for UAL involved a CLF-1A constant temperature magnetic agitator (Shanghai Lichen Bang xi Instrument Technology Co., Ltd., Shanghai, China). A 250 mL glass beaker was filled with a mixture of sulfuric acid and sodium chloride and heated to a predetermined temperature. Once the desired temperature was reached, a certain amount of waste catalyst containing Pd was added to the solution. An ultrasonic probe was positioned in the leaching solution, and ultrasonic waves with a frequency of 20 kHz were produced using an ultrasonic generator (kc-300W, Suzhou Kangzhou Electronics Co., Ltd., Suzhou, China) throughout the leaching experiments. The leaching process was carried out while simultaneously operating the ultrasonic generator and magnetic agitator. The experimental setup is illustrated in Figure 1. The reaction conditions, including leaching time, temperature, sulfuric acid concentration, liquid–solid ratio, and ultrasonic power, were carefully controlled. The reaction product was filtered, washed, and the Pd content in the filtrate was analyzed using an atomic absorption spectrophotometer (AAS). Each experiment was conducted in triplicate, and the average value was used as the final result. Pd recovery was calculated using the following equation:
where Y is the leaching efficiency of the metal (%); m0 and m1 represent the initial mass of the sample and the mass of the leaching residue, respectively (g); and ω0 and ω1 refer to the mass concentration of the metal in the sample before leaching and the leaching residue, respectively (%).
Y = (ω0 × m0 − ω1 × m1)/(ω0 × m0) × 100%
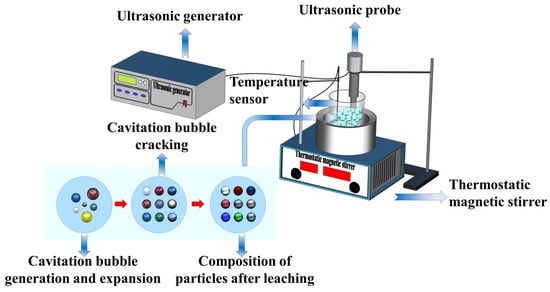
Figure 1.
Diagram illustrating the experimental setup for ultrasound-assisted leaching.
2.3. Analytical Methods
In order to analyze the leaching residue, a phase analysis and quantitative analysis were conducted using XRD (X’Pert PRO, Bruker, Karlsruhe, Germany) and XRF (ARL 9900, Suzhou, China). The micro-morphological characteristics of the solid residue were examined using SEM (Ultra55, Hitachi, Tokyo, Japan). To further investigate the chemical composition of the leached catalyst surface, EDS and XPS with a K-alpha source were utilized.
3. Results
3.1. Leaching Mechanism of Pd in H2SO4-NaCl System
The strong metal inertia of Pd makes its dissolution through acid alone challenging. This difficulty arises due to the weak affinity of hydrogen ions in acids for the external electrons of platinum group metal atoms. The electron affinity released upon electron capture is insufficient to overcome the energy required to break the metal bonds, thus preventing their rupture. However, the use of hot concentrated sulfuric acid, nitric acid, or other oxidizing acids in the presence of an oxidizing agent can facilitate the breakage of metallic bonds and the extraction of the outermost electrons from the platinum group metal atoms. A common approach to reduce the potential of the platinum group metal electrodes, improve oxidation capacity, and enhance solubility is to introduce appropriate complexing agents. These agents form complexes with platinum group metals, which have lower electrode potentials. For instance, the addition of chloride salts can stabilize Pd ions in solution by forming corresponding chlorine complexes. This stabilization mechanism enhances the solubility of Pd and contributes to improved dissolution characteristics. Hence, the H2SO4 + NaCl system was selected in this paper.
3.2. The Effect of Ultrasonic Power
Figure 2 illustrates the impact of ultrasonic power on the recovery rate of Pd. It can be observed that with the increase of ultrasonic power, the leaching rate of Pd exhibited a two-stage pattern of change. During the initial stage, the leaching rate of Pd rose sharply from 85.79% to 99% within the ultrasonic power range of 60 W to 200 W. This could be attributed to the mechanical effect of ultrasonic energy, which promotes the dispersion of solids, enhances the convective movement, and promotes material exchange between solids and liquids. In addition, ultrasonic waves can remove surface impurities, thereby improving the surface contact area between Pd metal and sulfuric acid molecules. Moreover, the cavitation effect and mechanical effect of ultrasonic were enhanced as the ultrasonic power increased, leading to an increased leaching rate of Pd metal. In the second stage, the leaching rate showed a slight decrease when the ultrasonic power exceeded 200 W, possibly due to a disturbance of the reaction and a weakening of the reaction due to a coalescence of bubbles produced by cavitation at high ultrasonic power. Therefore, 200 W was found to be the optimal ultrasonic power for leaching in our experiment.
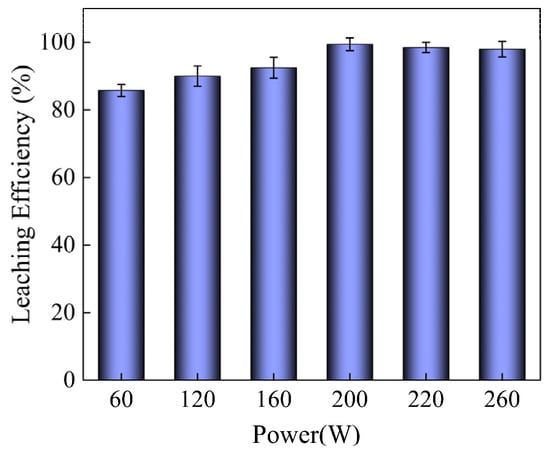
Figure 2.
The influence of ultrasonic power on Pd leaching under optimal conditions of ω(H2SO4) = 60%, liquid/solid ratio = 5:1, T = 60 °C, and time = 1 h.
3.3. Comparison between the Conventional and Ultrasound-Assisted Leaching
- a.
- The effect of H2SO4 concentration
The leaching solution’s concentration is a crucial factor affecting the leaching rate. As depicted in Figure 3a, there is a considerable increase in Pd recovery when the sulfuric acid concentration is increased from 40% to 60% for both the UAL and conventional leaching processes. However, when the concentration of sulfuric acid exceeds 60%, the leaching rate of Pd remains mostly unchanged. During the leaching process of Pd in the spent catalyst, the spent catalyst particles are continually eroded by sulfuric acid molecules, and the soluble mineral phase dissolves in the form of metal ions. Ultrasonic cavitation enhances Pd leaching efficiency, making it higher than conventional leaching. Acoustic cavitation phenomena occur when ultrasonic power is applied, creating high-speed micro-jets and high-pressure shock waves, mainly at the liquid–solid interface between the solution and palladium-containing spent catalyst. This promotes mass transfer between solid and liquid, particle diffusion, and uniformity of the leaching solution [27]. Therefore, a 60% sulfuric acid concentration is optimal.
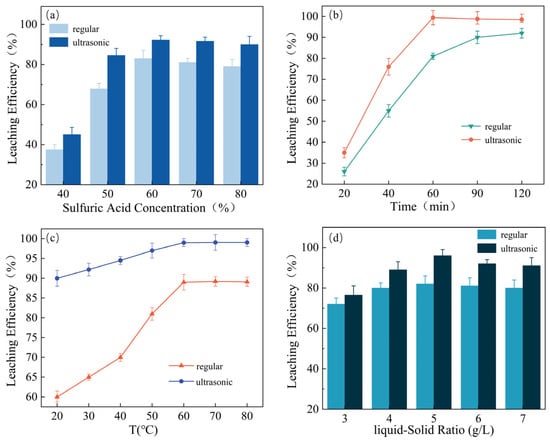
Figure 3.
The effects of H2SO4 concentration (a), time (b), temperature (c), and liquid–solid ratio (d) on the leaching rate of Pd. Each experimental variable was optimized while keeping the others constant at: 60 °C, 1 h, 60% H2SO4, and L/S = 5:1. All experiments were performed under 200 W ultrasonic power.
- b.
- The effect of leaching time
Figure 3b shows the effect of reaction time on the leaching rate of Pd under ultrasonic and conventional conditions. The results show that ultrasonic leaching significantly improves the Pd extraction rate compared to conventional leaching. Specifically, the leaching rate of Pd can reach up to 99% after 60 min of ultrasonic leaching, which is even higher than the conventional leaching rate after 120 min. Moreover, no significant improvement in the extraction rate was observed beyond 60 min of ultrasonic leaching. The reduced reaction time by half and the increased extraction rate of Pd can be attributed to the energy generated by ultrasonic vibration [28]. Ultrasonic waves cause a rapid rupture of the bubbles produced by the two opposite reactions, which enhances the appearance of separation and the diffusion rate of soluble substances in the liquid phase. Consequently, the liquid–solid contact interface is regenerated, the mass transfer efficiency improved, and the dissolution rate of Pd accelerated. Based on these results, the reaction time of 60 min was chosen as the experimental condition for subsequent experiments.
- c.
- The effect of leaching temperature
Increasing the system temperature during the leaching reaction effectively reduces the binding of mineral particles and enhances molecular diffusion [29]. In Figure 3c, we can see that the ultrasonic treatment resulted in a higher leaching rate of Pd compared to regular leaching under the same experimental conditions. Both leaching methods showed an increase in Pd leaching rate with increasing temperature. At 60 °C, the leaching rate of Pd under ultrasonic treatment was higher than regular leaching (99.12% and 89.09%, respectively). Interestingly, the leaching rate at 30 °C under ultrasonic conditions was even higher than that at 60 °C under conventional conditions. This could be due to the low heat transfer efficiency and utilization rate between the two phases at low temperatures. Ultrasonic treatment reduces thermal resistance and improves heat transfer efficiency [30]. However, the leaching efficacy remained relatively stable beyond 60 °C, suggesting that the reaction was not primarily influenced by chemical kinetics. The rise in leaching efficacy attributed to ultrasonic waves could be attributed to the expedited diffusion of reactants.
- d.
- The effect of liquid–solid ratio
The efficiency of ultrasonic cavitation is influenced by the solid content in the solution [31]. We therefore investigated the effect of the liquid–solid ratio on the leaching rate of Pd in our experiment. As shown in Figure 3d, the leaching rate of Pd increased continuously as the liquid–solid ratio increased. This is because a higher liquid–solid ratio reduces the viscosity of the pulp, improves the diffusion conditions, and ensures complete contact between the liquid–solid phases, resulting in a higher leaching rate of Pd. Moreover, higher liquid–solid ratios provide more H+ ions, which can facilitate the reaction process. When the liquid–solid ratio was 5, ultrasonic-assisted leaching yielded a 14% higher Pd extraction rate than conventional leaching. This suggests that an appropriate liquid–solid ratio and ultrasonic treatment promote the formation of nucleation centers at the liquid–solid interface between the Pd-containing solid catalyst and the leaching solution, leading to a significant enhancement in the hollow effect. No significant increase in the Pd extraction rate was observed for either leaching methods when the liquid–solid ratio exceeded 5, likely because Pd in the spent catalyst was already completely leached. Therefore, a liquid–solid ratio of 5:1 is optimal for the leaching of Pd from the spent catalyst.
3.4. Kinetic Analysis of the Leaching Process
The Pd leaching from the spent catalyst is a heterogeneous process occurring at the solid–liquid interface. The rate of heterogeneous reaction can be governed by either chemical reaction or diffusion. To analyze the kinetic parameters, the kinetic model of the shrinking core was utilized. The shrinking core model mathematically explains the mechanism of solid-state reactions, in which a chemical reaction gradually consumes a solid material, and the progress of the reaction happens through the diffusion of the reactants across a shrinking core surrounding the unreacted material. The chemical reaction and the diffusion reaction that occur simultaneously can be described by Equations (2) and (3), respectively:
where X is leaching efficiency (%); kc refers to the chemical reaction rate constant (min−1); t is the leaching time (min); and kd represents the diffusion reaction rate constant (min−1).
In any process with multiple steps happening in parallel, the slowest step determines the overall rate of the process. In this study, the data of UAL at various temperatures and times were fitted to Equations (2) and (3). The results are shown in Figure 4, revealing that the kinetic data supports the suitability of the product layer diffusion control model for Pd leaching in Ultrasonic-Assisted Leaching (UAL) systems, as opposed to the chemical reaction control model. The linear correlation coefficient for the chemical reaction control model was found to be below 0.98, while the correlation coefficient for the internal diffusion control model exceeded 0.99. These results indicate that the rate-limiting step in the leaching reaction rate is governed by diffusion.
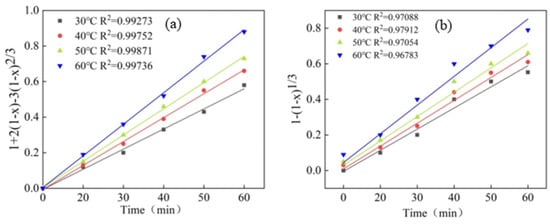
Figure 4.
The plots of vs. Time (a) and vs. Time (b) for the leaching of Pd from the spent catalyst at different temperatures. The rate constant, kd, can be determined from the slope of the trendline.
In order to further validate the effectiveness of the Pd leaching process under ultrasonic enhancement and diffusion control in a sulfuric acid system, the experimental data were substituted into Equations (2) and (3). The fitting results then were compared with those in Figure 5a,b. The linear correlation coefficient R2 for the diffusion control model surpassed that of the chemical reaction control model, with the fitting line in Figure 5a passing through the origin. Conversely, the fitting lines in Figure 5b deviate from the origin, which aligns with the diffusion control model.
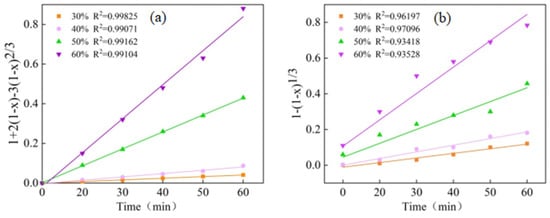
Figure 5.
The plots of and vs. time for the leaching of Pd from the spent catalyst at different H2SO4 concentrations. The rate constant, kd, can be determined from the slope of the trendline.
The Arrhenius equation was used to determine the activation energy of the leaching process:
where k denotes the rate constant (min−1); A represents the frequency parameter (min−1); Ea stands for the activation energy of the process (kJ/mol); and R denotes the universal gas constant (8.3145 J∙K−1∙mol−1).
lnk = lnA − Ea/RT
The dependence of lnk vs. 1000/T is presented in Figure 6, which shows that the activation energy for Pd leaching is 28.7 kJ/mol. The reaction is controlled by diffusion when the activation energy is less than 40 kJ/mol [32]. In this regard, Dong [33] also reported that the leaching process of silver containing waste catalyst was diffusion-controlled, and the activation energy was 38.83 kJ/mol. By considering the fitting coefficient and activation energy of R2, it can be concluded that the ultrasonic palladium leaching process is mainly controlled by diffusion. Ultrasonic stirring reduces the thickness of the diffusion layer, thereby increasing the diffusion rate and promoting the chemical reaction. Moreover, ultrasound generates high temperatures and pressures locally inside the solvent, which improves the reaction equilibrium constant [34]. Additionally, shock waves and micro-jets created by ultrasound break the surface of the catalyst, reducing the Ea of the reaction and improving the leaching efficiency of Pd [35]. Overall, ultrasonic power significantly enhances the leaching effect of Pd.
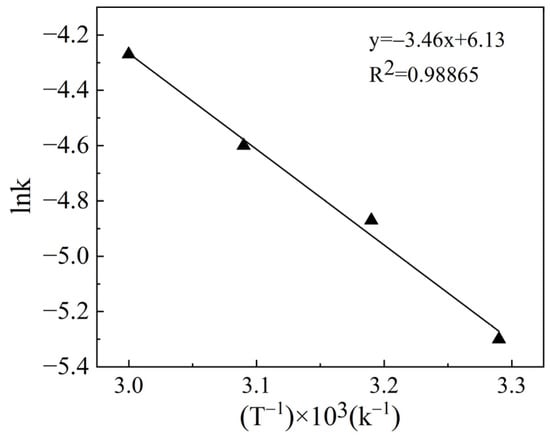
Figure 6.
The plot of lnk vs. 1/T for the Pd leaching rate from the spent catalyst.
3.5. Mechanism of Ultrasonic-Enhanced Leaching
The ultrasonic enhanced leaching process relies on the mechanical and thermal effects produced by ultrasonic cavitation. When ultrasonic waves propagate through a liquid, the liquid molecules experience periodic alternating sound fields. During the rarefaction phase of the acoustic waves, the liquid is subjected to tensile stress, leading to the formation of local negative pressure areas. Once the sound pressure reaches a sufficient level, the liquid or solid–liquid interface can rupture, resulting in the formation of small cavitation bubbles. In the compression phase of the sound waves, cavitation bubbles, created by the compressive stress of the liquid, rapidly collapse, generating localized high temperature and pressure. This phenomenon, known as ultrasonic cavitation, involves the formation, growth, enlargement, and collapse of these small bubbles over time [36]. The cavitation effect significantly influences the leaching process in the following ways: (a) Ultrasonic waves exhibit a pronounced skin effect on the solid surface. Cavitation-induced forces remove impurities that adhere to the surface of particles. Additionally, the strong micro-jets resulting from cavitation impact the solid surface, promoting the rupture of cracks and the refinement of particles. This process creates new reaction interfaces. (b) In the solid–liquid reaction systems, ultrasonic cavitation strongly stirs the solvent, enhancing the cross-phase diffusion transfer between the solid and liquid phases. This intensified mixing reduces the resistance to solute diffusion, ensures a higher degree of uniformity in the reaction solution, and improves the control of diffusion and reaction rates in the solid–liquid leaching process. In this study, Pd was coated with an alumina carrier, preventing contact with the sulfuric acid solution in conventional leaching conditions. Cavitation induced high temperatures and pressures in a short time, creating microcracks on the solid surface, destroying the reactant surface, promoting the rate of diffusion and interfacial reactions at the liquid–solid boundary, and increasing the dissolution rate of Pd. This is depicted in Figure 7.
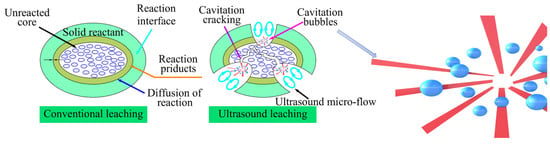
Figure 7.
The mechanism of ultrasound-enhanced leaching.
The XRD pattern of the ultrasonic leaching slag system is presented in Figure 8. The absence of characteristic peaks of Pd indicates that most of the Pd has leached into the solution. Only the presence of Al2O3 is observed, with no impurities such as sulfate, indicating the high resistance of α-Al2O3 to strong acids. High-purity alumina can be obtained by washing the product three times, which is a critical material for integrated circuits, ceramic sensors, aviation optics, and corrosion-resistant alumina ceramics [37]. Moreover, synthetic sapphire and ruby can also be produced using high-purity alumina. Therefore, the recovery of precious metals from spent catalysts not only prevents environmental pollution but also promotes resource recovery and utilization, leading to significant environmental and economic benefits.
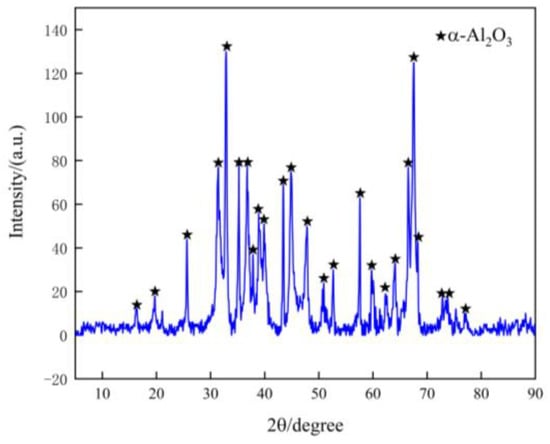
Figure 8.
XRD pattern of slag obtained after ultrasound-enhanced leaching of Pd.
The SEM-EDS analysis was carried out to investigate the effect of ultrasonic and conventional leaching on microstructure changes. Figure 9 illustrates the differences in the microstructure of leached slag between conventional and UAL under optimal conditions. The particles of conventional leaching slag are large and disorderly distributed. Hard and compact inclusions are formed on the surface, and the aggregation phenomenon can be observed. In contrast, the particle size of ultrasonic leaching slag is smaller, and the shear stress generated on the surface of solid particles causes material breakage and inclusion opening, which accelerates the mass transfer process and destroys grain growth, thus promoting the liquid–solid reaction.
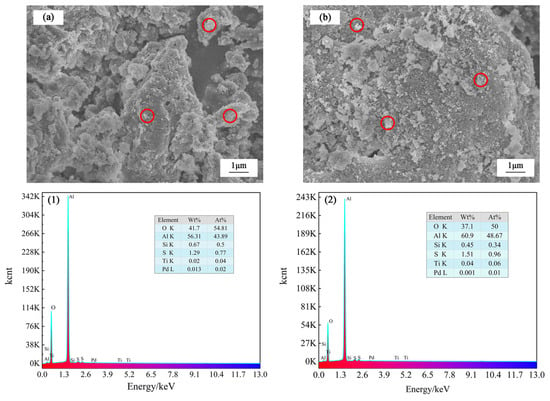
Figure 9.
SEM images of slag obtained after (a) conventional leaching, and (b) UAL. EDS images of slag obtained after (1) conventional leaching, and (2) UAL.
4. Conclusions
In conclusion, this research aimed to develop a process for the high-efficiency recovery of Pd from spent catalysts using ultrasound. The optimal conditions were found to be an ultrasonic power of 200 W, liquid–solid ratio of 5:1, leaching time of 1 h, leaching temperature of 60 °C, H2SO4 concentration of 60%, and 0.1 moles of NaCl, which resulted in 99% Pd leaching. Under the same experimental conditions, ultrasound-assisted leaching reduced the leaching time by half and increased the leaching rate by over 10% compared to conventional methods. The kinetic contraction kernel model confirmed that the leaching of Pd was controlled by diffusion with an activation energy of 28.7 kJ/mol. XRD and SEM-EDS analysis revealed that ultrasound opened the alumina coating, increased the contact area between Pd metal and H+, promoted the formation of a new reaction interface, and reduced the resistance of liquid–solid mass transfer, facilitating the dissolution and diffusion of Pd ions.
Overall, this research demonstrated that ultrasound-assisted leaching is an effective and efficient method for the recovery of Pd from spent catalysts. This process not only prevents environmental pollution but also realizes resource recovery and utilization, leading to significant environmental and economic benefits. Further studies could investigate the potential of this method for the recovery of other precious metals and explore its scalability for industrial applications.
Author Contributions
Methodology, J.W. and X.Z.; software, J.F.; validation, K.X.; formal analysis, S.M. and Q.G.; investigation, R.Z. and H.W.; resources, J.W.; data curation, J.W.; writing—original draft preparation, X.Z.; writing—review and editing, X.Z. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received the financial support from the National Key R&D Program (No: 2019YFC1907504), Liaoning Educational Committee General Program (LJKZ0166).
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
The authors thank the members of the research team for all their help in the process of testing, research, and thesis writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xie, X.; Liu, G.Q.; Zhang, F. Research on Recovery of Palladium from the Spent Pd-Al2O3 Catalyst. Compr. Util. Resour. China 2020, 38, 22–24. [Google Scholar]
- Ding, Y.J.; Zhang, S.G. Status and research progress on recovery of platinum group metals from spent catalysts. J. Eng. Sci. 2020, 42, 257–269. [Google Scholar]
- Dong, H.; Zhao, J.; Chen, J. Recovery of platinum group metals from spent catalysts: A review. Int. J. Miner. Process. 2015, 145, 108–113. [Google Scholar] [CrossRef]
- Paiva, A.P.; Ortet, O.; Carvalho, G.I. Recovery of palladium from a spent industrial catalyst through leaching and solvent extraction. Hydrometallurgy 2017, 171, 394–401. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sezaki, Y.; Hata, Y. Selective Recovery of Platinum (IV) from HCl Solutions Using 2-Ethylhexylamine as a Precipitant. Separations 2021, 8, 40. [Google Scholar] [CrossRef]
- De Corte, S.; Hennebel, T.; De Gusseme, B.; Verstraete, W.; Boon, N. Bio-palladium: From metal recovery to catalytic applications. Microb. Biotechnol. 2012, 5, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Gürsel, I.V.; Noël, T.; Wang, Q. Separation/recycling methods for homogeneous transition metal catalysts in continuous flow. Green Chem. 2015, 17, 2012–2026. [Google Scholar] [CrossRef]
- Molnár, Á.; Papp, A. Catalyst recycling—A survey of recent progress and current status. Coord. Chem. Rev. 2017, 349, 1–65. [Google Scholar] [CrossRef]
- Chen, J.P.; Wang, H.B.; Gong, W.X. Progress of Platinum Group Metals Recovery from Spent Catalystsin Petroleum and Chemical industries. Compr. Util. Resour. China 2017, 35, 69–71. [Google Scholar]
- Wang, L.; Chen, M. Policies and perspective on end-of-life vehicles in China. J. Clean. Prod. 2013, 44, 168–176. [Google Scholar] [CrossRef]
- Ueda, Y.; Morisada, S.; Kawakita, H. Selective Extraction of Platinum (IV) from the Simulated Secondary Resources Using Simple Secondary Amide and Urea Extractants. Separations 2021, 8, 139. [Google Scholar] [CrossRef]
- Fu, H.; Chen, Y.; Liu, T. Research on Hazardous Waste Removal Management: Identification of the Hazardous Characteristics of Fluid Catalytic Cracking Spent Catalysts. Molecules 2021, 26, 2289. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.N.; Xu, S.S.; Guo, S.K. National Hazardous Waste List. Shanghai Build. Mater. 2016, 194, 1–11. [Google Scholar]
- De Aberasturi, D.J.; Pinedo, R.; De Larramendi, I.R. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 505–513. [Google Scholar] [CrossRef]
- Ding, Y.J. An efficient leaching of palladium from spent catalysts through oxidation with Fe (III). Materials 2019, 12, 1205. [Google Scholar] [CrossRef]
- Hang, L.G. An integrated capture of copper scrap and electrodeposition process to enrich and prepare pure palladium for recycling of spent catalyst from automobile. Waste Manag. 2020, 108, 172–182. [Google Scholar]
- Chiang, K.C.; Chen, K.L.; Chen, C.Y. Recovery of spent alumina-supported platinum catalyst and reduction of platinum oxide via plasma sintering technique. J. Taiwan Inst. Chem. Eng. 2011, 42, 158–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Chu, W. Removal of vanadium from petroleum coke by microwave and ultrasonic-assisted leaching. Hydrometallurgy 2020, 191, 105–168. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, A.X.; Wang, Y.M. Experimental research in leaching of copper-bearing tailings enhanced by ultrasonic treatment. J. China Univ. Min. Technol. 2008, 18, 98–102. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, E.; Zhang, L. A comparison of ultrasound-augmented and conventional leaching of silver from sintering dust using acidic thiourea. Ultrason. Sonochem. 2017, 34, 222–231. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, Y.; Ju, S. Ultrasound-assisted leaching of cobalt and lithium from spent lithium-ion batteries. Ultrason. Sonochem. 2018, 48, 88–95. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Yin, S.; Pei, J.; Jiang, F. Ultrasound-assisted leaching of rare earths from the weathered crust elution-deposited ore using magnesium sulfate without ammonia-nitrogen pollution. Ultrason. Sonochem. 2018, 41, 156–162. [Google Scholar] [CrossRef]
- Zhang, R.L.; Zhang, X.F.; Tang, S.Z. Ultrasound-assisted HCl-NaCl leaching of lead-rich and antimony-rich oxidizing slag. Ultrason. Sonochem. 2015, 27, 187–191. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Waste catalyst utilization: Extraction of valuable metals from spent hydroprocessing catalysts by ultrasonic-assisted leaching with acids. Ind. Eng. Chem. Res. 2011, 50, 9495–9501. [Google Scholar] [CrossRef]
- Juanqin, X.U.E.; Xi, L.U.; Yewei, D.U. Ultrasonic-assisted oxidation leaching of nickel sulfide concentrate. Chin. J. Chem. Eng. 2010, 18, 948–953. [Google Scholar]
- Zhang, K.H.; Li, B.; Wu, Y.F. Recycling of indium from waste LCD: A promising noncrushing leaching with the aid of ultrasonicwave. Waste Manag. 2017, 64, 236–243. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Bhanvase, B.A.; Sonawane, S.H.; Gogate, P.R.; Pandit, A. A novel approach for continuous synthesis of calcium carbonate using sequential operation of two sonochemical reactors. Ultrason. Sonochem. 2017, 35, 124–133. [Google Scholar] [CrossRef]
- Zhang, L.B.; Guo, W.; Peng, J.H. Comparison of ultrasonic-assisted and regular leaching of germanium from by-product of zinc metallurgy. Ultrason. Sonochem. 2016, 31, 143–149. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Gao, H. Comparison of ultrasound-assisted and regular leaching of vanadium and chromium from roasted high chromium vanadium slag. JOM 2018, 70, 155–160. [Google Scholar] [CrossRef]
- Yan, J.W.; Pan, D.A.; Li, B. Application of ultrasonic enhancement in wet leaching process. Chin. J. Process Eng. 2020, 20, 1241–1247. [Google Scholar]
- Zhang, J.X.; Zou, X.; Niu, F.S. Kinetics Research of Sulfuric Acid Leaching from Blast Furnace Flue Dust Containing Zinc. Met. Mines 2017, 6, 80–84. [Google Scholar]
- Dong, X.L. Study on the Clean Leaching Process from Ag-Contained and the Regeneration of the Leaching Reagent Ce(IV). Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2018. [Google Scholar]
- Li, H.; Li, S.; Srinivasakannan, C. Efficient cleaning extraction of silver from spent symbiosis lead-zinc mine assisted by ultrasound in sodium thiosulfate system. Ultrason. Sonochem. 2018, 49, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Luque-Garcıa, J.L.; De Castro, M.D.L. Ultrasound: A powerful tool for leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Fu, L. Mechanism and kinetics analysis of valuable metals leaching from copper-cadmium slag assisted by ultrasound cavitation. J. Clean. Prod. 2022, 379, 134775. [Google Scholar] [CrossRef]
- Ma, R.J.; Ma, Y.W. Recycling Utilization of Secondary Resource Metals in Circular Economy. Min. Metall. Eng. 2014, 34, 68–72. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).