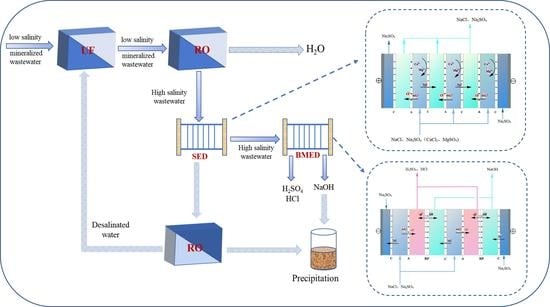
Zero Liquid Discharge and Resource Treatment of Low-Salinity Mineralized Wastewater Based on Combing Selectrodialysis with Bipolar Membrane Electrodialysis
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Pretreatment of Low-Salinity Mineralized Wastewater through UF + RO and UF + ED
2.3. Apparatus and Experimental Procedures
2.4. Analytical Methods
2.5. Data Analysis
3. Results and Discussion
3.1. Three-Stage ED Process for Salt Concentration
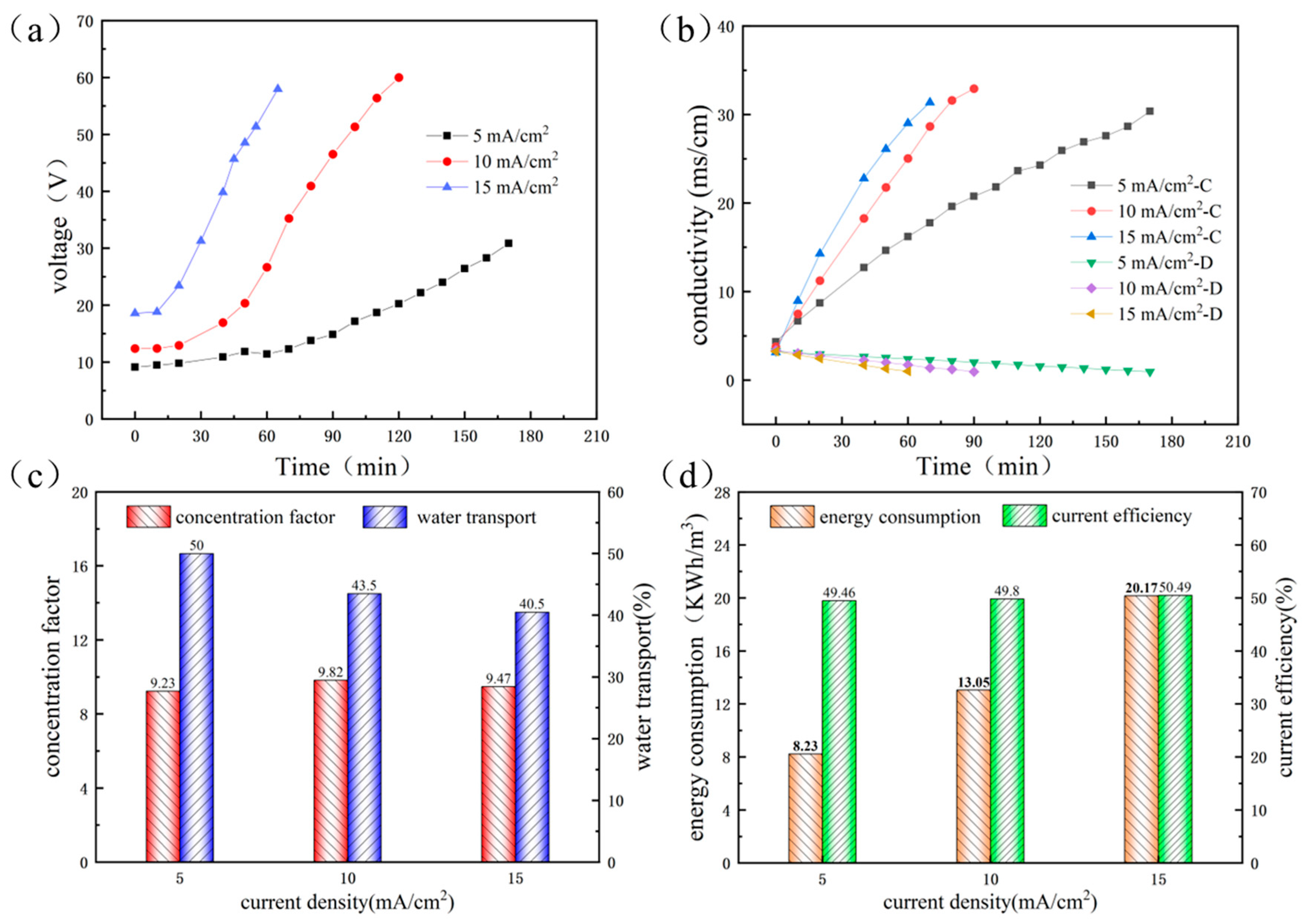
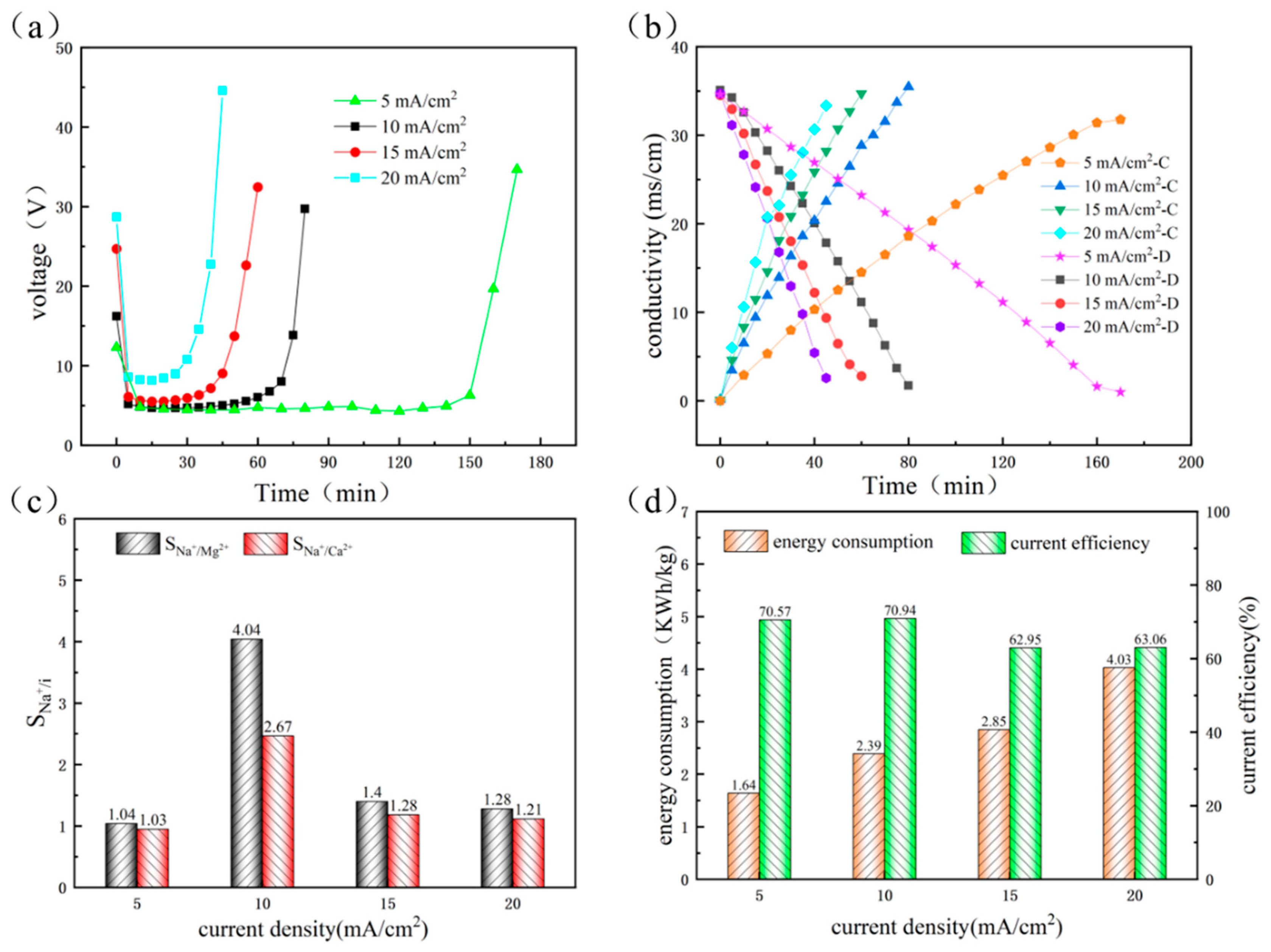
3.1.1. The Influence of Current Density in First-Stage ED Process
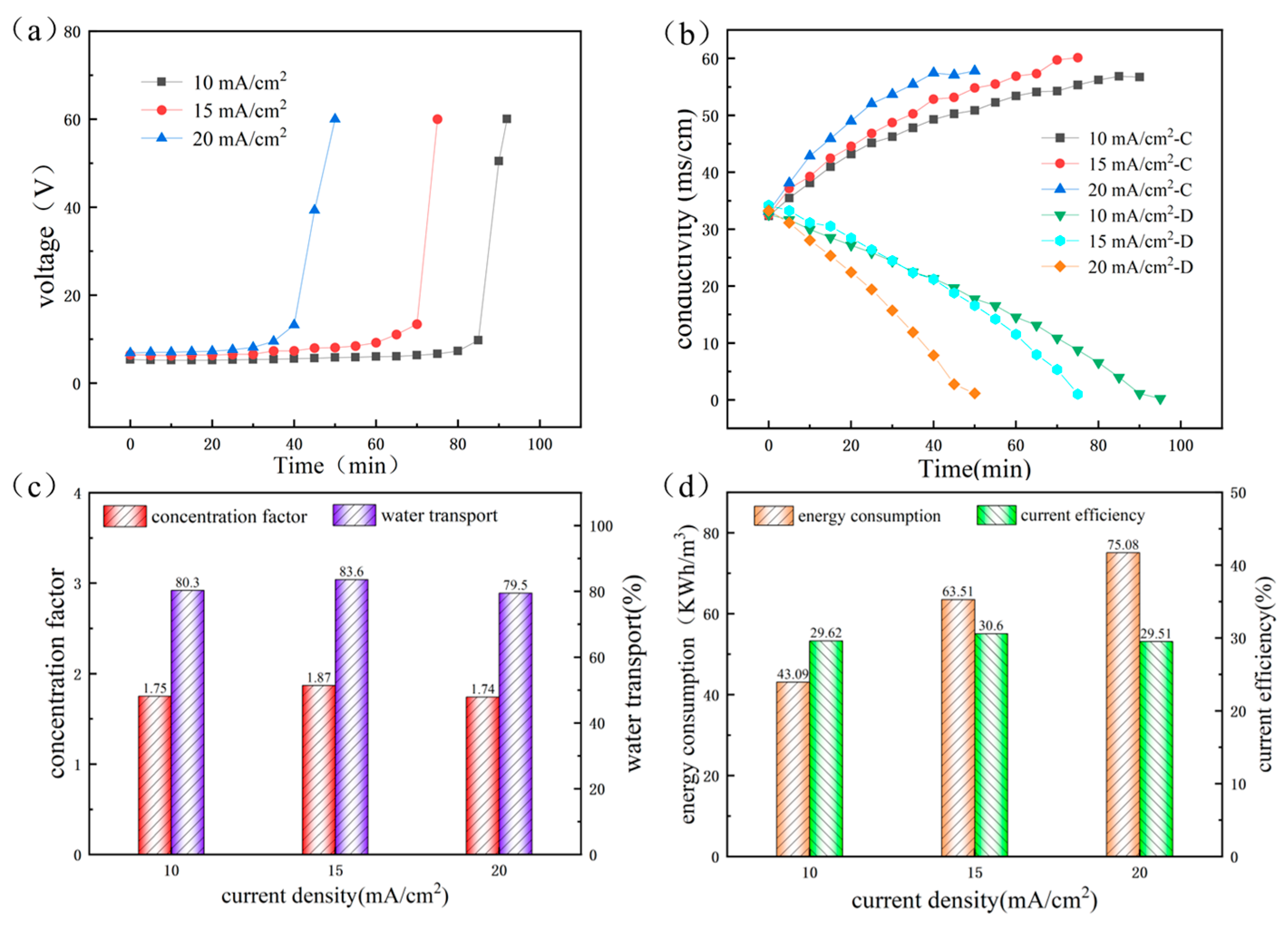
3.1.2. The Influence of Current Density in Second-Stage and Third-Stage ED Process
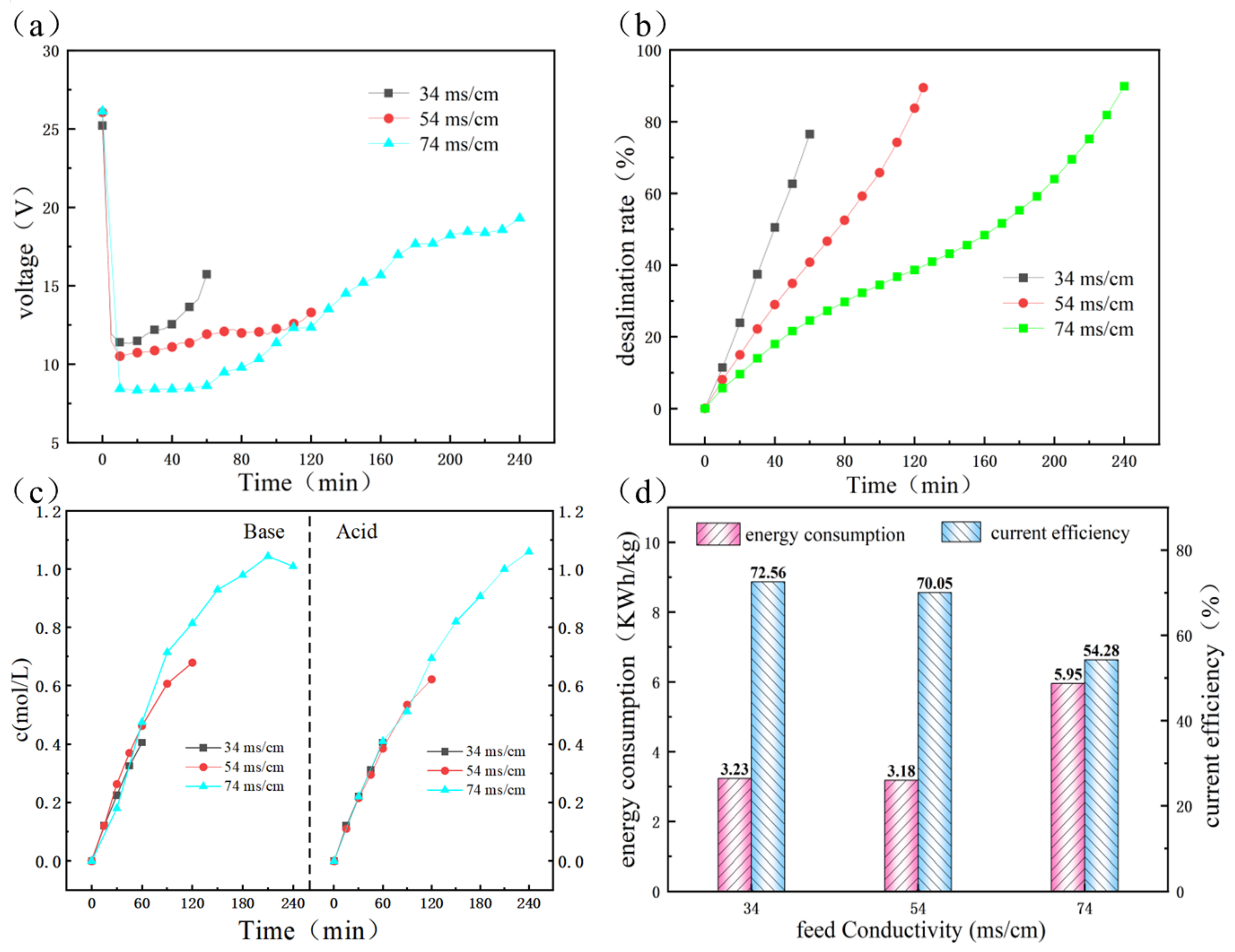
3.1.3. Performance of RO and ED
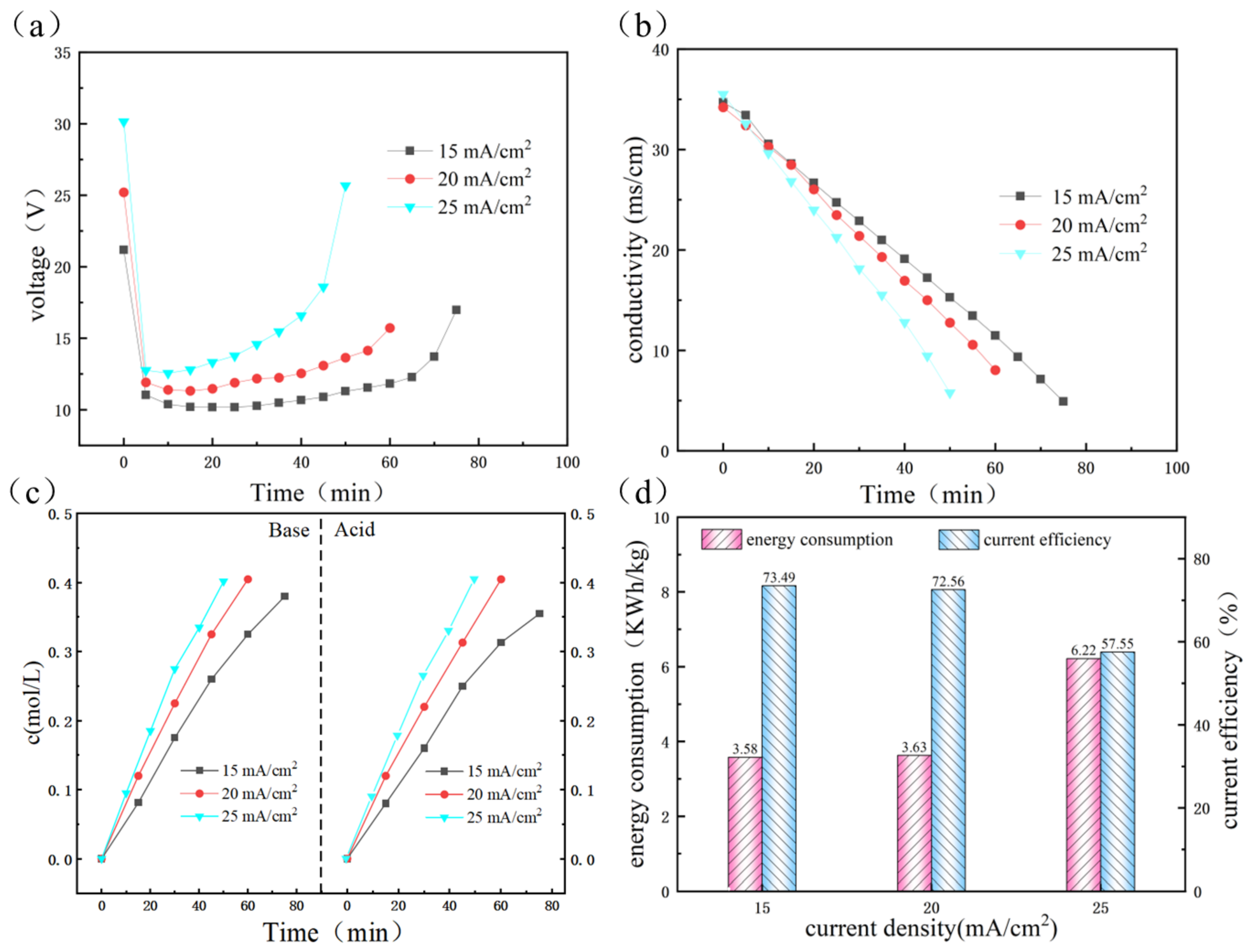
3.2. Purification of Concentrate Wastewater through SED Process
3.3. Production of High Purity Acid and Alkali with BMED
3.3.1. The Effect of Current Density
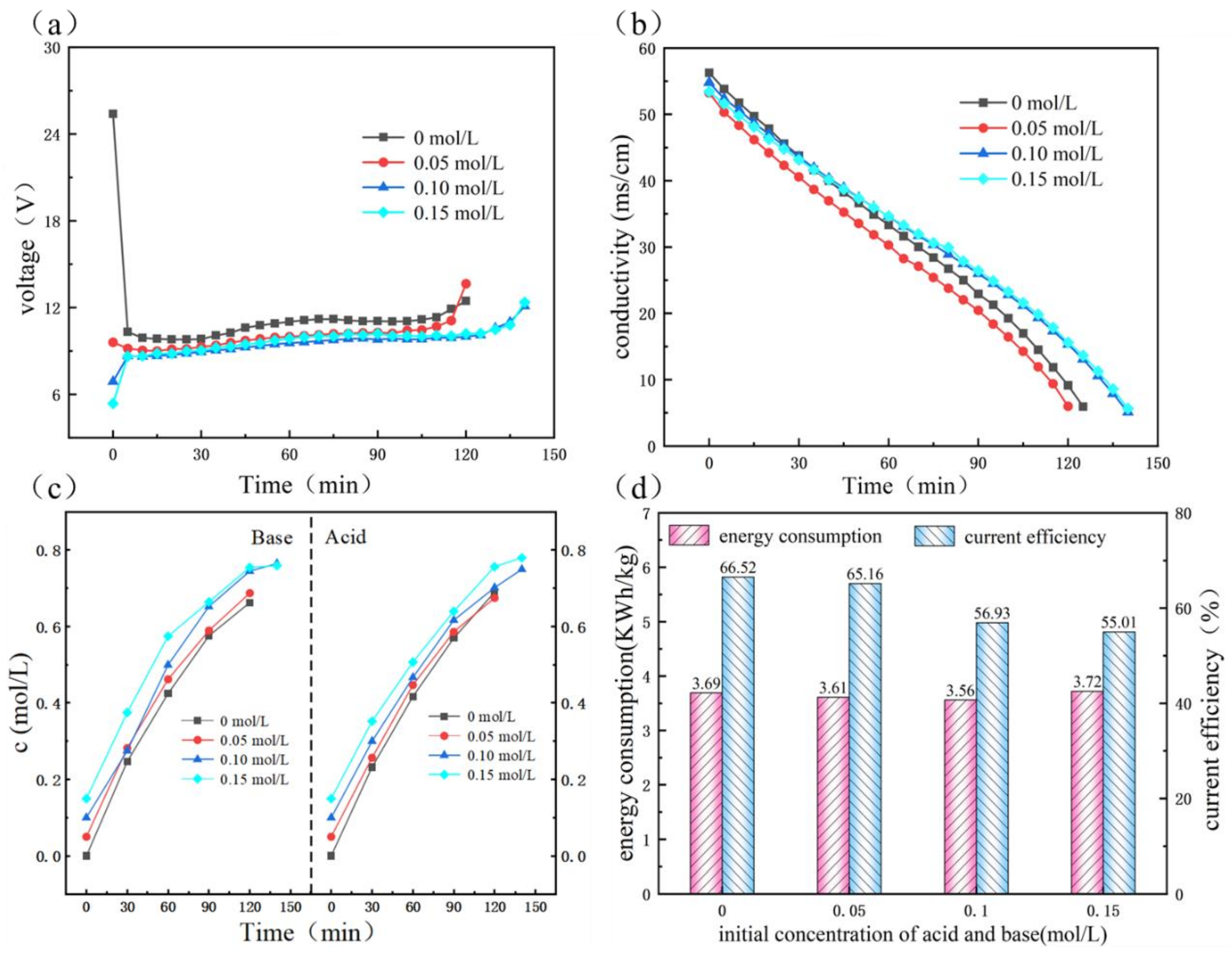
3.3.2. Effect of Initial Salt Concentration
3.3.3. Effect of Initial HCl and NaOH Contents
3.3.4. Effect of Flow Velocity
3.4. The Water Recovery Evaluation
3.5. Economic Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Gao, X.; Fan, A.; Fu, L.; Gao, C. An innovative beneficial reuse of seawater concentrate using bipolar membrane electrodialysis. J. Membr. Sci. 2014, 449, 119–126. [Google Scholar] [CrossRef]
- Gu, D.; Li, T.; Li, J.; Guo, Q.; Jiang, B.; Bian, W.; Yixiang, B. Current status and prospects of coal mine water treatment technology in China. Coal Sci. Technol. 2021, 49, 11–18. [Google Scholar]
- Jin, D.; Ge, G.; Zhang, Q.; Guo, Y.D. New energy-saving desalination technology of highly-mineralized mine water. Coal Sci. Technol. 2018, 46, 12–18. [Google Scholar]
- Chen, J.; Gu, M.; Zhou, Y.; Wan, D.; He, Q.; Shi, Y.; Liu, Y. Efficient nitrate and perchlorate removal from aqueous solution via a novel electro-dialysis ion-exchange membrane bioreactor. Chem. Eng. J. 2022, 430, 132952. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, H.; Wang, H.; Ladewig, B.P.; Yao, Z. A comprehensive review on the synthesis and applications of ion exchange membranes. Chemosphere 2021, 282, 130817. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, S.S.; Nosova, E.N.; Melnikova, E.D.; Zabolotsky, V.I. Reactive separation of inorganic and organic ions in electrodialysis with bilayer membranes. Sep. Purif. Technol. 2021, 268, 118561. [Google Scholar] [CrossRef]
- Zhang, Y.; Van der Bruggen, B.; Pinoy, L.; Meesschaert, B. Separation of nutrient ions and organic compounds from salts in RO concentrates by standard and monovalent selective ion-exchange membranes used in electrodialysis. J. Membr. Sci. 2009, 332, 104–112. [Google Scholar] [CrossRef]
- Qiu, Y.; Yao, L.; Tang, C.; Zhao, Y.; Zhu, J.; Shen, J. Integration of selectrodialysis and selectrodialysis with bipolar membrane to salt lake treatment for the production of lithium hydroxide. Desalination 2019, 465, 1–12. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Oppenheimer, J.; Adham, S.; Kumar, M. Innovative beneficial reuse of reverse osmosis concentrate using bipolar membrane electrodialysis and electrochlorination processes. J. Membr. Sci. 2009, 326, 392–399. [Google Scholar] [CrossRef]
- Tongwen, X.; Weihua, Y. Citric acid production by electrodialysis with bipolar membranes. Chem. Eng. Process. Process Intensif. 2002, 41, 519–524. [Google Scholar] [CrossRef]
- Nosova, E.; Achoh, A.; Zabolotsky, V.; Melnikov, S. Electrodialysis Desalination with Simultaneous pH Adjustment Using Bilayer and Bipolar Membranes, Modeling and Experiment. Membranes 2022, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ruan, X.; Kentish, S.E.; Li, G.; Xu, T.; Chen, G.Q. Production of lithium hydroxide by electrodialysis with bipolar membranes. Sep. Purif. Technol. 2021, 274, 119026. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Peng, Z. Evaluation of bipolar membrane electrodialysis for desalination of simulated salicylic acid wastewater. Desalination 2022, 537, 115866. [Google Scholar] [CrossRef]
- Melnikov, S.S.; Mugtamov, O.A.; Zabolotsky, V.I. Study of electrodialysis concentration process of inorganic acids and salts for the two-stage conversion of salts into acids utilizing bipolar electrodialysis. Sep. Purif. Technol. 2020, 235, 116198. [Google Scholar] [CrossRef]
- Du, C.; Du, J.R.; Zhao, X.; Cheng, F.; Ali, M.E.A.; Feng, X. Treatment of Brackish Water RO Brine via Bipolar Membrane Electrodialysis. Ind. Eng. Chem. Res. 2021, 60, 3115–3129. [Google Scholar] [CrossRef]
- Chaudhury, S.; Harlev, N.; Haim, O.; Lahav, O.; Nir, O. Decreasing Seawater Desalination Footprint by Integrating Bipolar-Membrane Electrodialysis in a Single-Pass Reverse Osmosis Scheme. ACS Sustain. Chem. Eng. 2021, 9, 16232–16240. [Google Scholar] [CrossRef]
- Miao, M.; Qiu, Y.; Yao, L.; Wu, Q.; Ruan, H.; Van der Bruggen, B.; Shen, J. Preparation of N,N,N-trimethyl-1-adamantylammonium hydroxide with high purity via bipolar membrane electrodialysis. Sep. Purif. Technol. 2018, 205, 241–250. [Google Scholar] [CrossRef]
- Yan, H.; Xu, C.; Li, W.; Wang, Y.; Xu, T. Electrodialysis To Concentrate Waste Ionic Liquids: Optimization of Operating Parameters. Ind. Eng. Chem. Res. 2016, 55, 2144–2152. [Google Scholar] [CrossRef]
- Nie, X.-Y.; Sun, S.-Y.; Sun, Z.; Song, X.; Yu, J.-G. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 2017, 403, 128–135. [Google Scholar] [CrossRef]
- Jiang, G.; Li, H.; Xu, M.; Ruan, H. Sustainable reverse osmosis, electrodialysis and bipolar membrane electrodialysis application for cold-rolling wastewater treatment in the steel industry. J. Water Process Eng. 2021, 40, 101968. [Google Scholar] [CrossRef]
- Qiu, Y.; Lv, Y.; Tang, C.; Liao, J.; Ruan, H.; Sotto, A.; Shen, J. Sustainable recovery of high-saline papermaking wastewater: Optimized separation for salts and organics via membrane-hybrid process. Desalination 2021, 507, 114938. [Google Scholar] [CrossRef]
- Qiu, Y.; Ruan, H.; Tang, C.; Yao, L.; Shen, J.; Sotto, A. Study on Recovering High-Concentration Lithium Salt from Lithium-Containing Wastewater Using a Hybrid Reverse Osmosis (RO)–Electrodialysis (ED) Process. ACS Sustain. Chem. Eng. 2019, 7, 13481–13490. [Google Scholar] [CrossRef]
- Peters, C.D.; Hankins, N.P. Osmotically assisted reverse osmosis (OARO): Five approaches to dewatering saline brines using pressure-driven membrane processes. Desalination 2019, 458, 1–13. [Google Scholar] [CrossRef]
- Fane, A.G. A grand challenge for membrane desalination: More water, less carbon. Desalination 2018, 426, 155–163. [Google Scholar] [CrossRef]
- Panagopoulos, A. A comparative study on minimum and actual energy consumption for the treatment of desalination brine. Energy 2020, 212, 118733. [Google Scholar] [CrossRef]
- Masigol, M.A.; Moheb, A.; Mehrabani-Zeinabad, A. An experimental investigation into batch electrodialysis process for removal of sodium sulfate from magnesium stearate aqueous slurry. Desalination 2012, 300, 12–18. [Google Scholar] [CrossRef]
- Peng, Z.; Sun, Y. Leakage circuit characteristics of a bipolar membrane electrodialyzer with 5 BP-A-C units. J. Membr. Sci. 2020, 597, 117762. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Wang, Q.; Feng, H.; Xu, T. Production of Lithium Hydroxide from Lake Brines through Electro–Electrodialysis with Bipolar Membranes (EEDBM). Ind. Eng. Chem. Res. 2014, 53, 6103–6112. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, X.; Feng, H.; Xu, T. In-situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: Continuous operation. Bioresour. Technol. 2013, 147, 442–448. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, M.; Pan, J.; Sotto, A.; Shen, J.; Gao, C.; Van der Bruggen, B. Process Economic Evaluation of Resource Valorization of Seawater Concentrate by Membrane Technology. ACS Sustain. Chem. Eng. 2017, 5, 5820–5830. [Google Scholar] [CrossRef]
- Strathmann, H.; Krol, J.J.; Rapp, H.-J.; Eigenberger, G. Limiting current density and water dissociation in bipolar membranes. J. Membr. Sci. 1997, 125, 123–142. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Peng, Z.; Liu, Y. Treatment of high salinity sulfanilic acid wastewater by bipolar membrane electrodialysis. Sep. Purif. Technol. 2022, 281, 119842. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Zhang, X.; Xu, T. Treatment of simulated brominated butyl rubber wastewater by bipolar membrane electrodialysis. Sep. Purif. Technol. 2011, 80, 196–201. [Google Scholar] [CrossRef]
- Wright, N.C.; Shah, S.R.; Amrose, S.E.; Winter, A.G. A robust model of brackish water electrodialysis desalination with experimental comparison at different size scales. Desalination 2018, 443, 27–43. [Google Scholar] [CrossRef]
- Walker, W.S.; Kim, Y.; Lawler, D.F. Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis—Part I: Sensitivity to superficial velocity. Desalination 2014, 344, 152–162. [Google Scholar] [CrossRef]
- Tanaka, Y. Concentration polarization in ion-exchange membrane electrodialysis—The events arising in a flowing solution in a desalting cell. J. Membr. Sci. 2003, 216, 149–164. [Google Scholar] [CrossRef]



| Membrane Type | Thickness (mm) | Burst Strength (MPa) | Area Resistance (Ω cm2) | Transport Number (%) | Temp (°C) |
|---|---|---|---|---|---|
| CAM | 0.20–0.34 | 0.6 | 6–8 | -- | 25–40 |
| AAM | 0.34–0.38 | >0.6 | 5–7 | -- | 25–40 |
| BPM | 0.28 | >1.0 | -- | -- | 25–40 |
| CSO | 0.1 | 0.15 | 2.3 | 97 | -- |
| Na+ (µg/L) | Mg2+ (µg/L) | K+ (µg/L) | Ca2+ (µg/L) | Cl− (µg/L) | SO42− (µg/L) | |
|---|---|---|---|---|---|---|
| Feed | 7829.75 | 70.2 | 74.01 | 91.01 | 3.96 | 7.82 |
| Conductivity (mS/cm) | Na+ (mg/L) | K+ (mg/L) | Mg2+ (mg/L) | Ca2+ (mg/L) | Cl− (mg/L) | SO42− (mg/L) | |
|---|---|---|---|---|---|---|---|
| RO | 34.34 | 10,794.2 | 133.5 | 113.6 | 131.2 | 7011.4 | 13,885.3 |
| ED | 35 | 10,807.1 | 136.5 | 108.7 | 128.2 | 7063.1 | 13,953.1 |
| 55.23 | 17,380.8 | 181 | 177 | 234.9 | 10,890.2 | 23,018.1 | |
| 74.63 | 25,458.1 | 284.6 | 264 | 331.9 | 16,614 | 32,830.6 |
| Operation Mode | Current Density (mA/cm2) | CFED | WT (%) | E (kWh/m3) | CE (%) |
|---|---|---|---|---|---|
| Third-stage ED | 15 | 1.48 | 56 | 81.95 | 20.41 |
| 20 | 1.46 | 58.5 | 87.52 | 19.83 | |
| 25 | 1.51 | 50 | 99.79 | 20.69 |
| Concentrate Factor | Energy Consumption (kWh/m3) | |
|---|---|---|
| RO | 10.9 | 3.52 |
| 5 mA/cm2 | 9.23 | 8.23 |
| 10 mA/cm2 | 9.82 | 13.05 |
| 15 mA/cm2 | 9.47 | 20.17 |
| Na+ (mg/L) | K+ (mg/L) | Mg2+ (mg/L) | Ca2+ (mg/L) | |
|---|---|---|---|---|
| a Feed | 10,807.87 | 136.51 | 108.70 | 128.23 |
| 5 mA/cm2 | 8567.78 | 84.41 | 81.19 | 97.23 |
| 10 mA/cm2 | 8306.20 | 68.92 | 20.31 | 36.96 |
| 15 mA/cm2 | 9839.89 | 85.74 | 69.45 | 90.65 |
| 20 mA/cm2 | 9032.80 | 100.91 | 69.55 | 88.53 |
| Ion | Acid (mg/L) | Base (mg/L) |
|---|---|---|
| Na+ | 320.3 | 17,320 |
| Mg2+ | 0 | 0 |
| Ca2+ | 0 | 8.95 |
| Cl− | 10,550.1 | 195.3 |
| SO42− | 22,680 | 30.2 |
| SED | BMED | |
|---|---|---|
| operating conditions | ||
| repeat unit | 3.00 | 4 |
| current density (mA/cm2) | 10.00 | 20 |
| flow rate (L/h) | 20.00 | 20 |
| working time (h/year) | 7200 | 7200 |
| effective membrane area (cm2) | 187.20 | 187.20 |
| practical membrane area (cm2) | 297.00 | 297.00 |
| feed conductivity (mS/cm) | 34.00 | 55.00 |
| energy | ||
| energy consumption (kWh/kg) | 1.00 | 3.51 |
| process capacity (kg/year) | 172.80 | 104.75 |
| electricity charge (USD/kWh) | 0.100 | 0.100 |
| energy consumption for acid/base production (USD/kg) | 0.100 | 0.351 |
| energy consumption for peripheral equipment (USD/kg) | 0.052 | 0.18 |
| total energy cost (USD/kg) | 0.152 | 0.53 |
| capital cost | ||
| membrane life (year) | 3 | 3 |
| bipolar membrane price (USD/m2) | 376.00 | 376 |
| ion-exchange membran | 165.00 | 165 |
| monoselective membrane price (USD/m2) | 260.00 | 260 |
| membrane cost (USD) | 50.49 | 85.24 |
| membrane stack cost (USD) | 75.74 | 127.86 |
| peripheral cost (USD) | 113.60 | 191.79 |
| total investment cost (USD) | 189.34 | 319.65 |
| total fixed cost (USD/year) | 63.11 | 106.55 |
| total fixed cost (USD/kg) | 0.365 | 1.02 |
| total process cost (USD/kg) | 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Cheng, X.; Sun, J.; Liu, J.; Liu, Z.; Wang, Y.; Pan, J. Zero Liquid Discharge and Resource Treatment of Low-Salinity Mineralized Wastewater Based on Combing Selectrodialysis with Bipolar Membrane Electrodialysis. Separations 2023, 10, 269. https://doi.org/10.3390/separations10040269
Zhao X, Cheng X, Sun J, Liu J, Liu Z, Wang Y, Pan J. Zero Liquid Discharge and Resource Treatment of Low-Salinity Mineralized Wastewater Based on Combing Selectrodialysis with Bipolar Membrane Electrodialysis. Separations. 2023; 10(4):269. https://doi.org/10.3390/separations10040269
Chicago/Turabian StyleZhao, Xueting, Xinhao Cheng, Jinshan Sun, Jialin Liu, Zhaofeng Liu, Yali Wang, and Jiefeng Pan. 2023. "Zero Liquid Discharge and Resource Treatment of Low-Salinity Mineralized Wastewater Based on Combing Selectrodialysis with Bipolar Membrane Electrodialysis" Separations 10, no. 4: 269. https://doi.org/10.3390/separations10040269
APA StyleZhao, X., Cheng, X., Sun, J., Liu, J., Liu, Z., Wang, Y., & Pan, J. (2023). Zero Liquid Discharge and Resource Treatment of Low-Salinity Mineralized Wastewater Based on Combing Selectrodialysis with Bipolar Membrane Electrodialysis. Separations, 10(4), 269. https://doi.org/10.3390/separations10040269