Abstract
Carbon nanotubes (CNTs) with hollow nanochannels have attracted much attention in preparing high-performance water treatment membranes. In this paper, the grafting polymer chains, including alkynyl terminated poly(methyl methacrylate) methacrylate (PMMA) single chain and PMMA-b-poly (ethylene glycol) methacrylate [P(PEGMA)] diblock molecular chains, were synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization. A UV-induced click reaction was used to graft different linear polymers onto the surface of magnetic thiol-functionalized carbon nanotubes (mCNTs-SH). The poly(vinylidene fluoride) (PVDF) composite ultrafiltration membrane within the oriented nanochannels was prepared using phase inversion and magnetic field orientation. TEM and XRD results confirmed that the magnetic carbon nanotubes grafted with a diblock molecular chain had good nano-dispersion and orientation array effects in PVDF composite ultrafiltration membrane. The water contact angle of the array mCNT-g-diblock molecular chain-based composite membrane was 48.5°, significantly enhancing the PEGMA chain segments. The composite membrane with CTNs’ nanochannels attained a higher water flux. As the diblock molecular chain grafted mCNTs oriented in the membrane, the water flux reached 17.6 LMH (five times greater than the pure PVDF membrane), while the molecular weight cut-off (MWCO) for PEG1400 rejection could reach higher than 80%.
1. Introduction
Ultrafiltration technology has been widely used in water purification, solution separation and concentration, and wastewater purification and reuse, such as oil-water separation and protein effluent separation [1]. Poly(vinylidene fluoride) (PVDF) is a common ultrafiltration membrane material with excellent chemical resistance and thermal stability. However, due to its strong hydrophobicity, organic matter is easily adsorbed on the membrane surface, which causes the membrane pore to be blocked and the water flux to rapidly decrease [2]. In recent years, multi-walled carbon nanotubes (MWCNTs) have been widely used in membrane materials due to their hollow and fast water channels. However, the dispersion of MWCNTs in organic solvents and aqueous solutions is very low due to the weak van der Waals force. Therefore, modification of MWCNTs to make them well dispersed in PVDF membranes is significant for high throughput and separation performance [3]. Recently, by depositing superparamagnetic iron oxide nanoparticles (Fe3O4), magnetic carbon nanotubes (mCNTs) have been oriented in some direction under the induced action of the magnetic field to maximize the effect of fast water channels [4]. In order to stabilize magnetic particles on the surface of CNTs, compatible polymer segments can be grafted on the CNTs’ surface [5]. It is possible to graft polymer chains with a desired structure onto CNTs’ surfaces using reversible addition-fracture chain transfer (RAFT) polymerization, thereby improving nanoscale dispersion and interface interactions between CNTs’ surfaces and the polymer chains. In the previous work [6], the polymethylmethacrylate (PMMA) molecule was linked to the surface of CNTs by using a click reaction after a RAFT synthesis of PMMA chains. CNTs with grafted PMMA chains demonstrated good compatibility with PVDF chains, resulting in improved nano-dispersion in PVDF. It should be noted that PMMA chains are relatively rigid, weakening the interface mutual effect between the PVDF chains and CNTs’ surface [7,8]. Recent research shows that grafting blocked polymer chains, including a rigid PMMA chain and flexible PEGMA chain, onto the CNTs’ surface enhanced the interfacial interaction between CNTs’ surfaces and mixed polymer chains [9,10].
It has been proved that CNT-based membranes offer many advantages, including enhanced water permeability, high selectivity, and anti-fouling properties [11]. Additionally, CNTs can load other inorganic nanoparticles and realize the modification of the membrane matrix through a synergistic effect. As reported [12], silica-coated multi-walled carbon nanotubes (SiO2-CNT) were embedded into the PVDF polymer matrix to develop a novel PVDF/SiO2-CNT nanocomposite membrane. It has been demonstrated that incorporating hydrophilic SiO2-CNT into the PVDF matrix increased the permeability from 303 to 377 L/m2∙h (25% enhancement) due to the improved dispersion of nanoparticles, which subsequently resulted in enhanced morphological properties. Thus, the modified CNTs make composited membranes exhibit better filtration properties and improved thermal stability and mechanical properties, significantly improving durability.
Because the disordered CNTs in the membrane matrix have limited water permeation promotion, the strategy to make the CNTs disperse in nano-scale and arrange in one direction (arrayed CNTs) would play an essential role in the higher performance polymeric membrane and surface properties. As reported [13], the CNTs deposited with magnetic particles and immobilized using block copolymers. This practical grafting method gave the CNTs’ surface a double layer and could be oriented in the polymer membrane matrix. The PMMA segments can become compatible with the PVDF chain matrix, while the P(PEGMA) segments enhance the CNT surface’s hydrophilicity. However, previous work shows that a small amount of CNT (<2 wt%) was incorporated into the membrane matrix because of limited nanoscale dispersion. Therefore, the higher amount of incorporation into the polymeric membrane is still a challenge.
In this study, a diblock molecular chain with rigid PMMA and flexible P(PEGMA) segments was grafted onto magnetically thiol-functionalized carbon nanotubes (mCNTs-SH) using a technique referred to as thiol-alkynyl click chemistry initiated by ultraviolet light [14]. The alkynyl-terminated poly (methyl methacrylate) and poly (ethylene methacrylate) block copolymers were synthesized using the benzoyl peroxide/dimethylaniline redox system as the free radical source at room temperature. Magnetic responsive CNTs with bilayer polymer interface were prepared by grafting P(PEGMA)-b-PMMA-alkynyl segment to sulfhydryl functionalized CNTs. The effects of grafted diblock molecular chains on CNTs’ surface and CNTs’ orientation by the magnetic introduction on the membrane structure and filtration performance were systematically investigated.
2. Materials and Methods
2.1. Materials
Multi-walled carbon nanotubes (MWCNTs) (diameter < 8 nm, length 0.5~2 μm) were purchased from Nanjing Xianfeng Technology Co., Ltd. (Nanjing, Jiangsu, China). Photoinitiator 1173 (CP) was obtained from Shanghai Guangyi Chemical Co., Ltd. (Shanghai, China). Poly(vinylidene fluoride) (PVDF-741) was supplied by Arkema (Châteauroux, France). N. N-dimethylacetamide (DMAc) (AR) was purchased from Sinopharm Chemical Reagents Co., Ltd. (Shanghai, China). Poly (ethylene glycol) methyl ether methacrylate (PEGMA) (AR) and methylmethacrylate (MMA) monomers were obtained from Shanghai Aladdin (Shanghai, China). RAFT reagent of dodecyl thiocarbonate 2-methyl-propylenyl propionate [15] (DMP-Alkynyl) (94.73% purity) was self-made in the laboratory. Azodiisobutyronitrile (AIBN) (98% purity) was purchased from Aladdin Reagent Co., Ltd. (Shanghai, China).
2.2. Synthesizing Magnetical CNTs Grafting Single and Diblock Molecular Chains
As reported [16], the terminal alkynylated polymethylmethacrylate (PMMA-alkynyl) and diblock molecular chains, which were copolymerized with poly (ethylene glycol) methacrylate [P(PEGMA)], were synthesized. The thiol-functionalized CNTs (CNTs-SH) [2] and Fe3O4 [17,18] were prepared by the reported method.
2.3. Orientation of Magnetical CNTs Grafting Molecular Chains in the Membrane
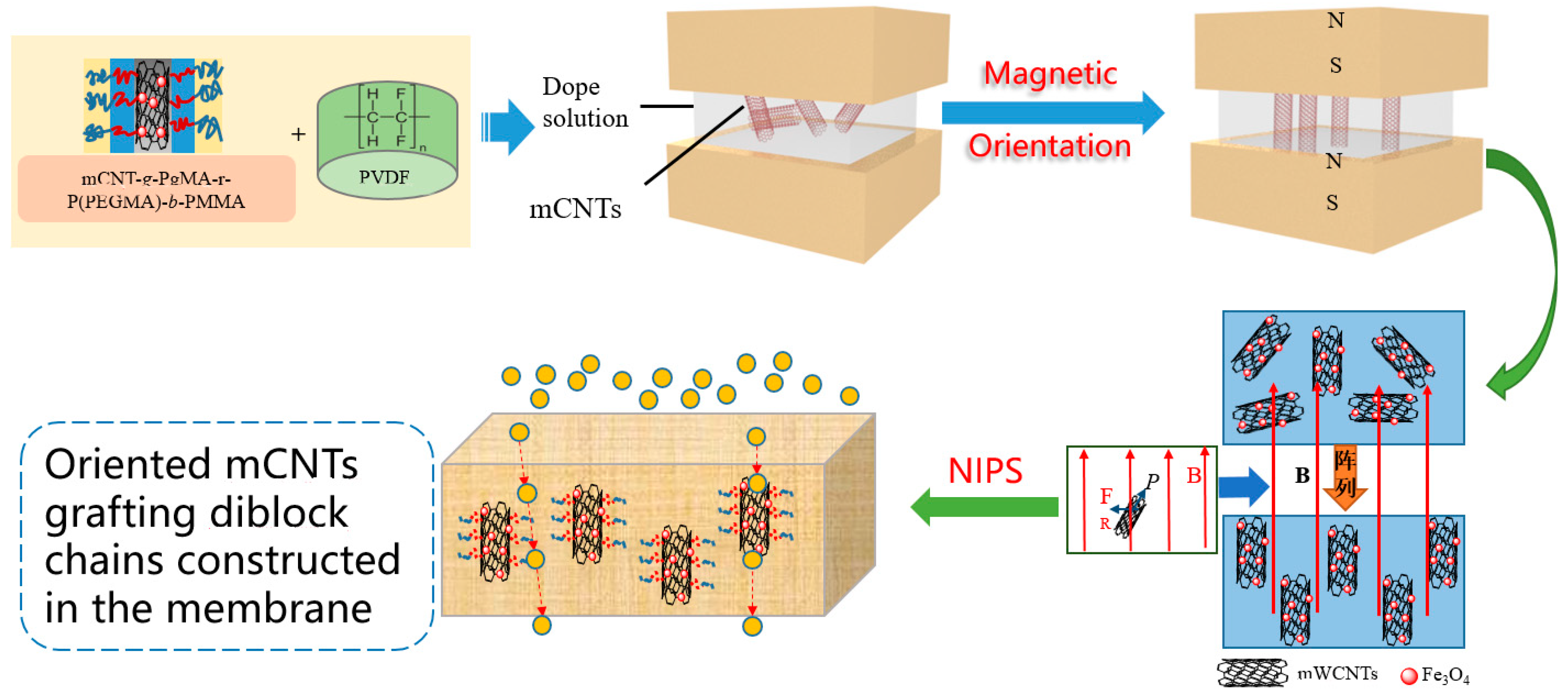
The orientation of magnetical CNTs grafting single and diblock molecular chains in the PVDF membrane was achieved by a magnetic field introducing (<0.65 T) interaction during the phase inversion process (as shown in Scheme 1). For example, an M2 sample was derived from 2 wt% mCNTs grafting PMMA/14wt% PVDF/84% DMAc solution. In order to disperse the grafted mCNTs in the dope solution, ultrasonic treatment was performed. After stirring continuously for 12 h at 70 °C, the dope solution was degassed before casting. The same process prepared membrane samples according to the composite and magnetic introduction in Table 1.
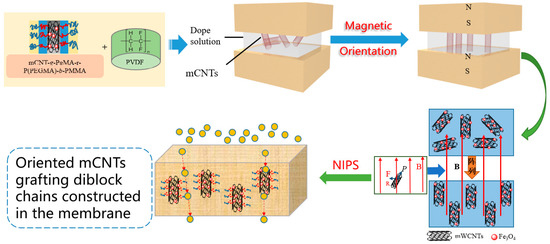
Scheme 1.
Preparation of PVDF composited membrane with orientation mCNTs.

Table 1.
PVDF composite membrane notation and its composition of dope solutions with or without magnetic field processing.
2.4. Characterizing the Structure and Morphology of the Magnetical CNTs Grafting Molecular Chains
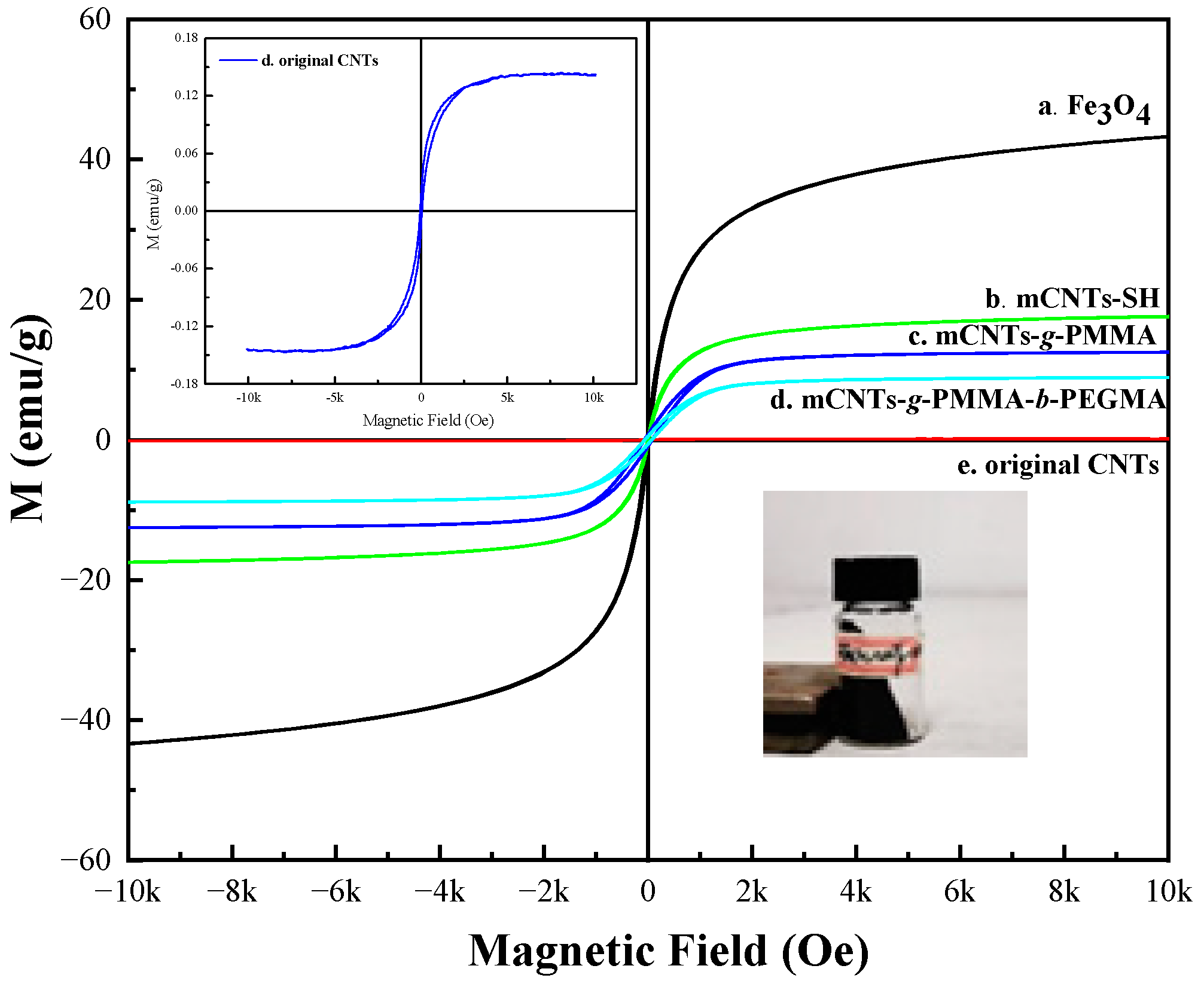
The CNTs’ surface morphology was observed by transmission electron microscopy (TEM) (JEOL, JEM 2100, Tokyo, Japan) and a field-emission scanning electron microscope (FE-SEM) (Quanta FEG 250, Hillsboro, OR, USA). Hysteresis loops of magnetical CNTs grafting molecular chains were obtained by vibration sample magnetometer (VSM) measurement (MPMS, XL-7, San Diego, CA, USA). The specific magnetic field range required for the test ranged from −10.000 Oe to +10.000 Oe under the ±3 T range of magnetic field. The original CNTs, mCNTs-SH, and Fe3O4 were also used as reference samples.
2.5. Characterizing the Basic Properties of the CNTs/PVDF Composited Membrane
The surface and section morphology of the PVDF composite membranes were observed after spraying with gold under 15.00 kV accelerating voltage. The membrane surface analysis was carried out on Nicolet iS50 (Thermo Fisher Company, Waltham, MA, USA) using total reflection (ATR) mode. The calculation for β-PVDF content in the crystalline phase was as follows [19].
where Aα And Aβ present the absorption peak area of the α-PVDF and β-PVDF at 761 cm−1 and 842 cm−1; Kβ/Kα = 1.26.
The melting crystallization of the PVDF in the composited membrane was characterized by a differential scanning calorimeter (DSC-214, Netzsch, Weimar, Germany). The temperature range under the N2 atmosphere is 20 °C~200 °C with a heating or cooling rate of 10 °C/min. The crystallinity (Xc) of PVDF is calculated by the melting enthalpy ratio between the membrane sample and the reference PVDF with 100% crystallinity [20].
The orientation of the CNTs in the PVDF composite membrane was determined using TEM (JEOL, JEM 2100, Tokyo, Japan). XRD (D8 Advanced X, Bruker, Ettlingen, Germany) was used to confirm the orienting degree of CNTs in the composite membrane. It was carried out in Cu Kα irradiation source at 25 °C scanning from 20° to 60° with a rate of 0.02°/s.
At room temperature, the membrane surface wettability was investigated by the water contact angle meter (JC2000D1, Shanghai Zhongchen Digital Technology Instrument Co., Ltd., Shanghai, China). Each point was measured from a 1.0 μL water droplet.
All the membrane samples’ porosity was measured by weighing method [18]. Before the test, the membrane sample was soaked in isobutanol for 24 h. After that, the weight changes in the membrane before and after soaking can be calculated. The porosity can be obtained as follows.
2.6. Filtration and Antifouling Properties of the CNTs/PVDF Composited Membrane
As in our previous work [21], the filtration test was carried out on the water flux filtration equipment. The flux of pure water was measured at 0.1 MPa, with a test area of 23.7 × 10−4 m2, in cross-flow mode, at 160 mL/min. Then the permeability can be calculated by Equation (2).
where PWP is the flux of the composite membrane (L·m−2·h−1); A is the membrane surface area (m2), and V is the collected volume of the permeating side (L) at a running time Δt. The poly(ethylene glycol) (PEG) rejection was measured in the same mode by changing the PEG molecular weight from 1000 to 1600 Da. The PEG rejection can be obtained from the following equation.
where Cf is the content of PEG in the feed side solution, g/L; Cp is the content of PEG in the permeate side solution, g/L; A is the membrane testing area.
As in previous work, the BSA absorption test on the PVDF composite membrane surfaces obtained the anti-fouling evaluation [21]. The UV spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) analyzed the BSA absorption difference before and after immersion. According to the remaining absorbed BSA amount, the BSA absorption on the membrane surface can be calculated.
where C0 is the concentration of BSA solution before immersion (μg/mL), C1 is the concentration of BSA solution after immersion (μg/mL), V is the BSA solution volume (mL), and A is the sample membrane area (cm2). According to the literature, BSA standard solution with 0.2 g/L concentration is prepared [22].
3. Results and Discussion
3.1. Surface Chemical Identification and Morphology Analysis for the Magnetically Grafted CNTs
CNTs’ Surface Morphologies Observation
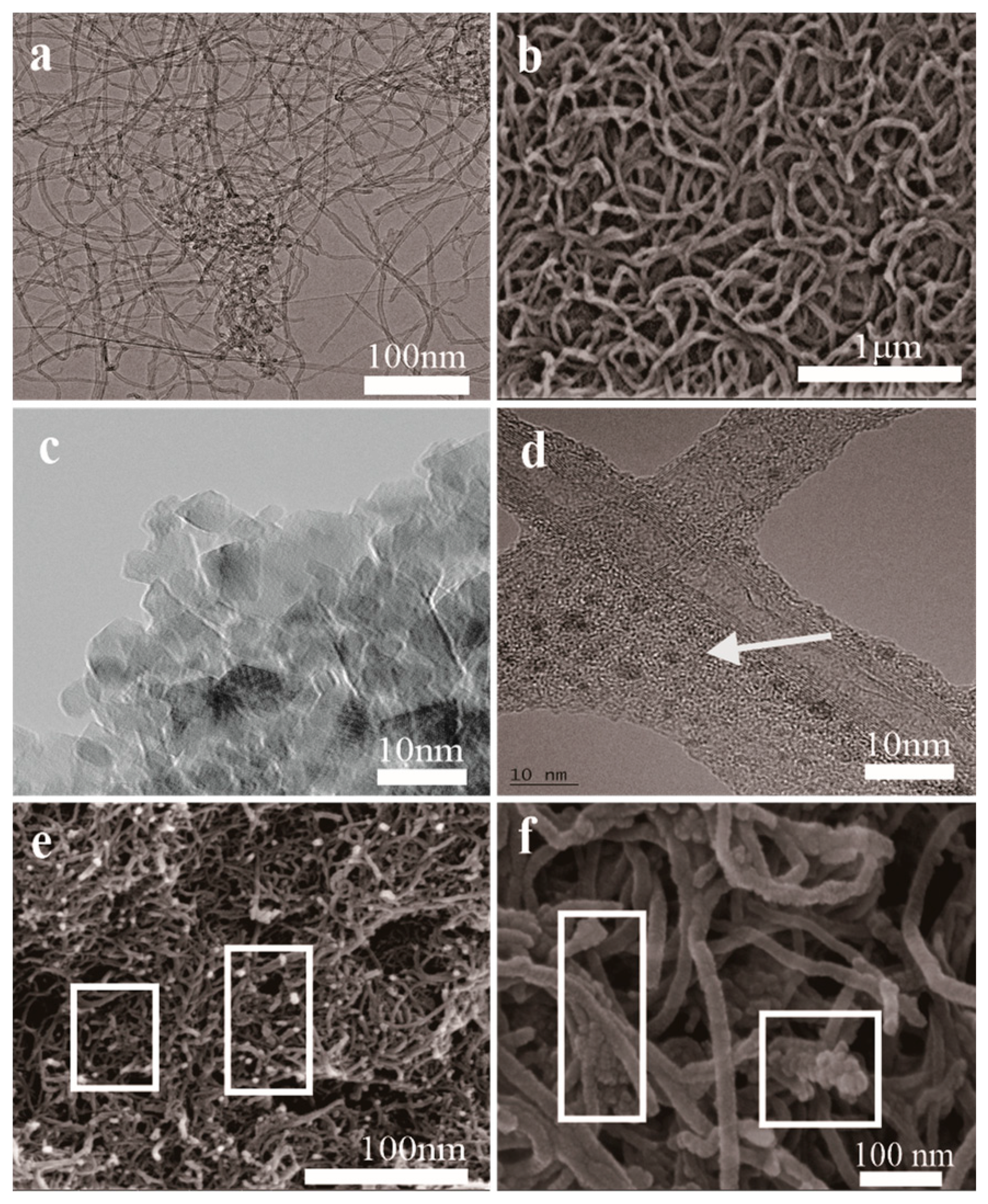
Compared with the original CNTs (Figure 1a) and mCNTs-SH (Figure 1b), the mCNT-g-PMMA (Figure 1e) and mCNT-g-block (Figure 1f) show obvious deposited Fe3O4 nanoparticles and grafted polymer chains on the CNTs’ surface. The Fe3O4 nanoparticles show about 5 nm diameter (Figure 1c) and deposit uniformly on the CNTs’ surface (Figure 1d). The length of CNTs-SH is much shorter than the original one because of the acidification treatment process. As shown in Figure 1d, the CNTs’ surface is grafted PMMA-b-P(PEGMA) chains, while the Fe3O4 nanoparticles are well deposited on the surface.
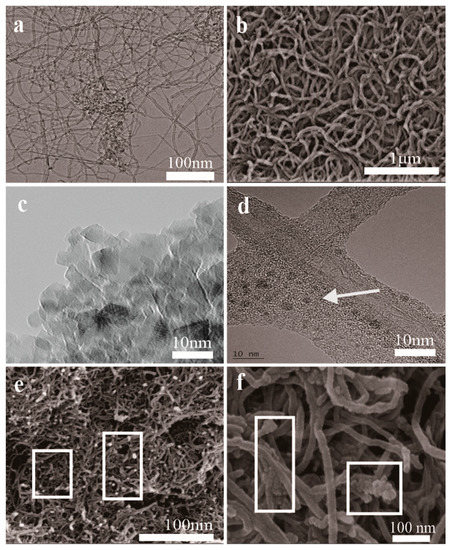
Figure 1.
SEM (b,e,f) and TEM (a,c,d) images of magnetically functionalized CNTs: (a) original CNTs; (b) mCNTs-SH; (c) Fe3O4; (d) mCNTs-g-Block; (e) mCNTs-g-PMMA; (f) mCNTs-g-Block.
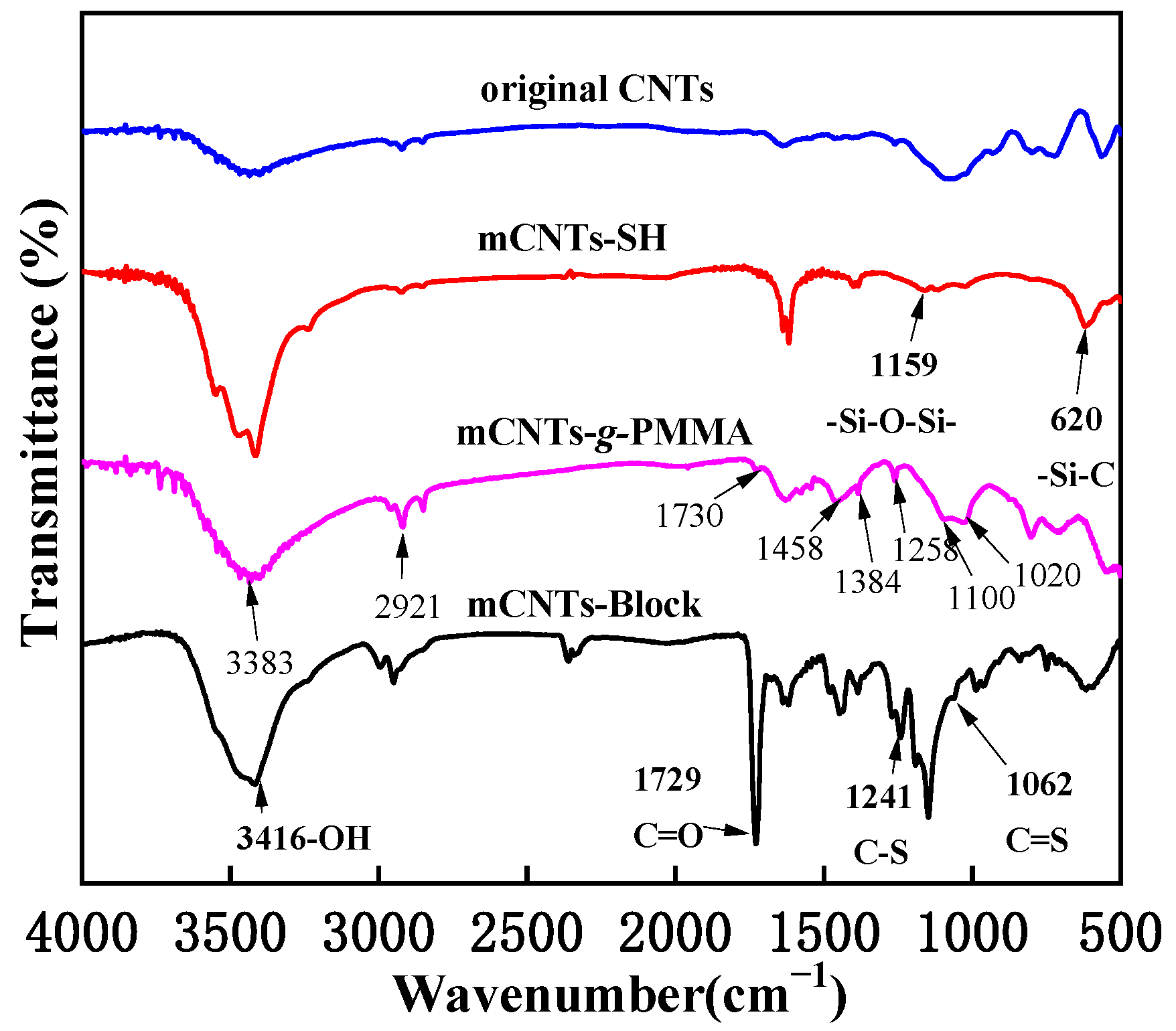
Figure 2 shows the FTIR spectra of magnetically functionalized CNTs. The absorption peaks at 1159 cm−1 and 660 cm−1 refer to stretching vibration peaks of -Si-O-Si- and -Si-C- groups produced by the thiolated process. These two new characteristic peaks also showed that the thiol group is successfully immobilized on the surface of CNTs. Compared with the original CNTs, the mCNTs-g-PMMA shows a strong C-H vibration peak of methylene (−CH2−) at about 2900 cm−1. There is a characteristic C-H peak of methyl (−CH3) at about 1460 cm−1 and 1350 cm−1, which indicates that PMMA is successfully grafted onto the surface of mCNTs. Following the click reaction between mCNTs-SH and alkynyl terminated diblock molecular chains, C-S and C=S characteristic peaks appeared at 1252 cm−1 and 1045 cm−1, respectively, corresponding to the thiocarbonate structure in the RAFT agent, indicating the preparation of mCNTs-block. In addition, the strength of–OH and carbonyl peaks at 3416 cm−1 and 1729 cm−1 were significantly enhanced, proving that the diblock P(PEGMA)-b-PMMA was achieved grafting onto the surface of CNTs.
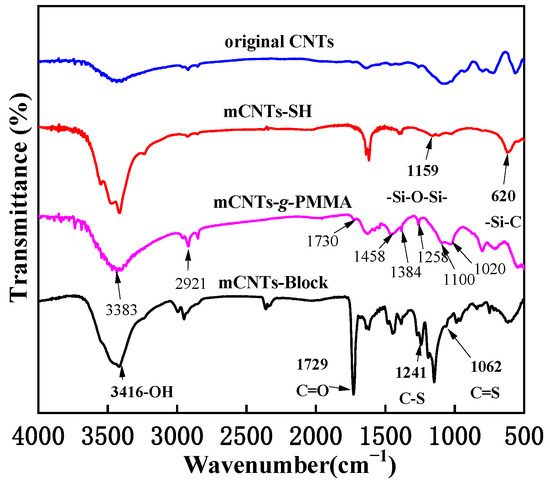
Figure 2.
FITR spectra of magnetically functionalized CNTs.
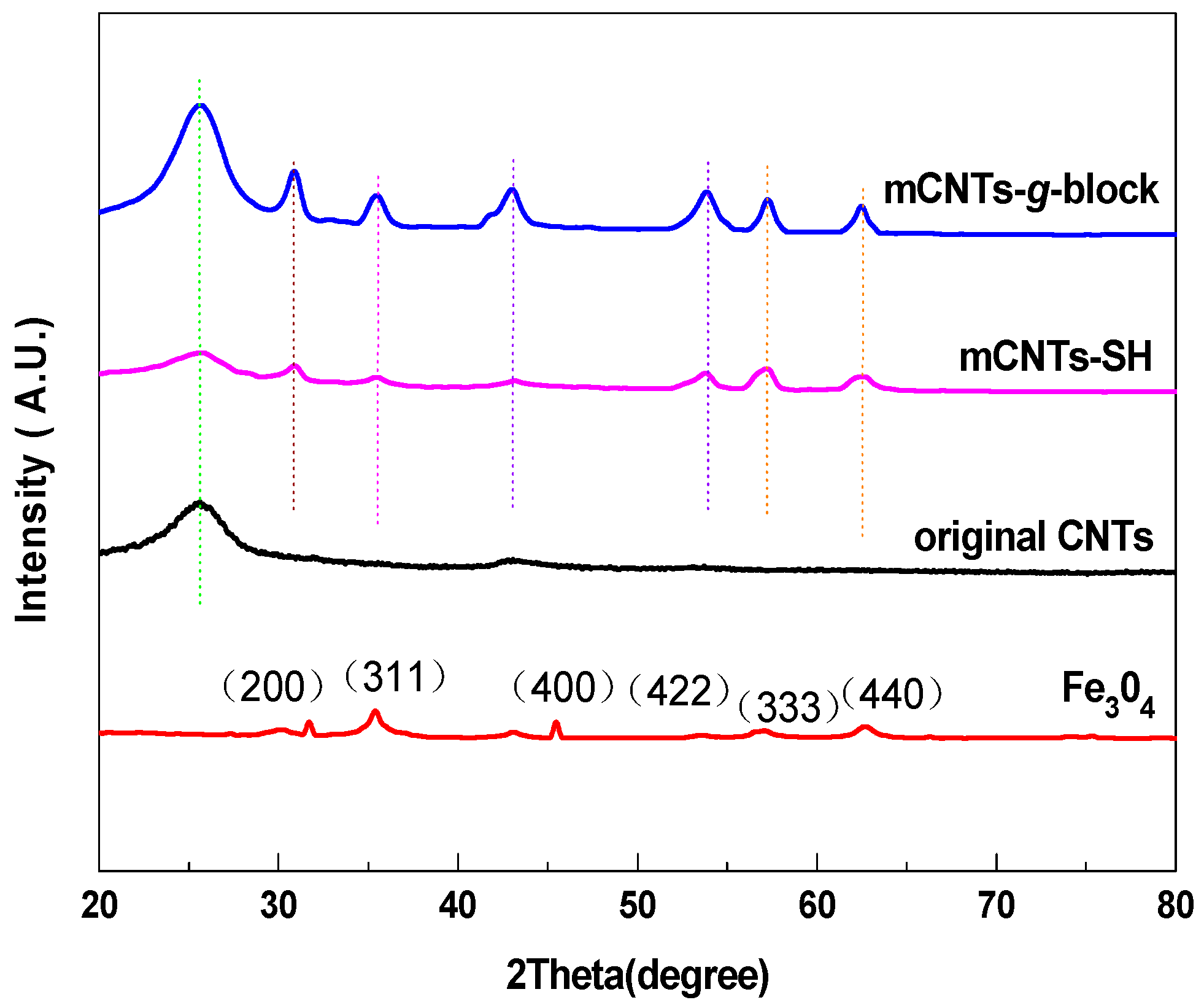
Figure 3 shows the XRD patterns of mCNTs-g-block, mCNTs-SH, mCNTs, and Fe3O4 particle samples. The Fe3O4 particles present peaks at 31.70°, 35.44°, 43.10°, 53.56°, 57.10°, and 62.66°, corresponding to the diffraction peaks of Fe3O4 at (200), (311), (400), (422), (333), and (440), respectively [2]. As these Fe3O4 particles are deposited on the CNTs, the characteristic peaks still keep in the samples, even after grafted single and diblock chains.
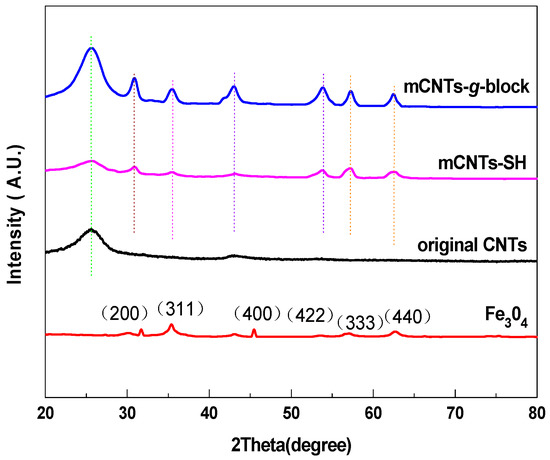
Figure 3.
XRD of magnetically functionalized CNTs and Fe3O4.
Figure 4 shows a hysteresis loop diagram of mCNTs, including –SH, single chains, and diblock chains grafting functionalized ones. All mCNT hysteresis curves pass through the origin, indicating that residual field strength (Br) and coercivity (HD) are equal to zero. The mCNTs and mCNTs-SH grafted with different polymer segments show superparamagnetism. Among them, the mCNTs-SH obtains a saturation magnetic field induction strength of 15.6 emu/g. The saturation magnetic induction strength of single-chain grafting mCNTs and diblock grafting mCNTs is 14.3 emu/g and 12.8 emu/g, respectively. Compared with mCNTs-SH, the saturation magnetic induction strength of the grafted mCNTs is reduced, which is caused by the reduction of the mass ratio of magnetic particles for grafting the polymer chain to the surface of mCNTs.
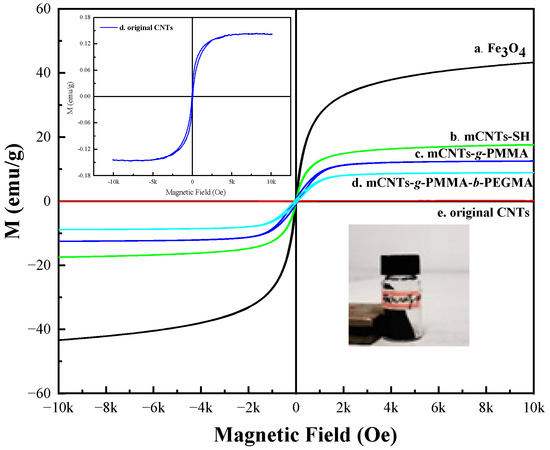
Figure 4.
Hysteresis loop diagram of magnetically functionalized CNTs.
3.2. Surface Property and Crystalline Phase Analysis for the PVDF Composited Membrane
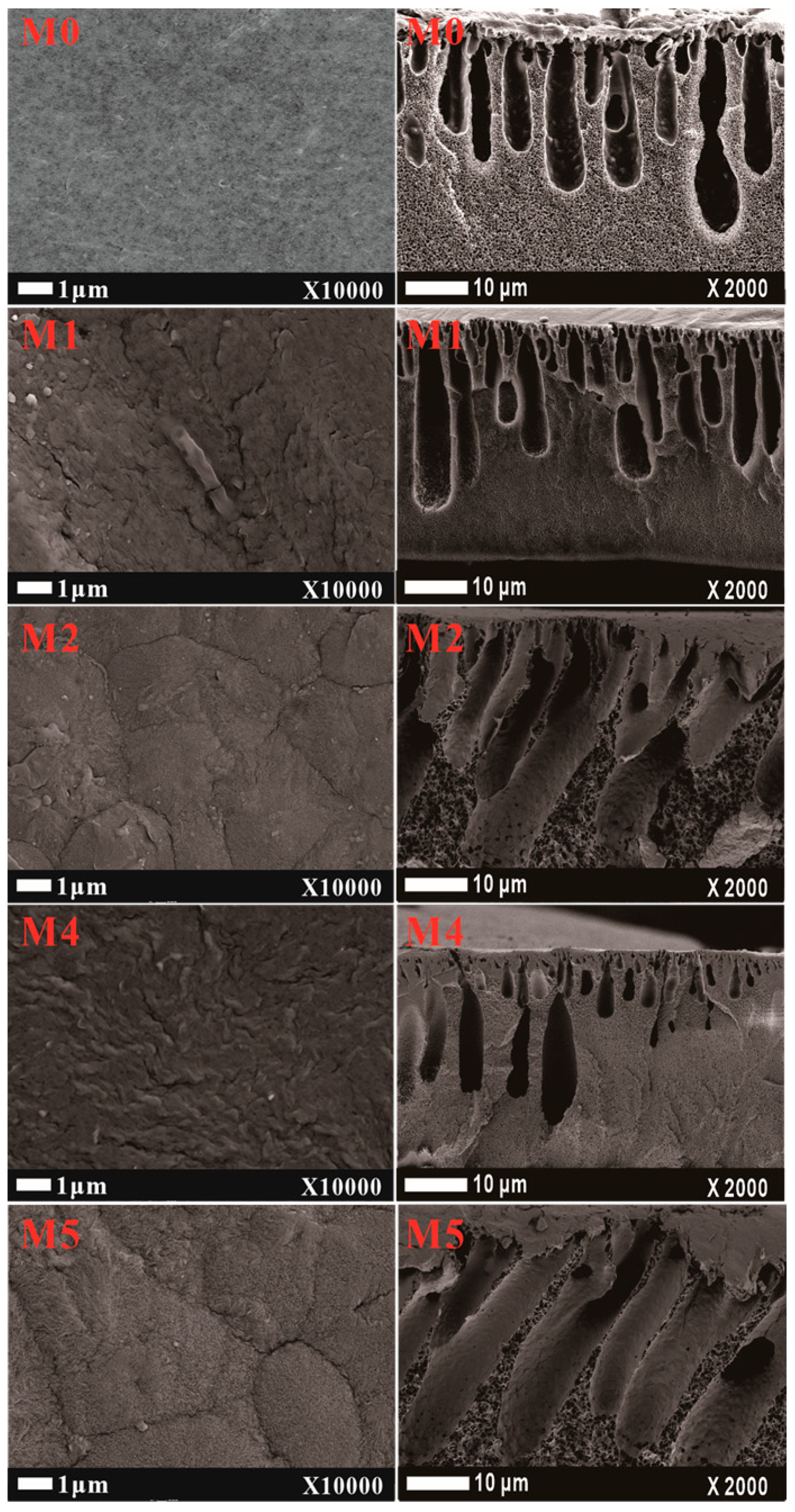
The SEM surface and cross-section of the PVDF composited membrane with mCNTs addition with or without magnetically orientating are illustrated in Figure 5. The morphology of the composited membrane significantly changes with the grafted mCNTs’ addition. The surface of pure PVDF membrane is relatively smooth and dense, and the cross-section structure is mostly spongy pores. After adding CNTs, the rough membrane surface is observed, and the finger-like pores are more obvious in the cross-section. Because of the interaction between PMMA molecular chains and PVDF chains, the mCNTs-g-PMMA favors pore formation during the NIPS process. Thus, the pore size of the M1 sample increased compared with the pure PVDF membrane (M0). As the diblock chains grafting mCNTs are incorporated into the membrane (M3), the roughness of the composite membrane is more evident. The pore size in the cross-section increases, and the finger-like pore becomes longer. The pore structure changes can be attributed to the more hydrophilic PEGMA chain segment’s interaction, making the phase inversion faster [23]. The surface and cross-section structure are not changed after magnetically orientating (M2 and M4).
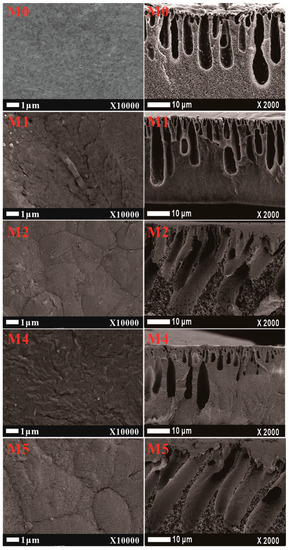
Figure 5.
The SEM surface and cross-section images of PVDF composited membranes with oriented or unoriented mCNTs grafting single and diblock chains. (The M0, M1, M2, M4, and M5 sample information is listed in Table 1.)
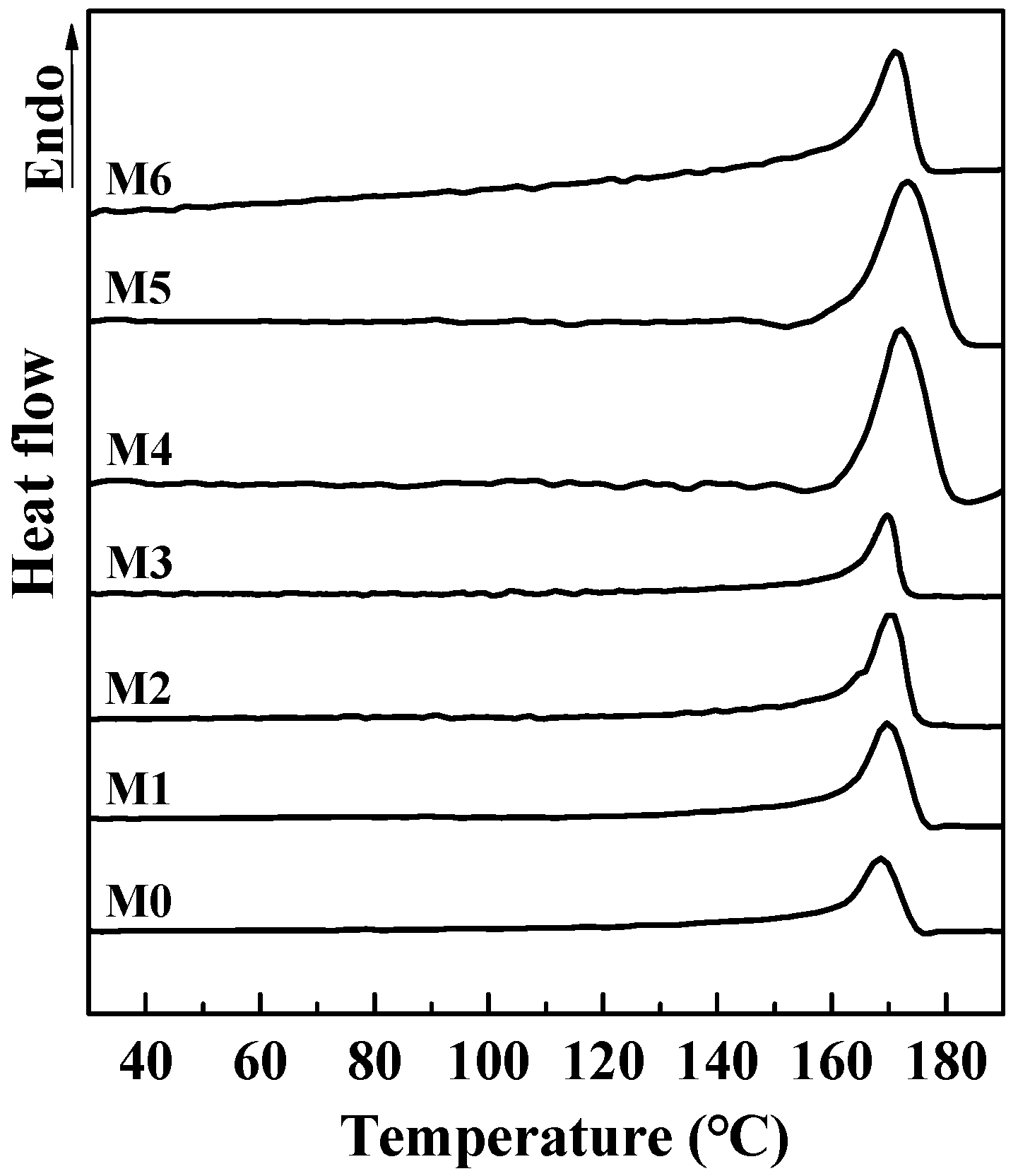
Figure 6 shows DSC melting curves of PVDF composited membranes incorporated with different amounts of mCNTs grafting single and diblock chains and membranes with oriented or unoriented mCNTs. For the pure PVDF membrane, the melting peak is at 169 °C. With the addition of CNTs, the melting peak shifts to a higher temperature, especially for the M4 and M5 membrane samples. This shift can be attributed to the enhancement of β-PVDF formation. FTIR results will discuss the detailed identification.
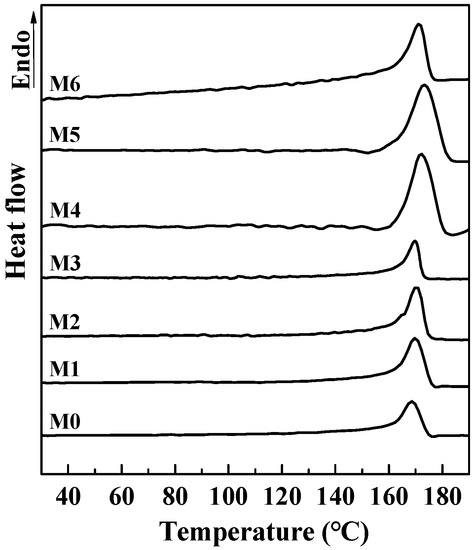
Figure 6.
DSC curves of PVDF composited membranes with various amounts of mCNTs grafting single and diblock chains and membranes with oriented or unoriented mCNTs. (The M0, M1, M2, M3, M4, M5 and M6 sample information is listed in Table 1.)
Table 2 reports the DSC melting data from Figure 6. The crystallinity of pure PVDF membrane is only 15.5%, and, with the addition of mCNTs, the crystallinity of the PVDF in the membrane is significantly improved. The 5 wt% addition of mCNTs grafting single and diblock chains in the PVDF composite membrane (M3, M4) increases the crystallinity to 24.5% and 35.2%, respectively. The PVDF crystallinity shows a small increase with the increase of grafted mCNTs’ addition, indicating an enhancement in crystallization by these mCNTs. However, when the mCNT-g-PMMA-b-P(PEGMA) is higher than 5 wt% (M6), the PVDF crystallinity in the composite membrane comes down (27.4 wt%). It is suggested that a high grafted mCNTs addition hinders the polymer crystallization.

Table 2.
DSC melting data of PVDF composited membranes with different amounts of mCNTs grafting single and diblock chains and membranes with oriented or unoriented mCNTs.
The PVDF crystallinity in the oriented mCNTs’ composite membrane (M2, M5) rises by about 1%, compared with those unoriented mCNTs’ composite membranes (M1, M4). The increased crystallinity can be attributed to the excellent β-PVDF nucleation effect by the oriented mCNTs. Thus, the M2 and M5 membrane samples achieve a higher melting temperature. As shown in Table 2, the mCNT-g-PMMA-b-P(PEGMA) has a more dominant enhancement on PVDF crystallization. PEGMA segments’ more flexible chain movement enhances the interaction between PVDF and mCNTs’ grafting diblock chains, favoring the PVDF crystallization.
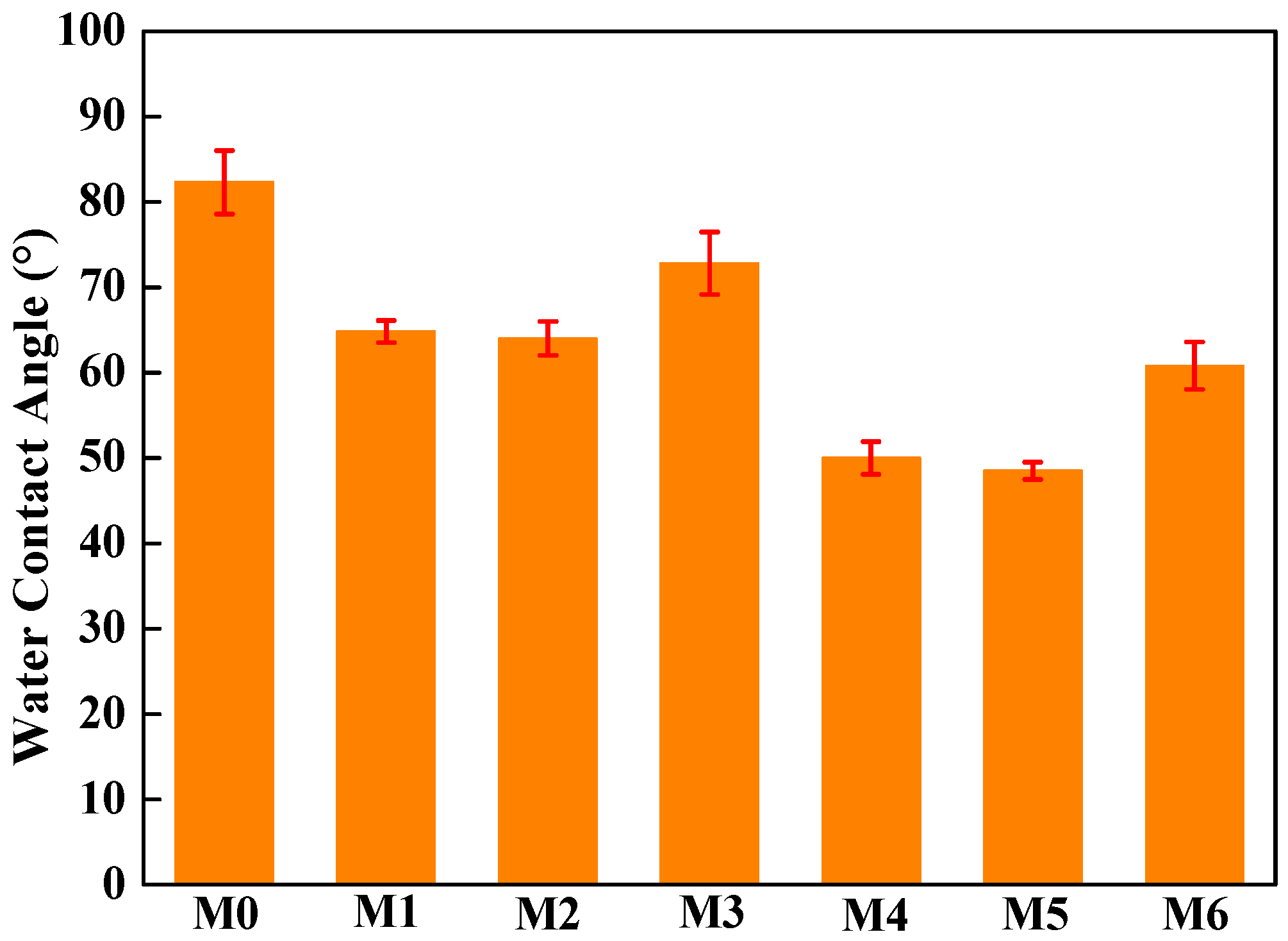
The water contact angle in Figure 7 shows the hydrophilicity of the different PVDF/mCNTs composite membranes. The pure PVDF membrane has a hydrophobic surface with a water contact angle of 82.3°. With the addition of the grafted mCNTs, the water contact angle decreases because of the hydrophilic polymer chain’s effect. Compared with mCNTs grafting single chains, the mCNT grafting diblock chains have more hydrophilicity enhancement because of the more hydrophilic PEGMA chains. For example, when 2 wt% mCNTs-g-PMMA is added, the water contact angle of M1 and M2 membranes decreases to 65°. When 5 wt% of mCNT grafting diblock chains was added, the water contact angle of M4 and M5 membranes went down to 50°. As the grafted mCNTs amount increases (in M3 and M6), the water contact angle shows a small rise again, which can be attributed to the agglomeration of mCNTs. For the composite membranes with grafted mCNTs before or after orientation, the hydrophilicity shows no change.
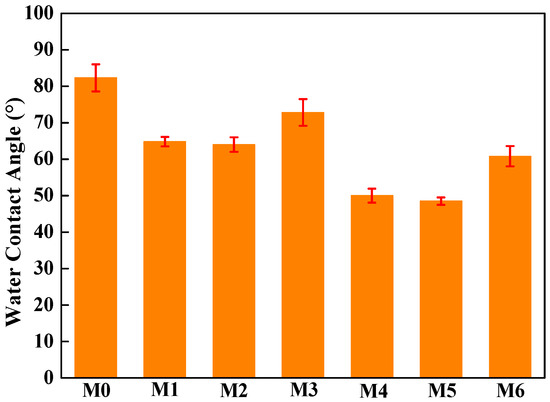
Figure 7.
Water contact angle of PVDF composited membranes with oriented or unoriented mCNTs grafting single and diblock chains and membranes with oriented or unoriented mCNTs.
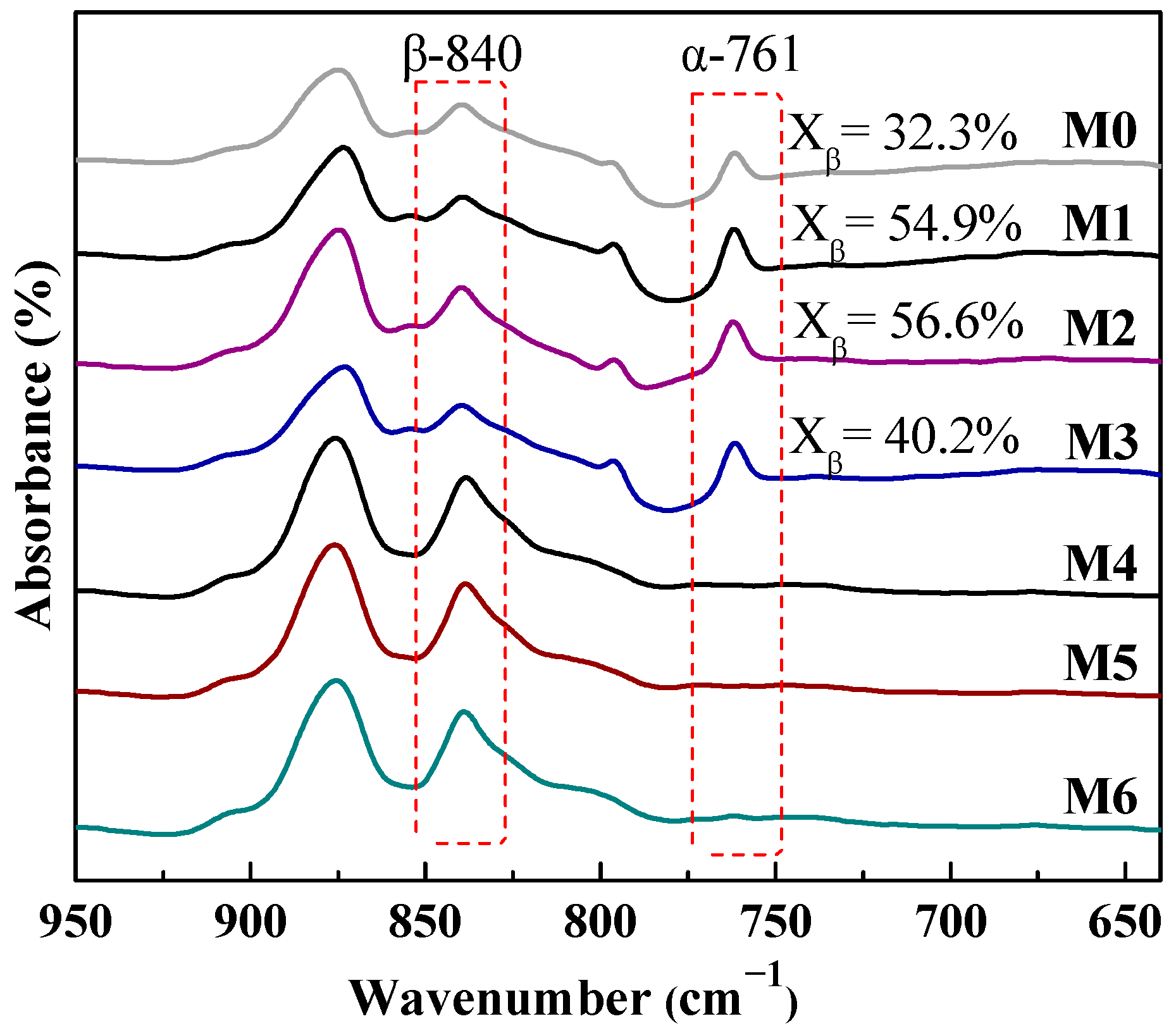
Figure 8 shows that the pure PVDF membrane (M0) has characteristic peaks at 761 cm−1 and 840 cm−1, which refer to α and β-PVDF crystalline phase. When adding 2~5 wt% mCNTs-g-PMMA into PVDF membrane, the α-PVDF still predominates in the crystalline phase. The β-PVDF content in the whole crystallinity increases to 40~56%.
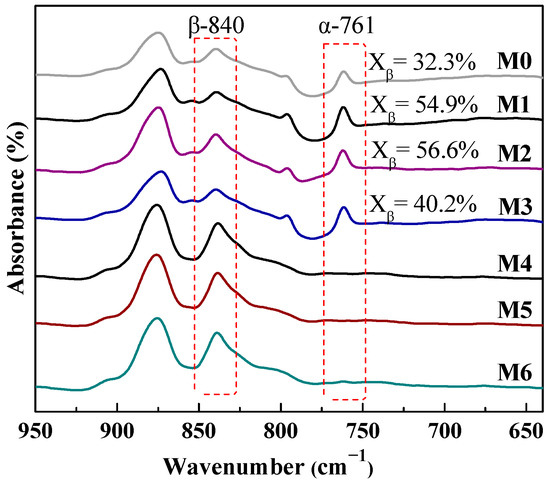
Figure 8.
FITR spectra of PVDF composited membranes with oriented or unoriented mCNTs grafting single and diblock chains and membranes with oriented or unoriented mCNTs.
When the mCNTs grafting diblock chains are added to the PVDF membrane (M4, M5 and M6), only β-PVDF exists in the crystalline phase. This result confirms that the mCNT grafting diblock chains favor the β-PVDF formation during the membrane preparation, the reason why higher melting peaks are obtained in the M4 and M5 samples (Figure 5). The composite membrane with oriented mCNTs (M2 and M5) shows no change compared with those without orientation (M1 and M4).
3.3. PVDF Composited Membrane Performance
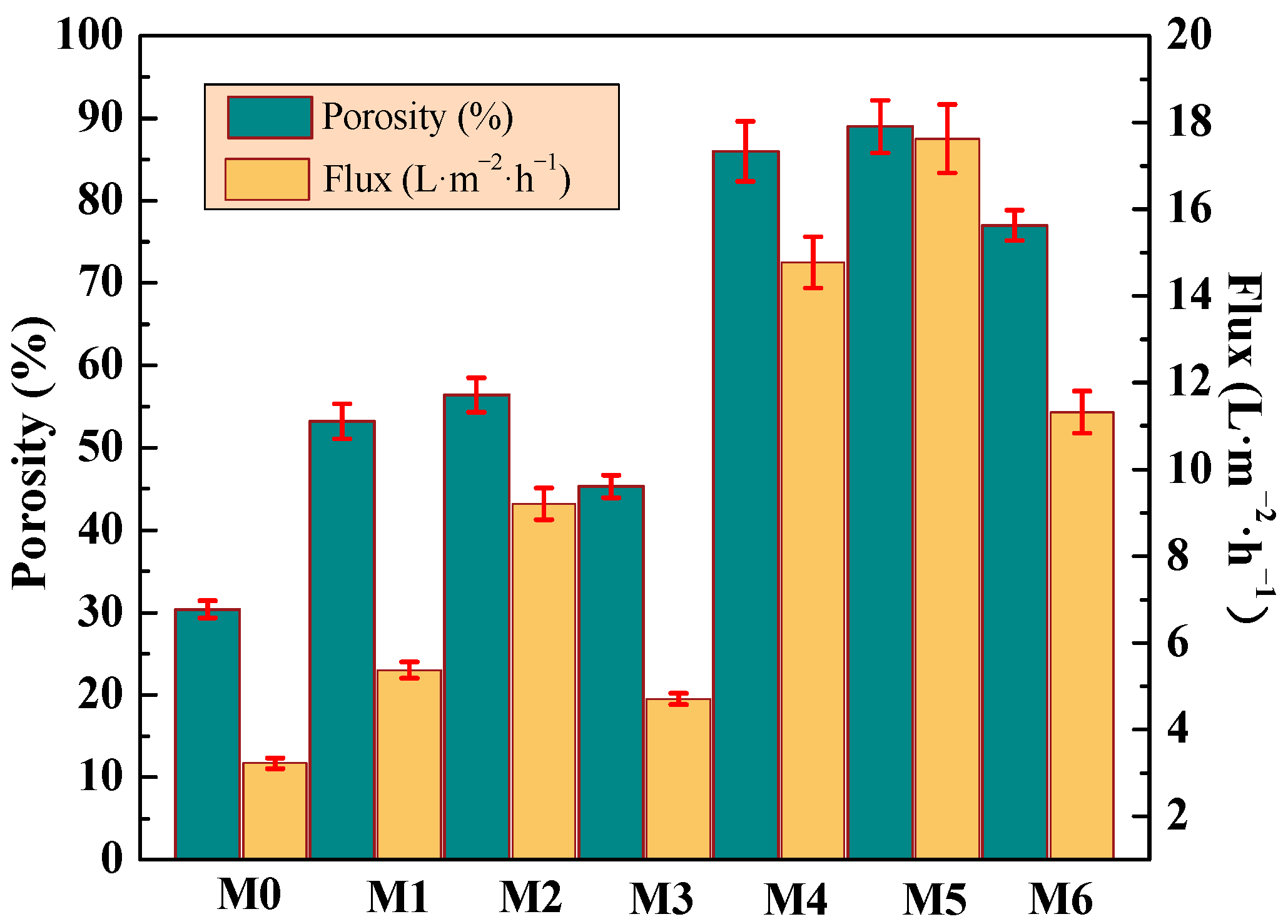
In Figure 9, with the addition of mCNTs grafting single and diblock chains, the porosity of the PVDF composite membrane increases from 30% (M0) to 53% and 85%, respectively. This increase occurs because hydrophilic mCNTs grafting diblock chains promote the pore density of the membrane and thus obtain high porosity. Due to the hydrophobicity of PVDF, the pure water flux of M0 sample is only 3.2 L/m2·h. The magnetical orientation of the grafted mCNTs promoted the formation of highly oriented nanochannels in the composite membranes. When the amount of mCNTs-g-PMMA is 2 wt%, the pure water flux increases to 5.3 LMH (M1) and to 10 LMH (M2) after the magnetical orientation. Due to the affinity between the hydrophilic segment PEGMA in mCNT grafting diblock chains and water molecules, the hydrophilicity is further enhanced. When the addition amount is 5 wt%, the pure water flux of the composite ultrafiltration membrane reaches the maximum value of 14 LMH (M4). The pure water flux rises up to 17.6 LMH in the magnetically oriented sample (M5). Due to the magnetic field-induced orientation, efficient nano-water channels were constructed, and high-performance composite membranes were obtained. Similarly, when the grafted mCNTs content increases (M3 and M6), the porosity and flux decrease somewhat.
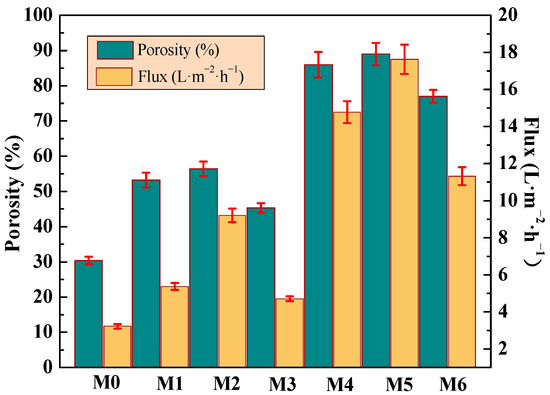
Figure 9.
Porosity, and pure water flux of the membranes incorporated with different amounts of mCNTs grafting single and diblock chains and membranes with oriented or unoriented mCNTs.
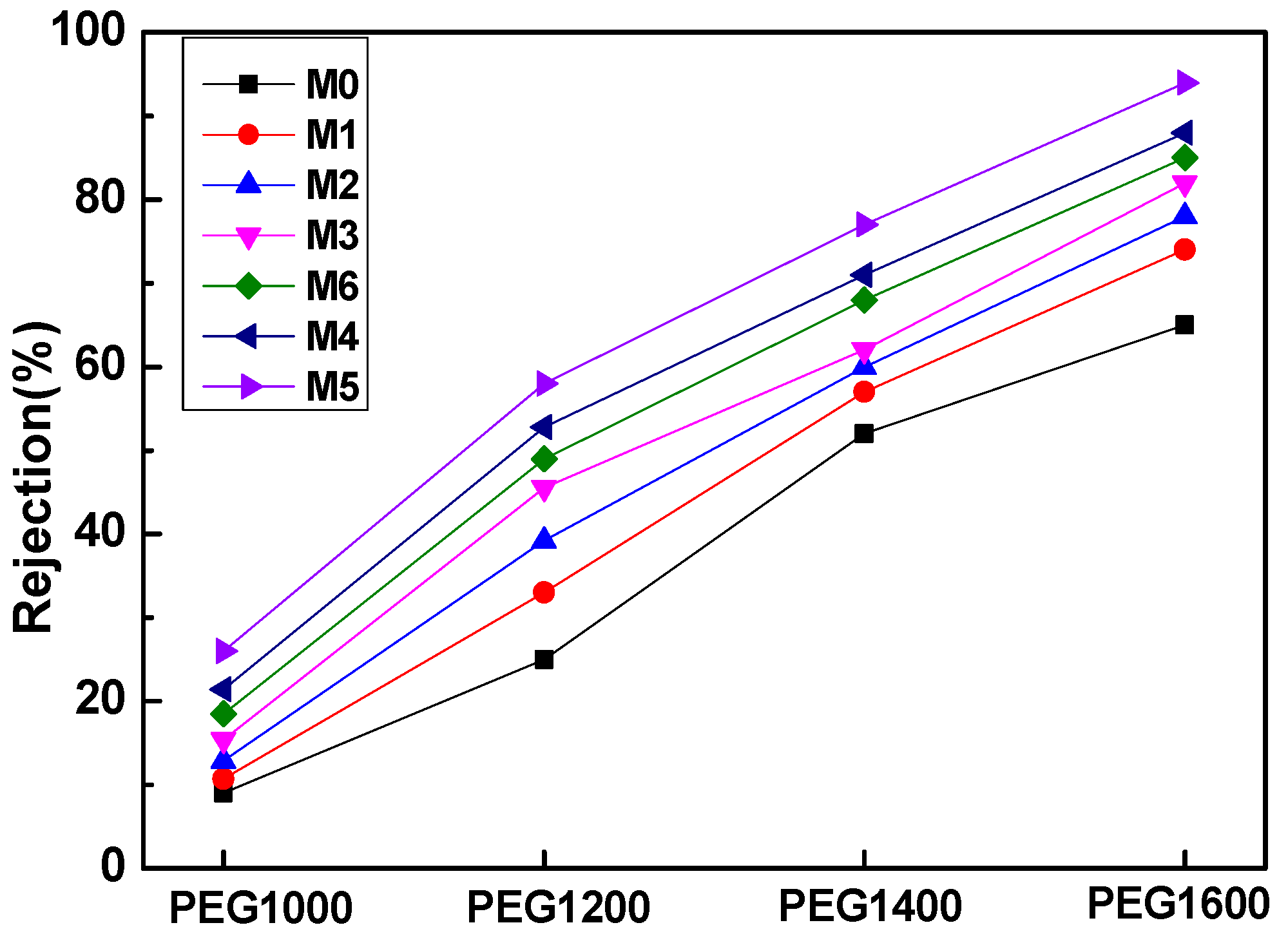
In Figure 10, the various PEG with different molecular weight cut-offs were used to evaluate the PVDF composite membanes’ rejection propensity. All prepared membranes can be applied for ultrafiltration membranes because even the PEG1600 rejection ranges from 60~90%. With the addition of mCNTs grafting single or diblock chains, the rejection percentage of different PEG1600 increased to 97%, compared to that of the pure PVDF membrane of 65% (M0). The increased PEG rejection can be assigned to the CNTs’ nanochannels’ effect. The mCNTs grafting diblock chains have significantly improved PEG rejection due to the well-dispersed nanochannels in the PVDF matrix, especially for the magnetically oriented one (M5). This result confirms that the magnetically functionalized mCNTs endow an excellent nanochannels’ effect on the membrane performance. The decreased water flux for M3 and M6 membranes can be attributed to the poorly dispersed mCNTs with grafted polymer chains. The water contact angle results appear in Figure 7. The hydrophilicity of M3 and M6 membranes indicates a small increase. As the grafted mCNTs amount increases (in M3 and M6), the water contact angle again attains a small rise, which can be attributed to the agglomeration of mCNTs. The hydrophilicity shows no change for the composite membranes with grafted mCNTs before or after orientation. The decreased water flux can be attributed to the poorly dispersed mCNTs with grafted polymer chains. As for M3 membranes, the content of the grafted mCNTs was higher than the M2 sample; the aggregated mCNTs cannot perform the nanochannels. Thus, the water flux decreased (Figure 9). The PEG rejection of the M3 membrane can be attributed to the smaller porosity in the membrane (Figure 9).
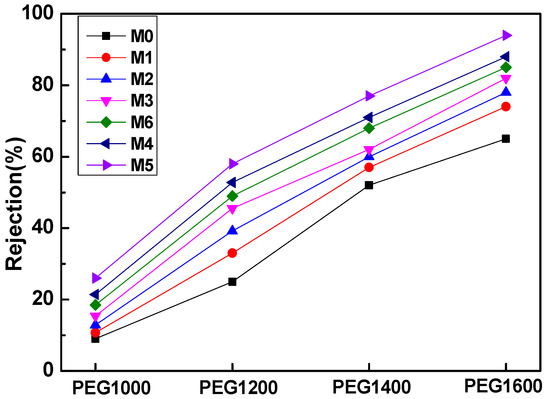
Figure 10.
Rejection characterization of four different molecular weight polyethylene glycol (PEG) with different membrane samples.
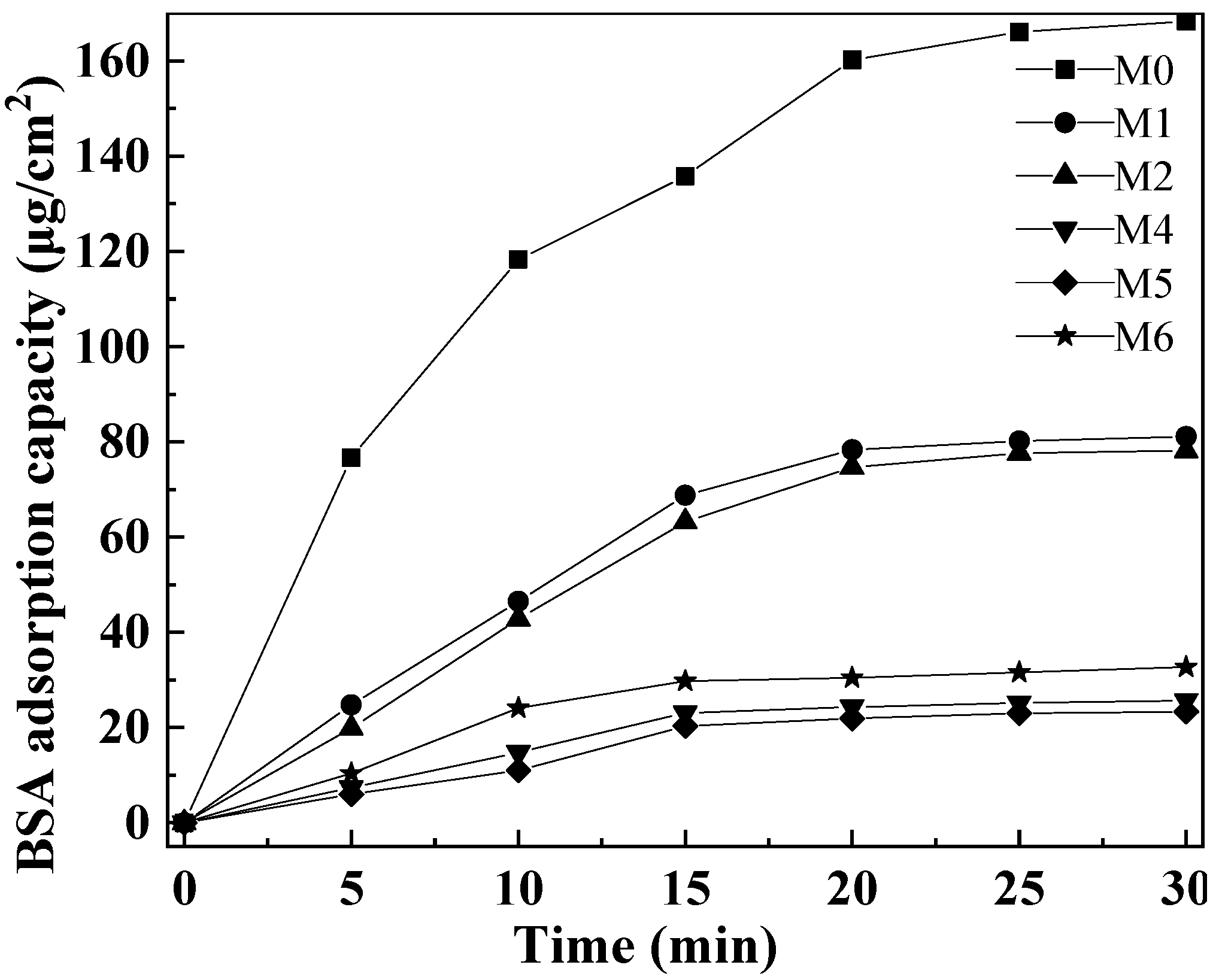
Figure 11 shows the BSA adsorption data of PVDF composite membranes with different amounts of mCNTs grafting single and diblock chains and membranes with oriented or unoriented mCNTs. The pure PVDF membrane M0 reaches a BSA adsorption capacity of 168.3 μg/cm2 because of its hydrophobicity. With the addition of mCNT-g-PMMA, the hydrophilicity increases, making the BSA adsorption amount reach the balance of 81.1 μg/cm2. The BAS absorption value decreased as mCNT grafting diblock chains were added to the composite membrane due to the more hydrophilic PEGMA chain segment. Thus, the BSA adsorption capacity of M4 and M6 is, respectively, 25.7 μg/cm2 and 32.7 μg/cm2. Due to the strong agglomeration of magnetic nanoparticles contained in mCNTs, the hydrophilic nanoparticles partially agglomerated, which reduced the anti-BSA adsorption effect of M6 composite ultrafiltration membrane with 10 wt% of mCNT-g-PMMA-b-P(PEGMA). Additionally, the composite membrane with oriented mCNTs has little enhanced BSA absorption, which may be due to the more hydrophilic polymer segments enriched in the membrane surface.
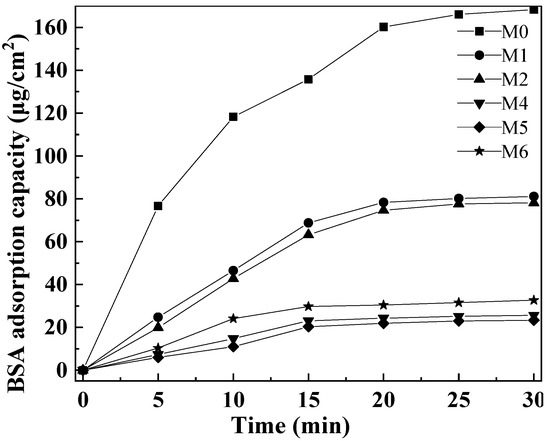
Figure 11.
BSA dynamic adsorption of the composited membranes with various amounts of mCNTs grafting single and diblock chains and membranes with oriented or unoriented mCNTs.
3.4. The Orientation Identification of Grafted mCNTs in the Membrane Matrix
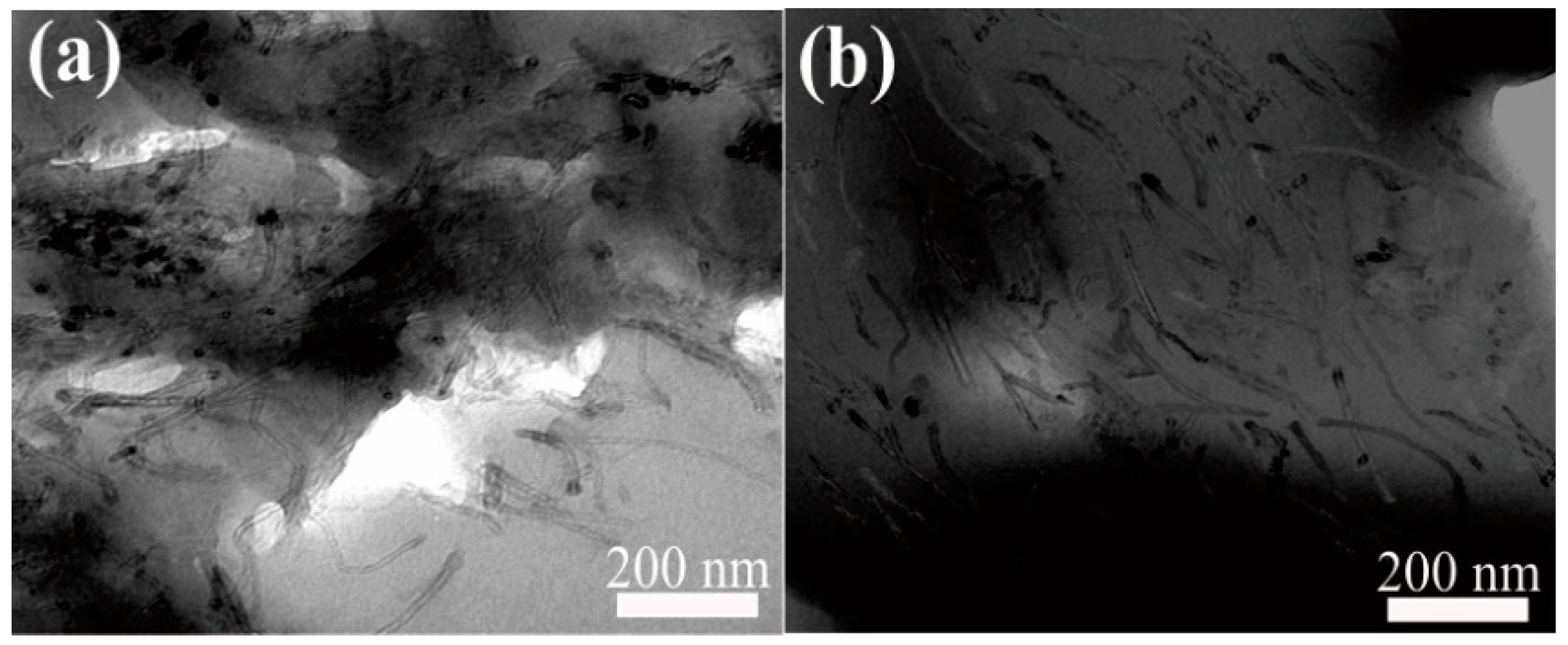
Figure 12 shows the cross-sectional TEM images of the membrane (M5) with 5 wt% oriented and non-oriented mCNTs grafting diblock chains. Before the orientation by the magnetic field, the mCNT grafting diblock chains are haphazardly dispersed in the PVDF membrane matrix (Figure 12a). After orienting by the magnetic introduction, the mCNT grafting diblock chains can be oriented in the same direction in the PVDF matrix membrane, indicating that the ordered nanochannels were achieved, as shown in Figure 12b.
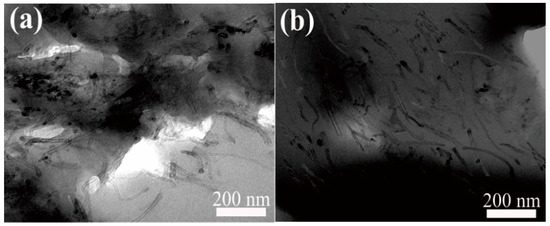
Figure 12.
Cross-sectional TEM of PVDF composite membranes with 5 wt% oriented (a) VS. non-oriented mCNTs grafting diblock chains (b).
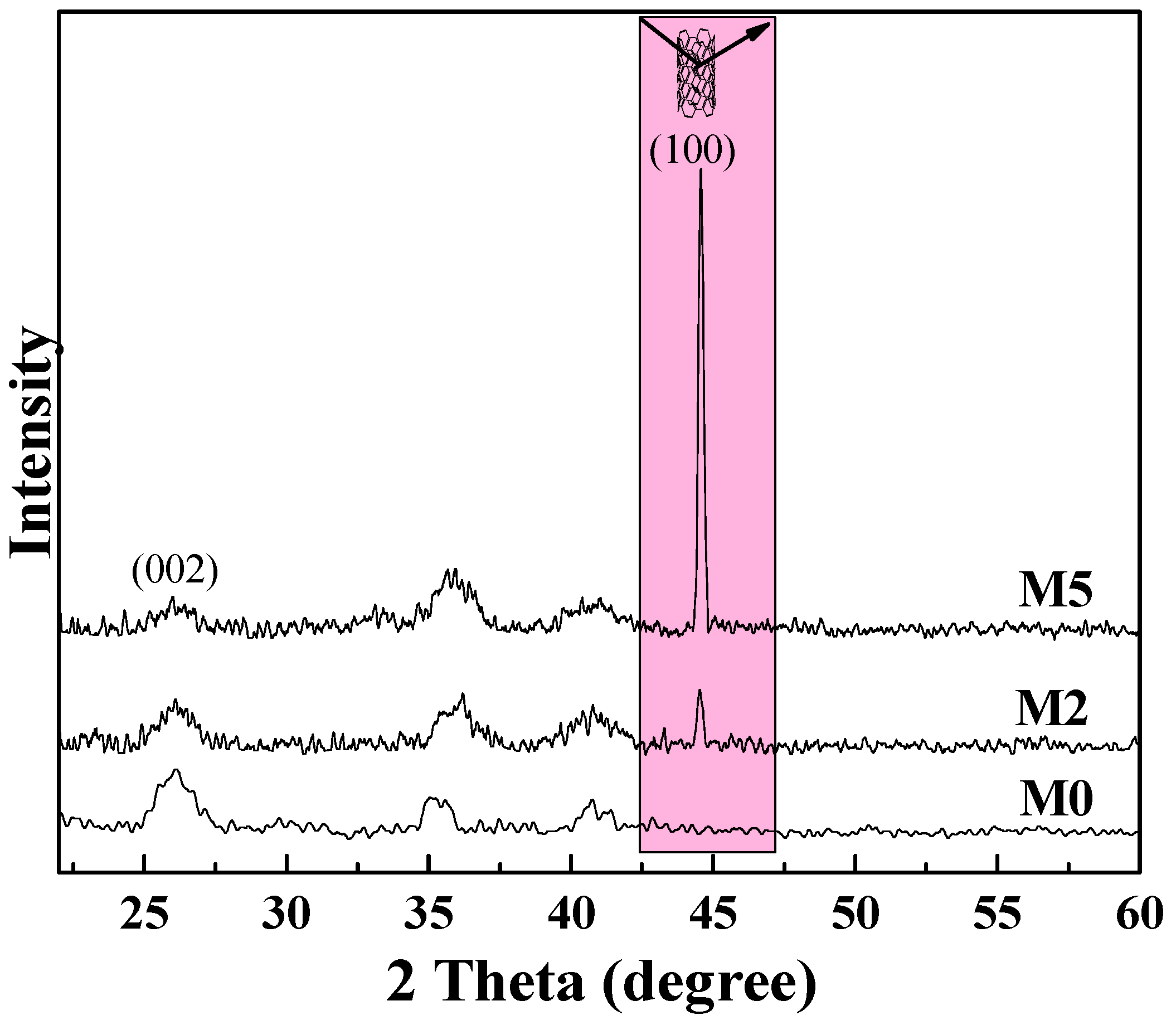
The XRD pattern of the M5 membrane (as shown in Figure 13) confirms the vertical orientation of mCNTs in the matrix membrane. The pure PVDF membrane (M0) has a strong α-PVDF crystal phase peak at (002) crystal plane. When the addition of mCNT-g-PMMA-b-P(PEGMA) is 5 wt%, the oriented mCNTs weakened the α-PVDF crystal in the composite membrane. This result agrees with the FTIR and DSC results above. The diffraction peak at 46° is related to the relative content of mCNTs with vertical orientation [10]. The diffraction peak at 45° of M5 is much stronger than that of M4 membrane, which indicates that there are many vertically oriented mCNTs in the M5 composite membrane, proving the construction of its oriented nan channels in this composite membrane.
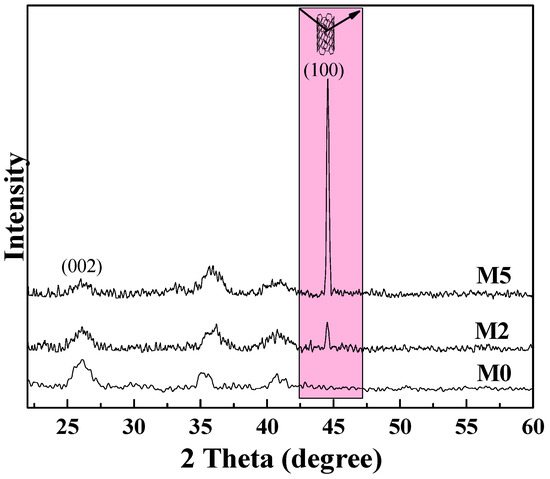
Figure 13.
XRD diagram of PVDF composite membranes with 5 wt% oriented or non-oriented mCNTs grafting diblock chains.
4. Conclusions
The magnetically functionalized CNTs, including mCNTs grafting single and diblock chains, were synthesized in this work. To realize the interface design structure, the two copolymers were grafted onto magnetized mCNT-SH. The magnetized mCNTs-SH, mCNTs-g-PMMA, and magnetized mCNTs grafting diblock chains have superparamagnetism and can obtain orientation under a magnetic field introduction. The original PVDF membrane illustrates a higher water contact angle (82°), and the pure water flux was also low (3.2 L/m2·h). The mCNTs’ interface layer structure can enhance the membrane’s hydrophilicity. For the composite membrane composed of mCNTs grafting single and diblock chains, introducing more hydrophilic PEGMA chains can reduce the water contact angle to 48.5° and make the pure water flux reach the maximum of 17.6 L/m2·h. When mCNT is oriented in the membrane, the magnetically introduced orientation can construct a no-water channel, significantly improving the ultrafiltration membrane’s water flux. The BSA absorption results showed that the membrane incorporating mCNTs grafting diblock chains has an excellent antifouling propensity with the lowest BSA absorption. These results can be attributed to the more hydrophilic PEGMA chains in the grafted mCNTs. The TEM and XRD results confirmed that the oriented mCNTs had been achieved.
Author Contributions
Data collation, X.S. and W.M.; Formal analysis, X.S., W.M. and P.P.; Funding acquisition, W.M.; Investigation, X.S. and W.M.; Methodology, S.Y., B.M. and H.Y.; Project management, W.M.; Writing-original draft, X.S. and W.M.; Writing–Reviewing and Editing, X.S., W.M., F.G. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (21406017), The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP). This research was funded by Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_3036, KYCX22_3010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zou, D.; Chen, X.; Drioli, E.; Ke, X.; Qiu, M.; Fan, Y. Facile co-sintering process to fabricate sustainable antifouling silver nanoparticles (AgNPs)-enhanced tight ceramic ultrafiltration membranes for protein separation. J. Membr. Sci. 2020, 593, 117402. [Google Scholar] [CrossRef]
- Ma, W.; Song, X.; Yin, S.; Peng, H.; Yang, H.; Gong, F.; Liu, C.; Zhong, J. Grafting an amphiphilic block copolymer to magnetic-functionalized carbon nanotubes and their nanochannels in membranes. ACS Appl. Polym. Mater. 2021, 3, 6468–6478. [Google Scholar] [CrossRef]
- Suhartono, J.; Pertiwi, D.S.; Noersalim, C.; Yulianingsih, D.; Sofianti, F.; Saptoro, A.; Chafidz, A. Characteristics and performances of blended polyethersulfone and carbon-based nanomaterial membranes: Effect of nanomaterial types and air exposure. Chem. Eng. Technol. 2020, 43, 1630–1637. [Google Scholar] [CrossRef]
- Li, G.; Feng, L.; Zhai, Z.; Wang, F. Study on enhancing effect of carbon nanotubes on the interlayer strength of 3D printing of plastic powder. AIP Adv. 2020, 10, 065106. [Google Scholar] [CrossRef]
- Du, C.; Li, M.; Cao, M.; Feng, S.; Guo, H.; Li, B. Enhanced thermal and mechanical properties of polyvinlydene fluoride composites with magnetic oriented carbon nanotube. Carbon 2018, 126, 197–207. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, Y.; Li, Y.; Zhang, P.; Cao, Z.; Yang, H.; Liu, C.; Tao, G.; Gong, F.; Matsuyama, H. Synthesis of hydrophilic carbon nanotubes by grafting poly(methyl methacrylate) via click reaction and its effect on poly(vinylidene fluoride)-carbon nanotube composite membrane properties. Appl. Surf. Sci. 2018, 435, 79–90. [Google Scholar] [CrossRef]
- Li, B.; Zhou, J.; Xu, X.; Yu, J.; Shao, W.; Fang, Y.; Lu, X. Solvent quality affects chain conformational order at the polymer surface revealed by sum frequency generation vibrational spectroscopy. Polymer 2013, 54, 1853–1859. [Google Scholar] [CrossRef]
- John, J.; Klepac, D.; Didović, M.; Sandesh, C.J.; Liu, Y.; Raju KV, S.N.; Pius, A.; Valić, S.; Thomas, S. Main chain and segmental dynamics of semi interpenetrating polymer networks based on polyisoprene and poly(methyl methacrylate). Polymer 2010, 51, 2390–2402. [Google Scholar] [CrossRef]
- Regev, C.; Belfer, S.; Holenberg, M.; Fainstein, R.; Parola, A.H.; Kasher, R. Fabrication of poly(ethylene glycol) particles with a micro-spherical morphology on polymeric fibers and its application in high flux water filtration. Sep. Purif. Technol. 2019, 210, 729–736. [Google Scholar] [CrossRef]
- Chen, G.E.; Sun, W.G.; Kong, Y.F.; Wu, Q.; Sun, L.; Yu, J.; Xu, Z.L. Hydrophilic Modification of PVDF microfiltration membrane with poly (Ethylene Glycol) dimethacrylate through surface polymerization. Polym. Plast. Technol. Eng. 2018, 57, 108–117. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Kwiecinska-Mydlak, A. Recent advances in water and wastewater treatment using membranes with carbon nanotubes. Membr. Water Treat. 2022, 13, 259–290. [Google Scholar]
- Demirel, E.; Dadashov, S. Fabrication of a novel PVDF based silica coated multi-walled carbon nanotube embedded membrane with improved filtration performance. Chem. Eng. Commun. 2022, 209, 1009–1034. [Google Scholar] [CrossRef]
- An, X.Y.; Wu, H.C.; Li, Y.J.; He, X.W.; Chen, L.X.; Zhang, Y.K. The hydrophilic boronic acid-poly(ethylene glycol) methyl ether methacrylate copolymer brushes functionalized magnetic carbon nanotubes for the selective enrichment of glycoproteins. Talanta 2020, 210, 120632. [Google Scholar] [CrossRef]
- Li, G.; Zheng, H.; Bai, R. A facile strategy for the preparation of azide polymers via room temperature RAFT polymerization by redox initiation. Macromol. Rapid Commun. 2009, 30, 442–447. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, Y.; Zhu, Z.; Guo, L.; Cao, Z.; Xia, Y.; Yang, H.; Gong, F.; Zhong, J. Synthesis of poly(methyl methacrylate) grafted multiwalled carbon nanotubes via a combination of RAFT and alkyne-azide click reaction. Appl. Sci. 2019, 9, 603. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Feng, Y.; Liao, Y.; Ye, Y.; Xie, X.; Mai, Y.W. Ultralow-carbon nanotube-toughened epoxy: The critical role of a double-layer interface. ACS Appl. Mater. Interfaces 2018, 10, 1204–1216. [Google Scholar] [CrossRef]
- Hosono, T.; Takahashi, H.; Fujita, A.; Joseyphus, R.J.; Tohji, K.; Jeyadevan, B. Synthesis of magnetite nanoparticles for AC magnetic heating. J. Magn. Magn. Mater. 2009, 321, 3019–3023. [Google Scholar] [CrossRef]
- Luo, H.; Huang, Y.; Wang, D. The crystallization and crystal transition of PVDF in PAN nano-tube. Polymer 2013, 54, 4710–4718. [Google Scholar] [CrossRef]
- Xu, S.; Ma, W.; Yang, H.; Cao, Z.; Gong, F.; Liu, C. Cross-Linking Combined with Surfactant Bilayer Assembly Enhances the Hydrophilic and Antifouling Properties of PTFE Microfiltration Membranes. Separtions 2022, 9, 2. [Google Scholar] [CrossRef]
- María, N.; Patil, Y.; Polymeropoulos, G.; Peshkov, A.; Rodionov, V.; Maiz, J.; Hadjichristidis, N.; Müller, A.J. (PVDF)2(PEO)2 miktoarm star copolymers: Synthesis and isothermal crystallization leading to exclusive β-phase formation. Eur. Polym. J. 2022, 179, 111506. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, S.; Li, M.; Xue, A.; Zhang, Y.; Zhao, Y.; Zhong, J.; Yang, D. PVDF/palygorskite composite ultrafiltration membranes: Effects of nanoclay particles on membrane structure and properties. Appl. Clay Sci. 2019, 181, 105171. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Koushkbaghi, S.; Hosseini, S.S.; Darabi, M.; Yousefi, A.; Irani, M. Metal organic framework nanoparticles loaded-PVDF/chitosan nanofibrous ultrafiltration membranes for the removal of BSA protein and Cr(VI) ions. J. Mol. Liq. 2020, 317, 113934. [Google Scholar] [CrossRef]
- Lin, Y.C.; Tseng, H.H.; Wang, D.K. Uncovering the effects of PEG porogen molecular weight and concentration on ultrafiltration membrane properties and protein purification performance. J. Membr. Sci. 2021, 618, 118729. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).