Detection of Periodontal Pathogens Based on an Integrated Continuous Flow PCR and Capillary Electrophoresis Microfluidic Chip
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of the PCR Solution
2.3. Fabrication of CF-PCR-MCE Microfluidic Chip
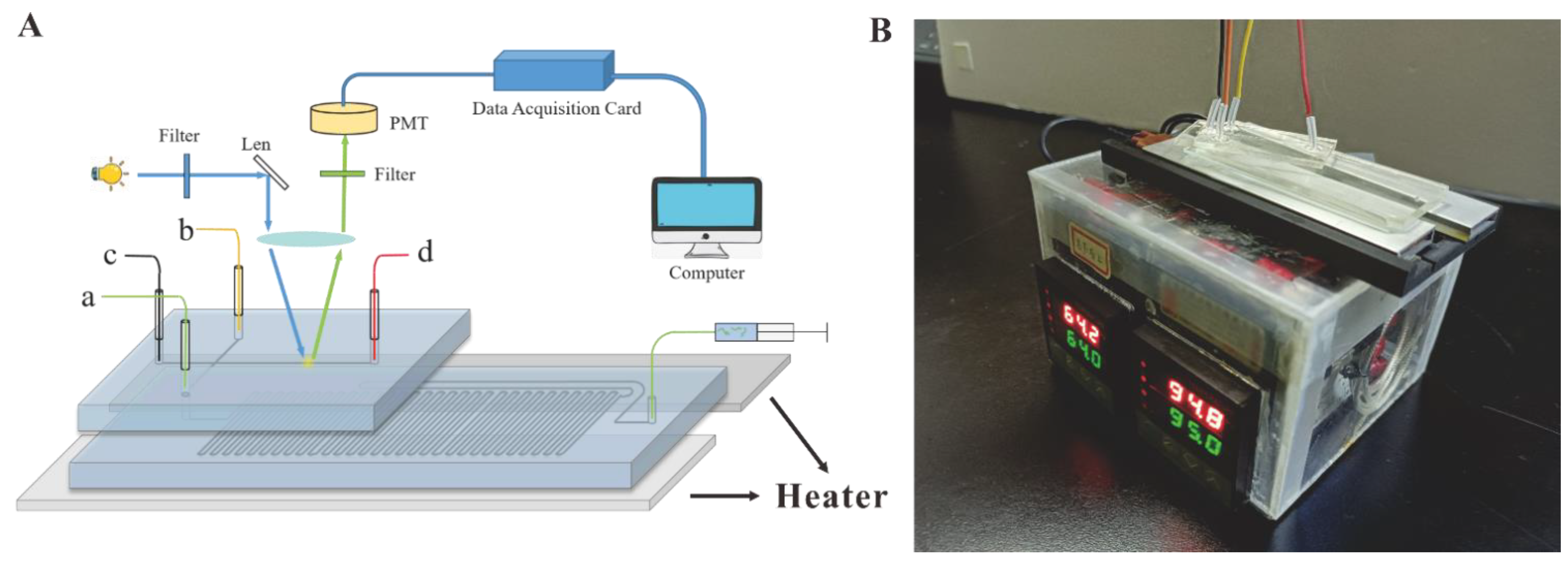
2.4. Construction of the CF-PCR-MCE System
2.5. The Self-Built Capillary Electrophoresis System
2.6. Experimental Protocol for Coating the Microchannels
3. Results and Discussion
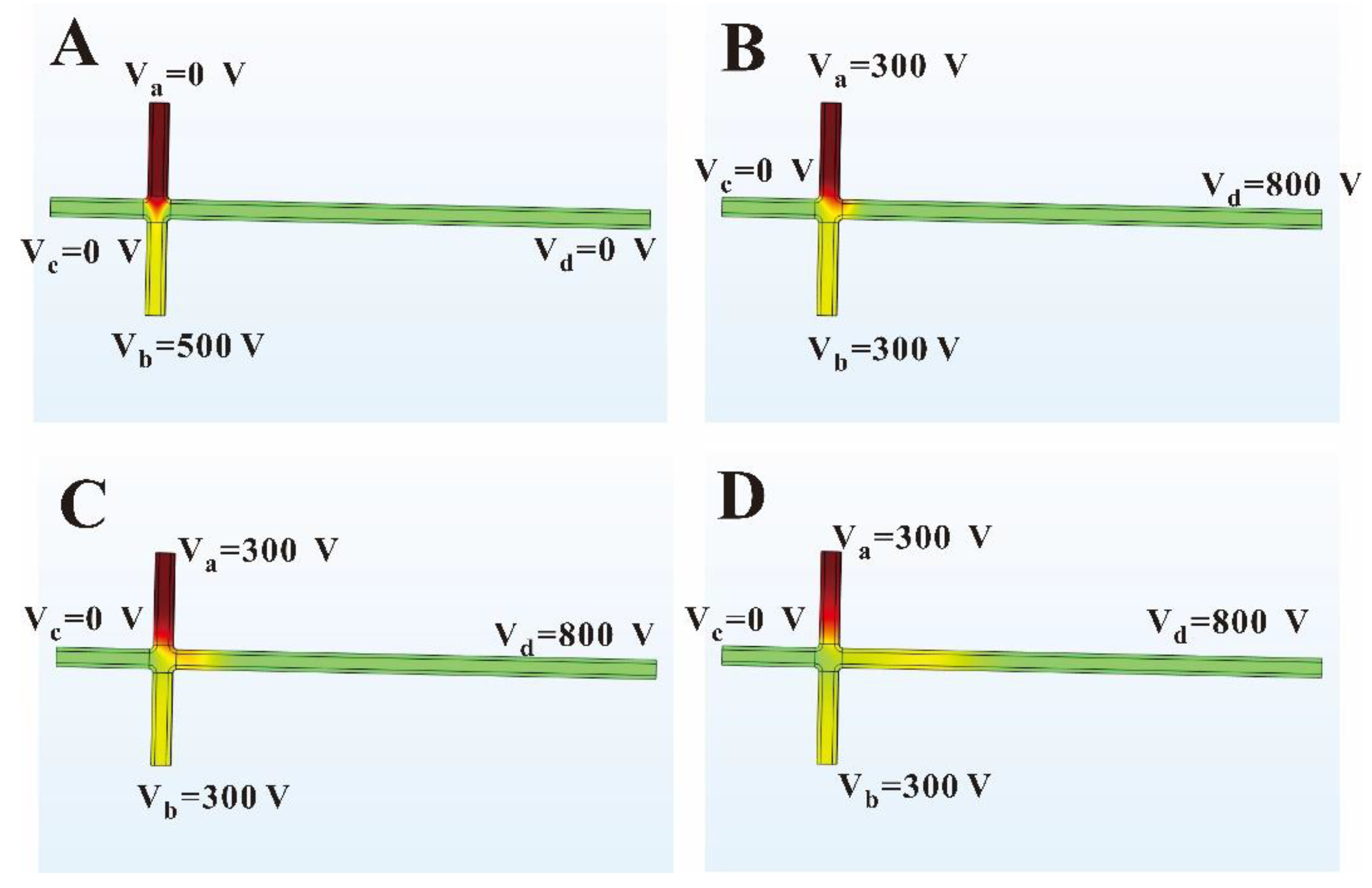
3.1. Simulation of Sample Flow State
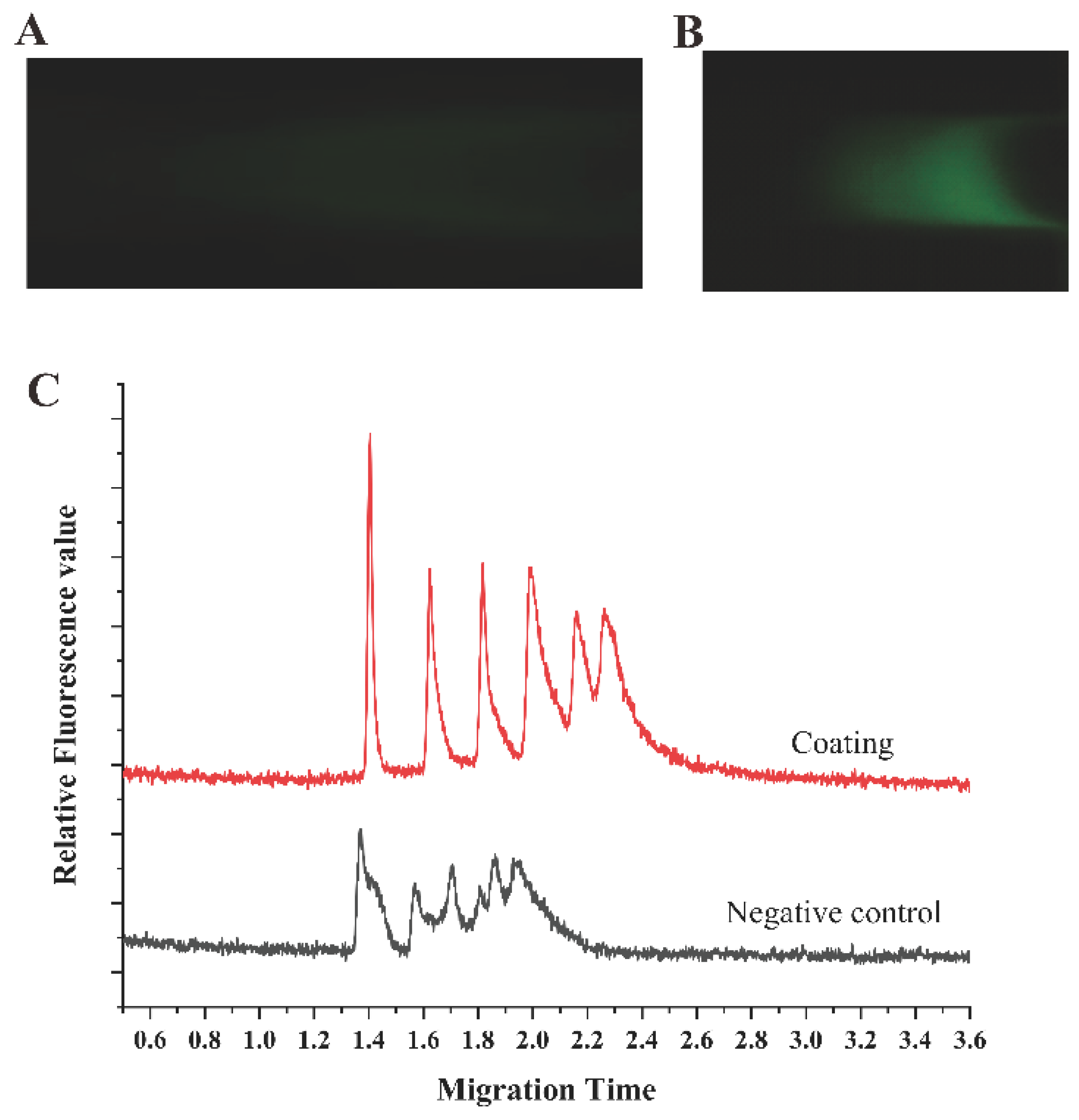
3.2. The Impact of Coating on Electrophoresis
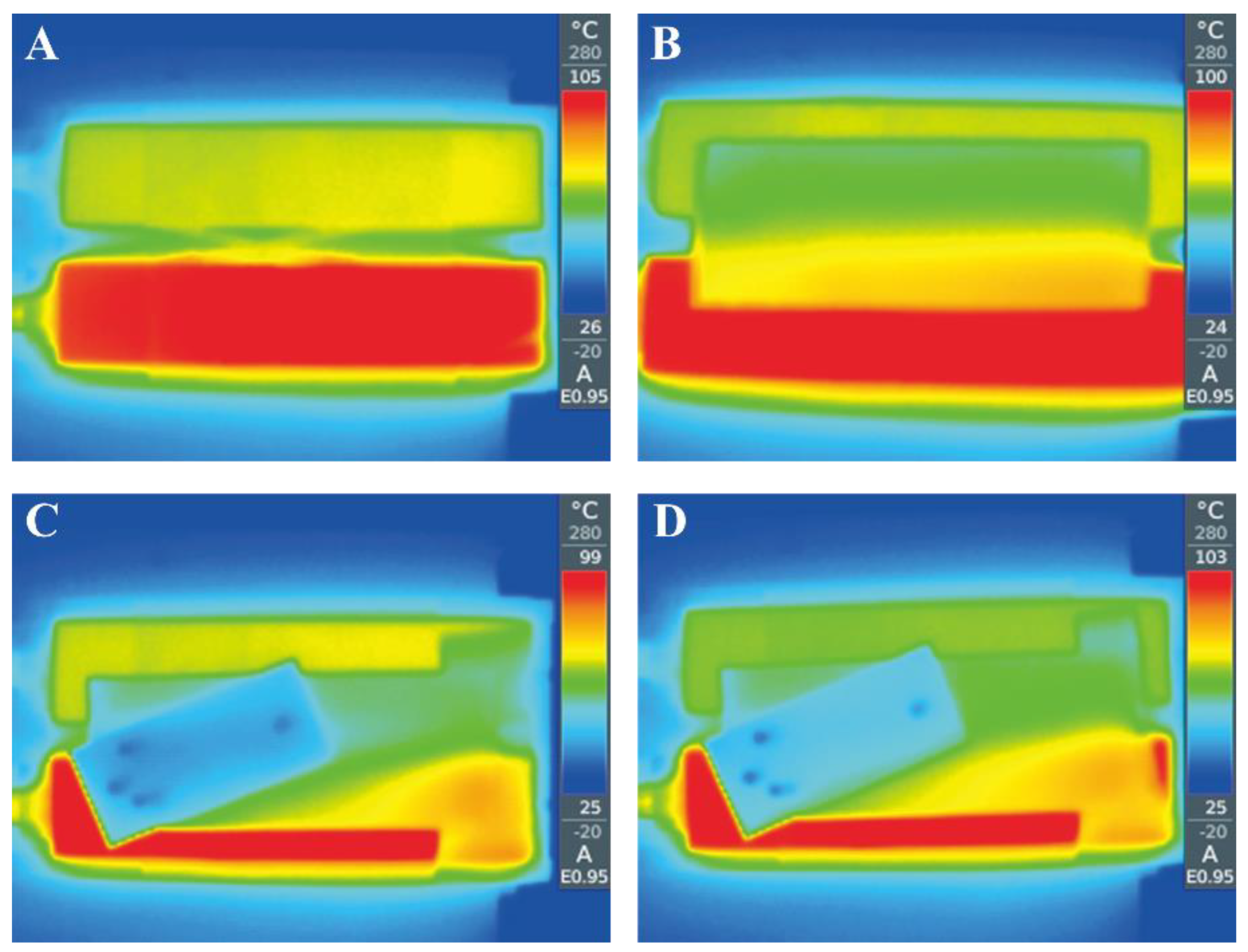
3.3. Temprerature Distribution in the CF-PCR-MCE Chip
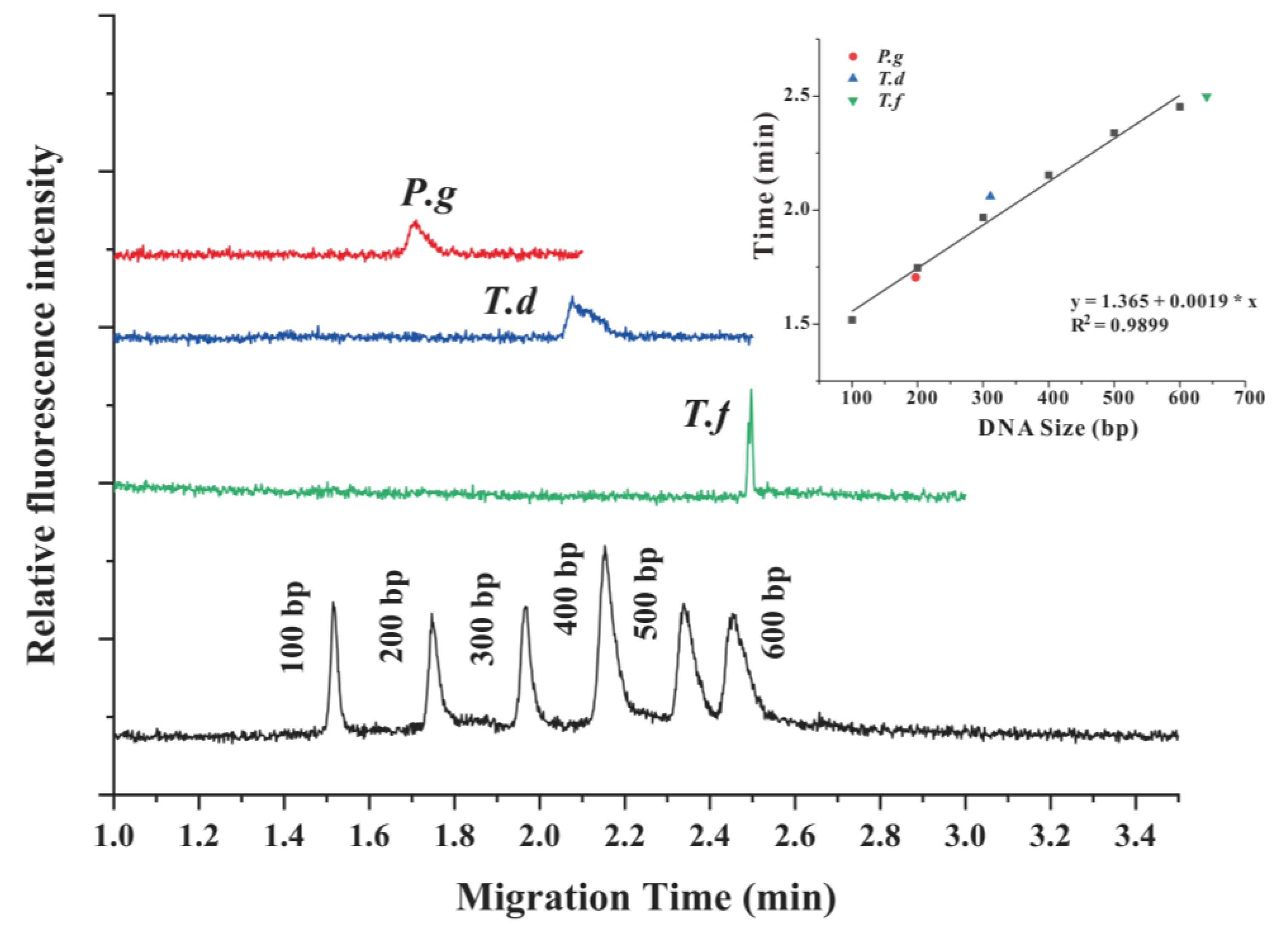
3.4. Amplification of P.g, T.d, and T.f in the CF-PCR-MCE System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mullis, K.; Faloona, F.; Scharf, S.; Saiki, R.; Horn, G.; Erlich, H. Specific enzymatic amplification of DNA in vitro: The polymerase chain reaction. Cold Spring Harb. Symp. Quant. Biol. 1986, 51, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hong, H.; Yoon, S.H. Diagnostic Performance of CT and Reverse Transcriptase Polymerase Chain Reaction for Coronavirus Disease 2019: A Meta-Analysis. Radiology 2020, 296, E145–E155. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, R.; Aherfi, S.; Wurtz, N.; Grimaldier, C.; Van Hoang, T.; Colson, P.; Raoult, D.; La Scola, B. Correlation between 3790 quantitative polymerase chain reaction–positives samples and positive cell cultures, including 1941 severe acute respiratory syndrome coronavirus 2 isolates. Clin. Infect. Dis. 2021, 72, e921. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.U.; de Mello, A.J.; Manz, A. Chemical Amplification: Continuous-Flow PCR on a Chip. Science 1998, 280, 1046–1048. [Google Scholar] [CrossRef]
- Qi, H.; Wang, X.; Chen, T.; Ma, X.; Zuo, T. Fabrication and characterization of a polymethyl methacrylate continuous-flow PCR microfluidic chip using CO2 laser ablation. Microsyst. Technol. 2009, 15, 1027–1030. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Lee, N.Y. Glass-polytetrafluoroethylene-glass based sandwich microdevice for continuous-flow polymerase chain reaction and its application for fast identification of foodborne pathogens. Talanta 2018, 176, 544–550. [Google Scholar] [CrossRef]
- Li, Z.; Ju, R.; Sekine, S.; Zhang, D.; Zhuang, S.; Yamaguchi, Y. All-in-one microfluidic device for on-site diagnosis of pathogens based on an integrated continuous flow PCR and electrophoresis biochip. Lab Chip 2019, 19, 2663–2668. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Wang, P.; Tao, C.; Zheng, L.; Sekine, S.; Zhuang, S.; Zhang, D.; Yamaguchi, Y. Multiplex amplification of target genes of periodontal pathogens in continuous flow PCR microfluidic chip. Lab Chip 2021, 21, 3159–3164. [Google Scholar] [CrossRef]
- Yang, B.; Wang, P.; Li, Z.; Tao, C.; You, Q.; Sekine, S.; Zhuang, S.; Zhang, D.; Yamaguchi, Y. A continuous flow PCR array microfluidic chip applied for simultaneous amplification of target genes of periodontal pathogens. Lab Chip 2021, 22, 733–737. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Gao, Z.; Sekine, S.; You, Q.; Zhuang, S.; Zhang, D.; Feng, S.; Yamaguchi, Y. Lower fluidic resistance of double-layer droplet continuous flow PCR microfluidic chip for rapid detection of bacteria. Anal. Chim. Acta 2023, 1251, 340995. [Google Scholar] [CrossRef]
- Jiang, X.; Shao, N.; Jing, W.; Tao, S.; Liu, S.; Sui, G. Microfluidic chip integrating high throughput continuous-flow PCR and DNA hybridization for bacteria analysis. Talanta 2014, 122, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Chen, P.; Huang, X.; Li, S.; Liu, B. Microfluidic chip electrophoresis for biochemical analysis. J. Sep. Sci. 2019, 43, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, S.A.; Alshabi, A.M.; Shaikh, I.A.; Orabi, M.A.A.; Abdel-Wahab, B.A.; Walbi, I.A.; Habeeb, M.S.; Khateeb, M.M.; Shettar, A.K.; Hoskeri, J.H. In Vitro Cytotoxicity and Spectral Analysis-Based Phytochemical Profiling of Methanol Extract of Barleria hochstetteri, and Molecular Mechanisms Underlying Its Apoptosis-Inducing Effect on Breast and Lung Cancer Cell Lines. Separations 2022, 9, 298. [Google Scholar] [CrossRef]
- Shimomura-Kuroki, J.; Yamashita, K.; Shimooka, S. Tannerella forsythia and the HLA-DQB1 allele are associated with susceptibility to periodontal disease in Japanese adolescents. Odontology 2009, 97, 32–37. [Google Scholar] [CrossRef]
- Zhuang, L.-F.; Watt, R.M.; Mattheos, N.; Si, M.-S.; Lai, H.-C.; Lang, N.P. Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and peri-implant tissues. Clin. Oral Implants Res. 2014, 27, 13–21. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Llama-Palacios, A.; Fernández, E.; Figuero, E.; Marín, M.J. An in vitro biofilm model associated to dental implants: Structural and quantitative analysis of in vitro biofilm formation on different dental implant surfaces. Dent. Mater. 2014, 30, 1161–1171. [Google Scholar] [CrossRef]
- Singh, S. Triple-layer electrodes Dielectrophoretic chip design by Comsol Multiphysics 4.0. J. Integr. Sci. Technol. 2013, 1, 28–29. [Google Scholar]
- Barman, U.; Sen, A.K.; Mishra, S.C. Theoretical and numerical investigations of an electroosmotic flow micropump with interdigitated electrodes. Microsyst. Technol. 2013, 20, 157–168. [Google Scholar] [CrossRef]
- Baños, R.D.; Arcos, J.C.; Bautista, O.; Méndez, F.; Merchán-Cruz, E.A. Mass transport by an oscillatory electroosmotic flow of power-law fluids in hydrophobic slit microchannels. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 9. [Google Scholar] [CrossRef]
- Farahinia, A.; Jamaati, J.; Niazmand, H. Investigation of slip effects on electroosmotic mixing in heterogeneous microchannels based on entropy index. SN Appl. Sci. 2019, 1, 728. [Google Scholar] [CrossRef]
- Kawamata, T.; Yamada, M.; Yasuda, M.; Seki, M. Continuous and precise particle separation by electroosmotic flow control in microfluidic devices. Electrophoresis 2008, 29, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Zhang, L.; Tao, D.; Liang, Y.; Zhang, W.; Zhang, Y. Miniaturized two-dimensional capillary electrophoresis on a microchip for analysis of the tryptic digest of proteins. J. Sep. Sci. 2008, 31, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Acunha, T.; Reguera, M.I.; Navarro, R.; Redondo, J.A.; Reinecke, H.; Gallardo, A.; Cifuentes, A. Potential of pro-dendronic polyamines with modulated segmental charge density as novel coating for fast and efficient analysis of peptides and basic proteins by capillary electrophoresis and capillary electrophoresis-mass spectrometry. Electrophoresis 2015, 36, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Lee, H.K.; Lin, B.; Yeung, E.S. Spatial temperature gradient capillary electrophoresis for DNA mutation detection. Electrophoresis 2015, 22, 3683–3687. [Google Scholar]
| Primers | The Nucleotide Sequence (5′---3′) | Amplicons | Bacteria |
|---|---|---|---|
| PG197-F | TGTAGATGACTGATGGTGAAAACC | 197 bp | Porphyromonas gingivali |
| PG197-R | ACGTCATCCCCACCTTCCTC | ||
| TD311-F | AAGGCGGTAGAGCCGCTCA | 311 bp | Treponema denticola |
| TD311-R | AGCCGCTGTCGAAAAGCCCA | ||
| TF641-F | GCGTATGTAACCTGCCCGCA | 641 bp | Tannerela forsythia |
| TF641-R | TGCTTCAGTGTCAGTTATACCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Huang, J.; Tao, C.; Li, Z.; Zhang, D.; Yamaguchi, Y. Detection of Periodontal Pathogens Based on an Integrated Continuous Flow PCR and Capillary Electrophoresis Microfluidic Chip. Separations 2023, 10, 271. https://doi.org/10.3390/separations10040271
Yang B, Huang J, Tao C, Li Z, Zhang D, Yamaguchi Y. Detection of Periodontal Pathogens Based on an Integrated Continuous Flow PCR and Capillary Electrophoresis Microfluidic Chip. Separations. 2023; 10(4):271. https://doi.org/10.3390/separations10040271
Chicago/Turabian StyleYang, Bo, Jiaxin Huang, Chunxian Tao, Zhenqing Li, Dawei Zhang, and Yoshinori Yamaguchi. 2023. "Detection of Periodontal Pathogens Based on an Integrated Continuous Flow PCR and Capillary Electrophoresis Microfluidic Chip" Separations 10, no. 4: 271. https://doi.org/10.3390/separations10040271
APA StyleYang, B., Huang, J., Tao, C., Li, Z., Zhang, D., & Yamaguchi, Y. (2023). Detection of Periodontal Pathogens Based on an Integrated Continuous Flow PCR and Capillary Electrophoresis Microfluidic Chip. Separations, 10(4), 271. https://doi.org/10.3390/separations10040271







