Green and Fast Extraction of Chitin from Waste Shrimp Shells: Characterization and Application in the Removal of Congo Red Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis and Characterization of Chitin
2.3. Congo Red Adsorption
2.4. Adsorption Isotherms
2.5. Kinetics and Thermodynamic Parameters of the Adsorption Process
2.6. Desorption Studies
3. Result and Discussion
3.1. Characterization of Materials Adsorbents
3.1.1. Chitin Extraction and Composition
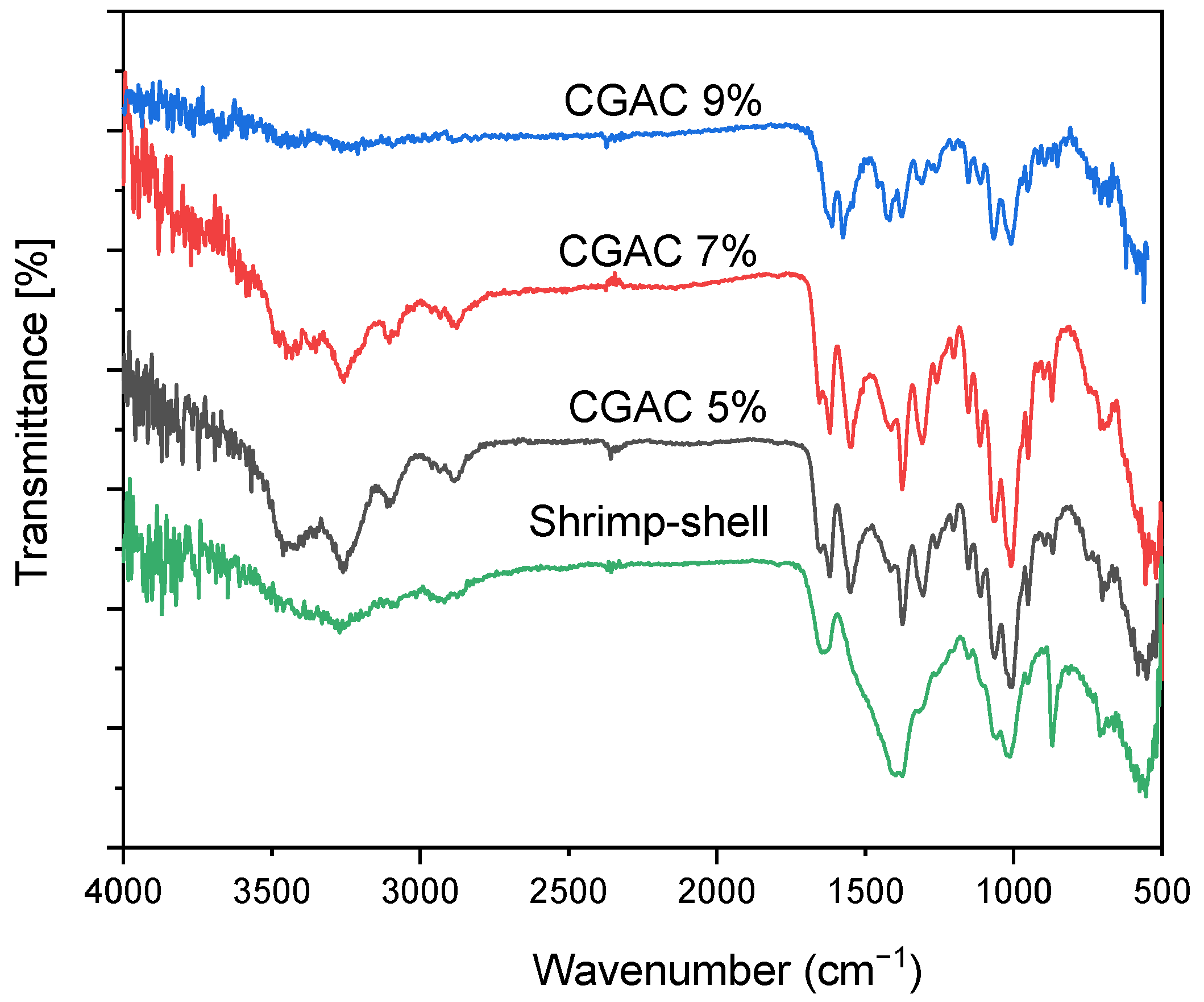
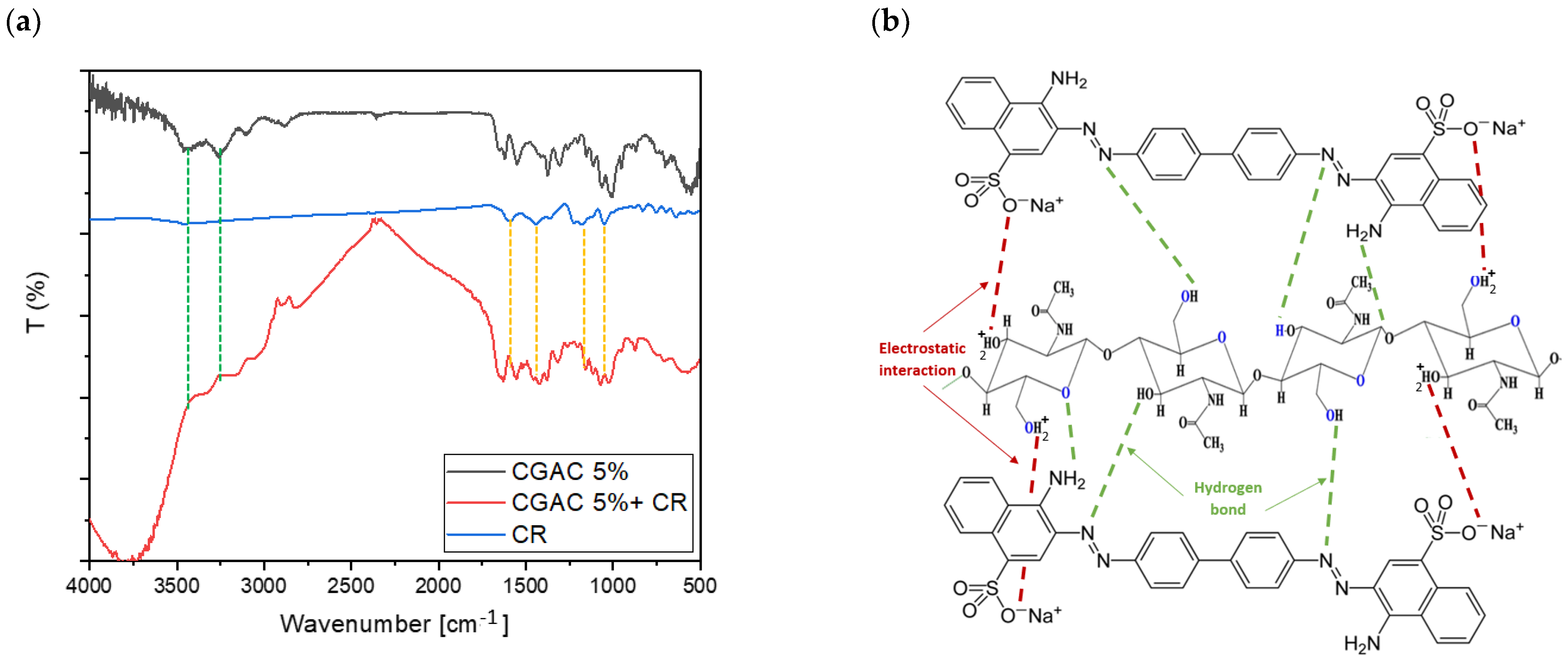
3.1.2. FT-IR Analysis
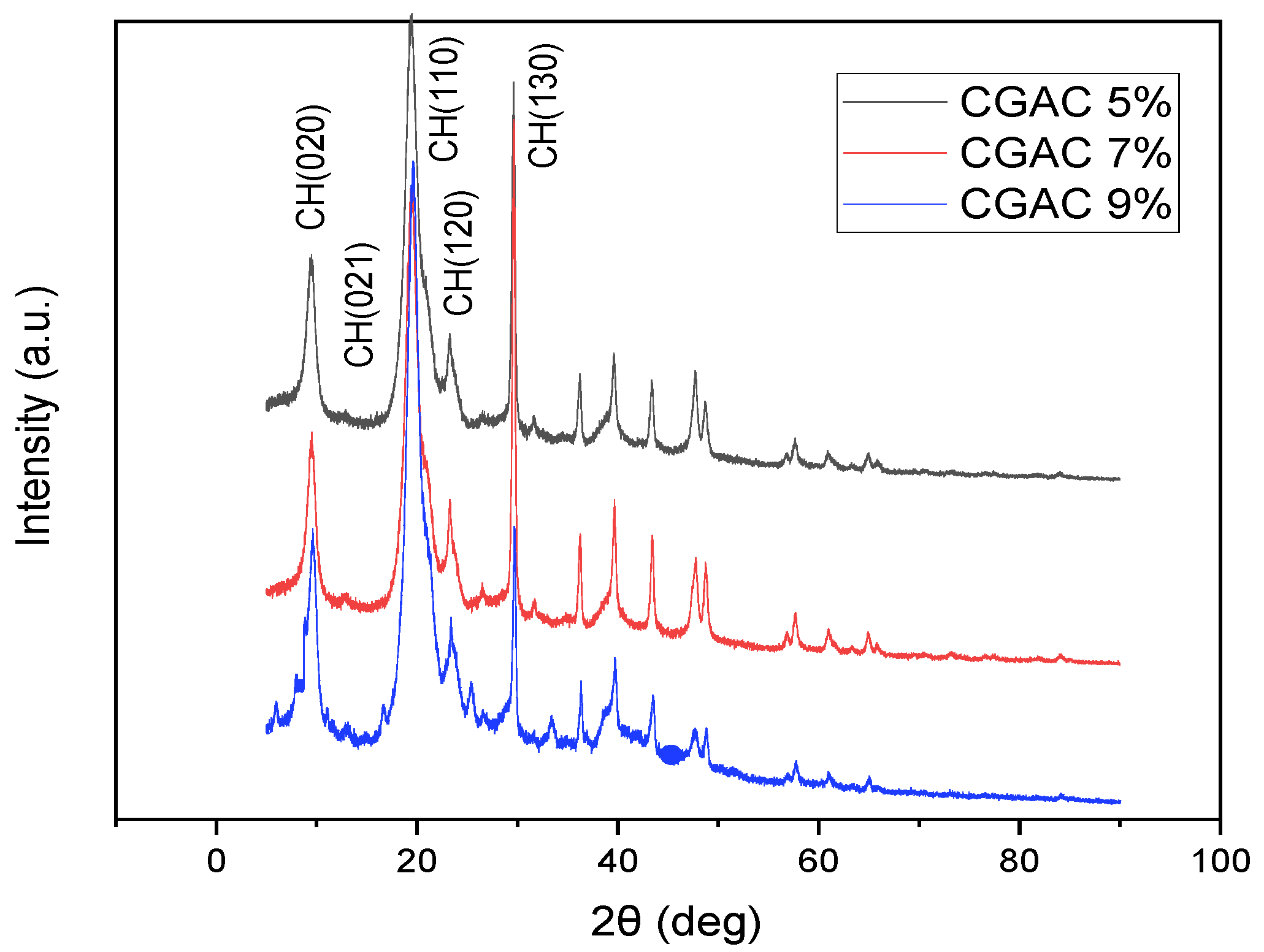
3.1.3. X-ray Diffraction Analysis of Chitin
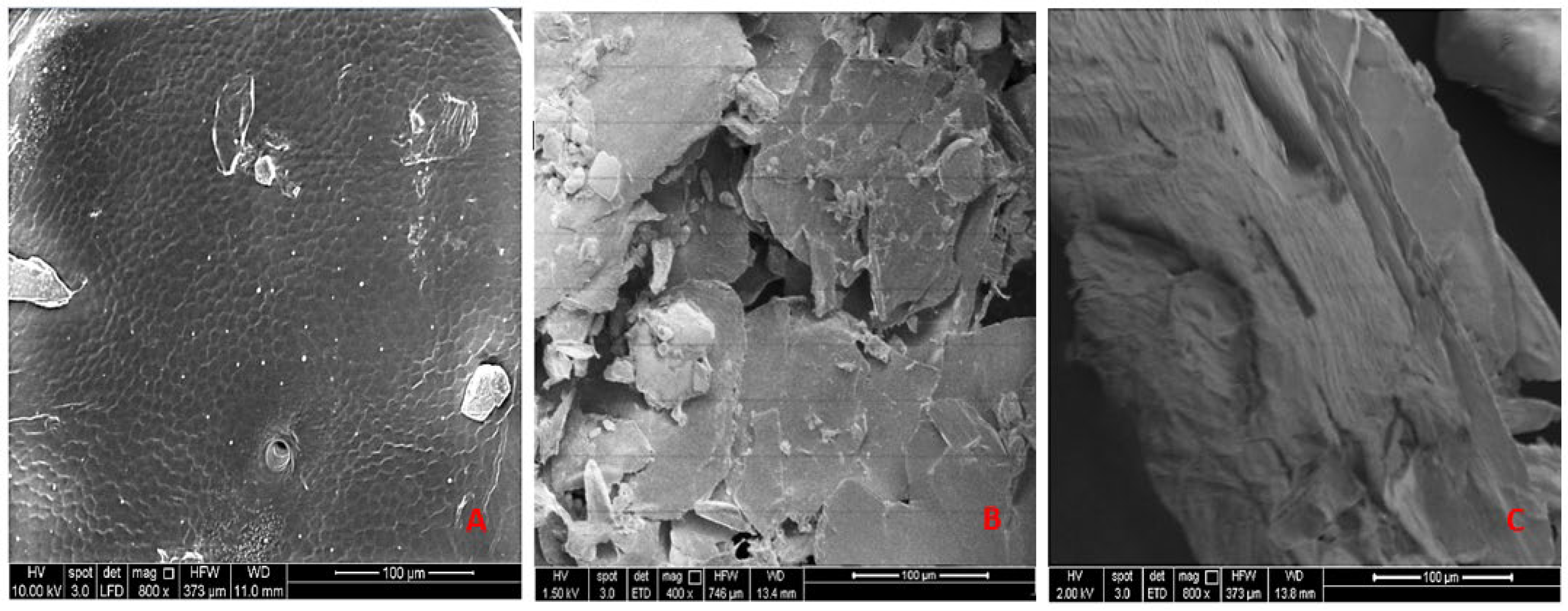
3.1.4. Scanning Electron Microscopy (SEM)
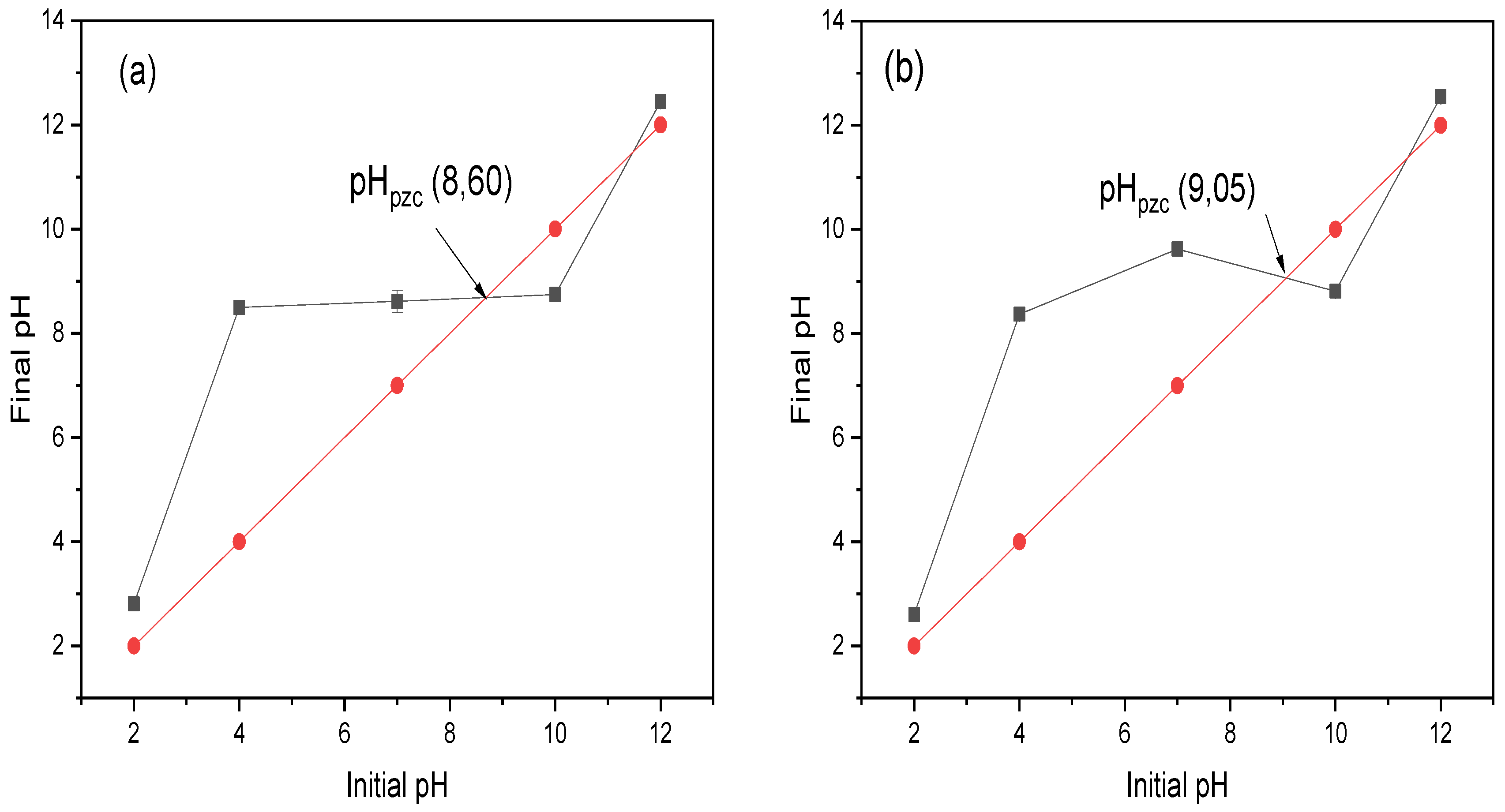
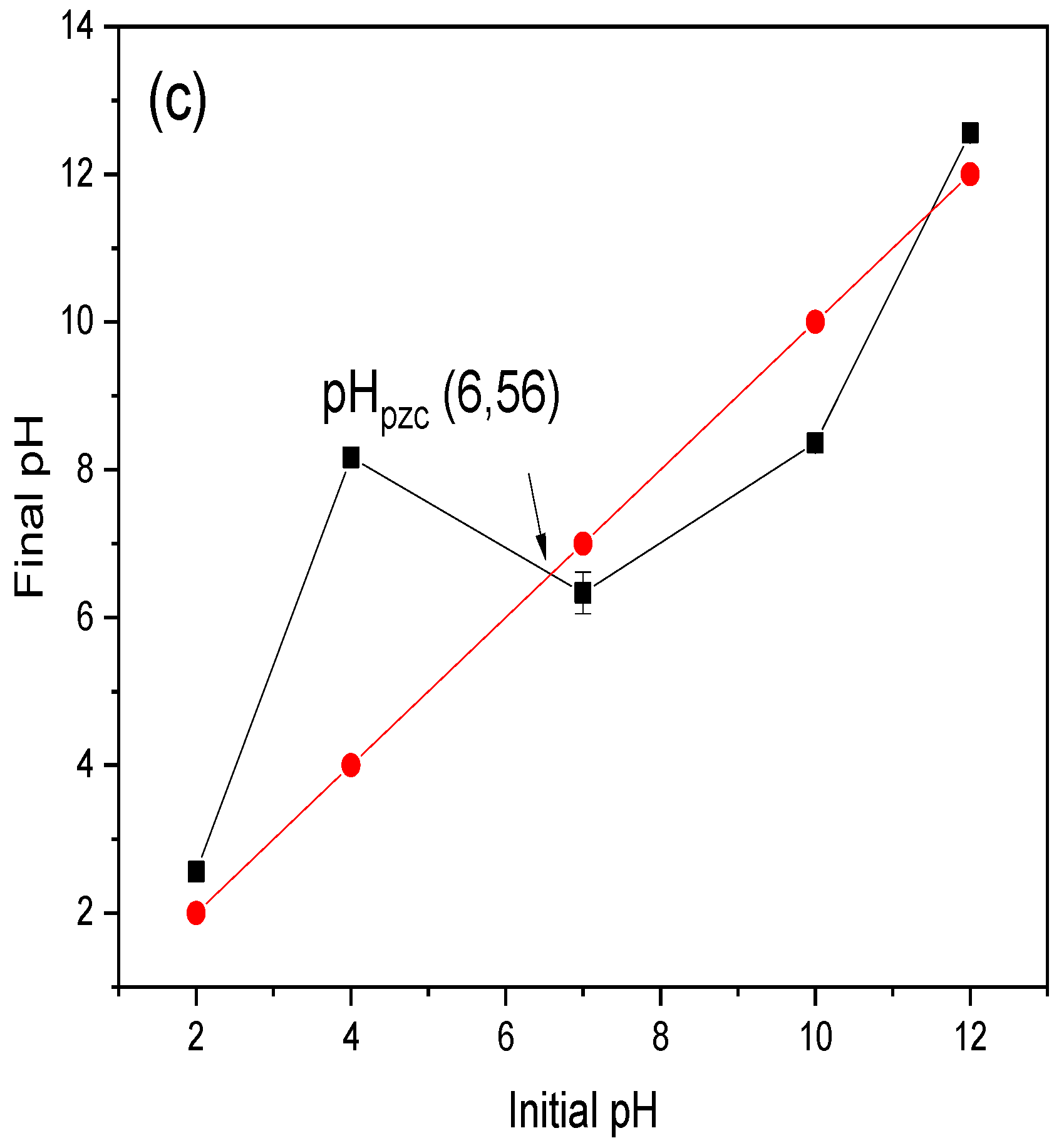
3.2. Effect of Initial Concentration, pH, and Temperature in CR Dye Adsorption
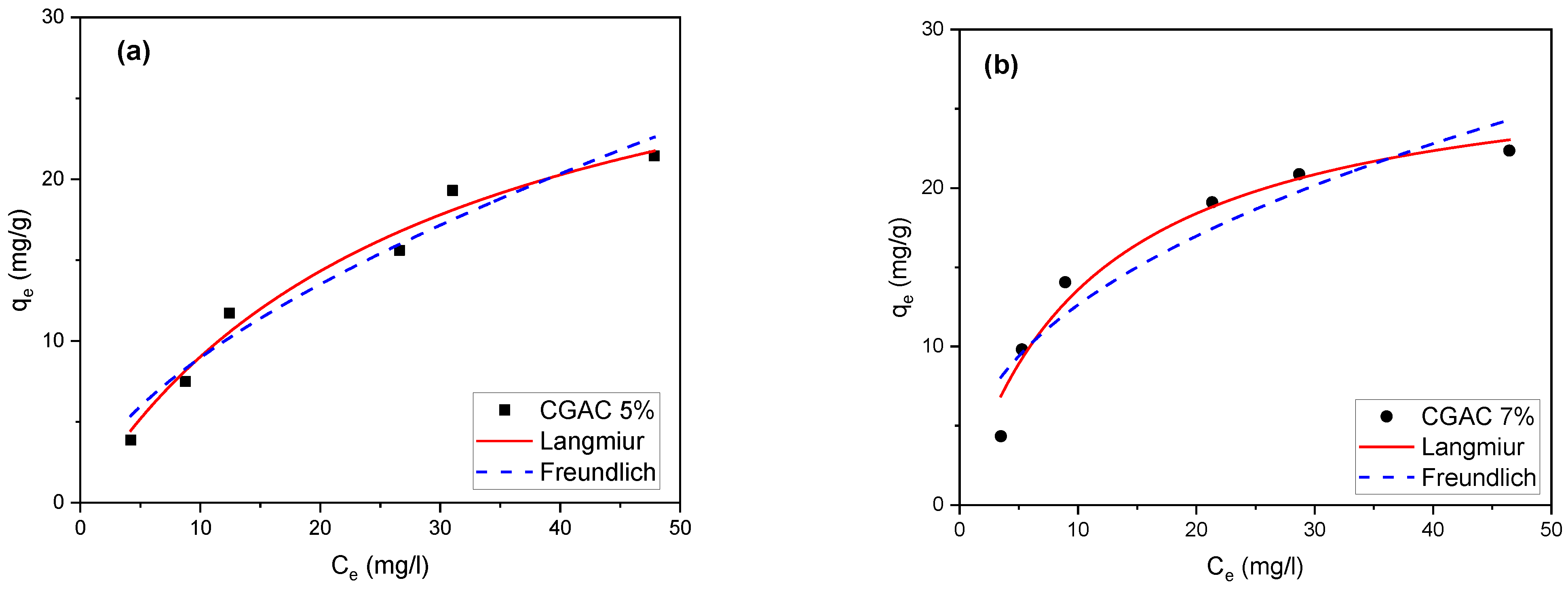
3.3. Adsorption Isotherms Studies
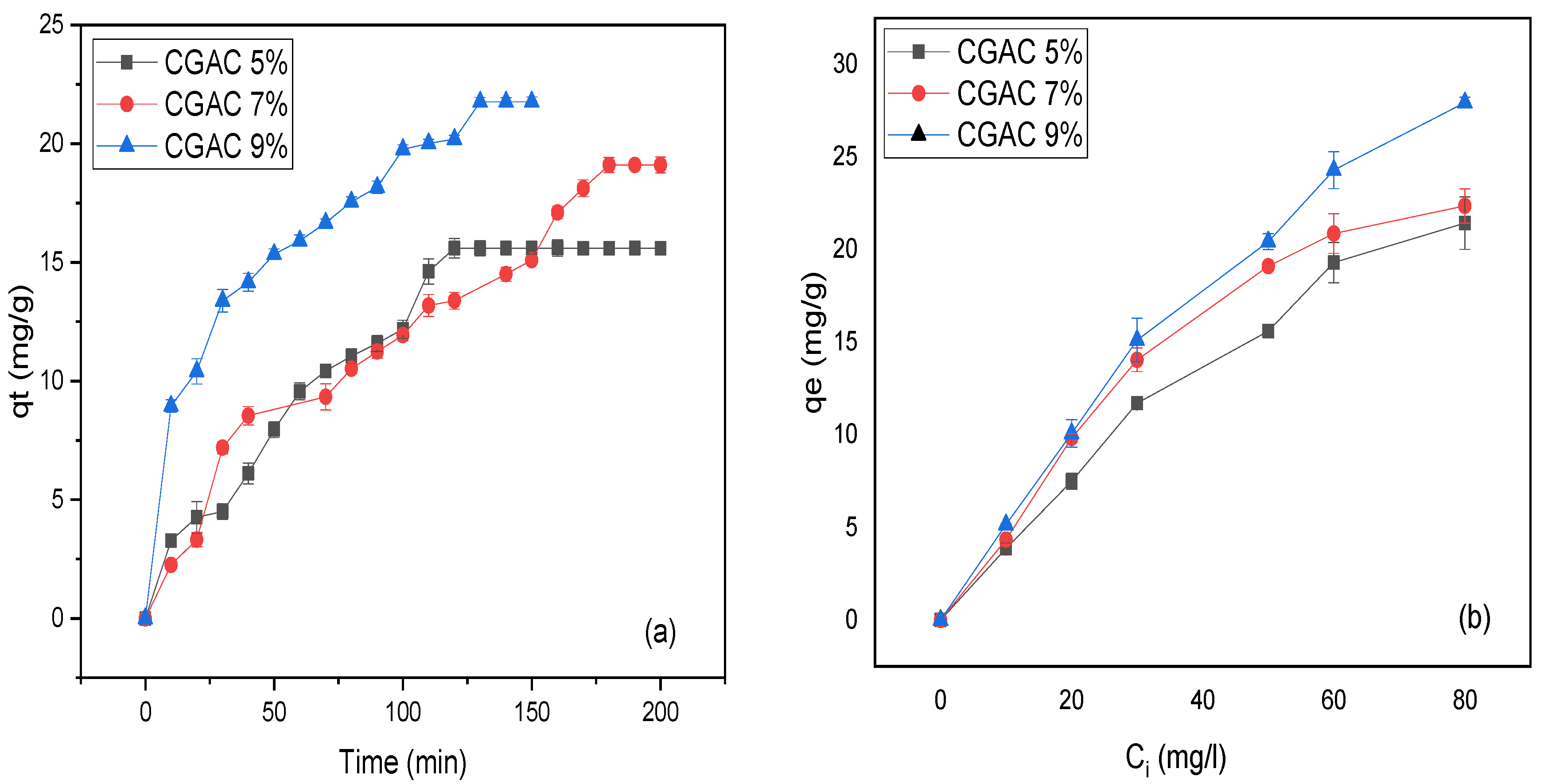
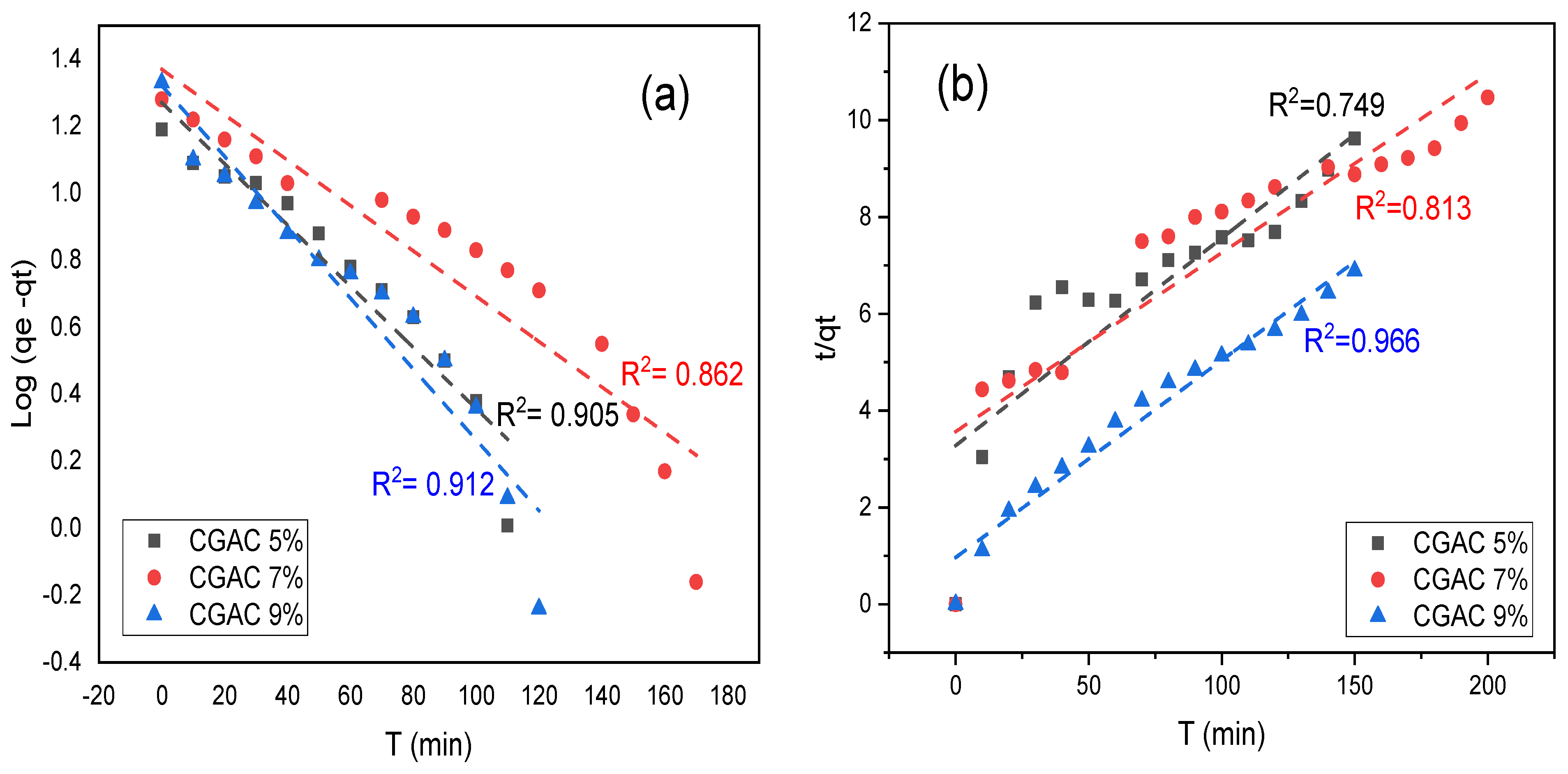
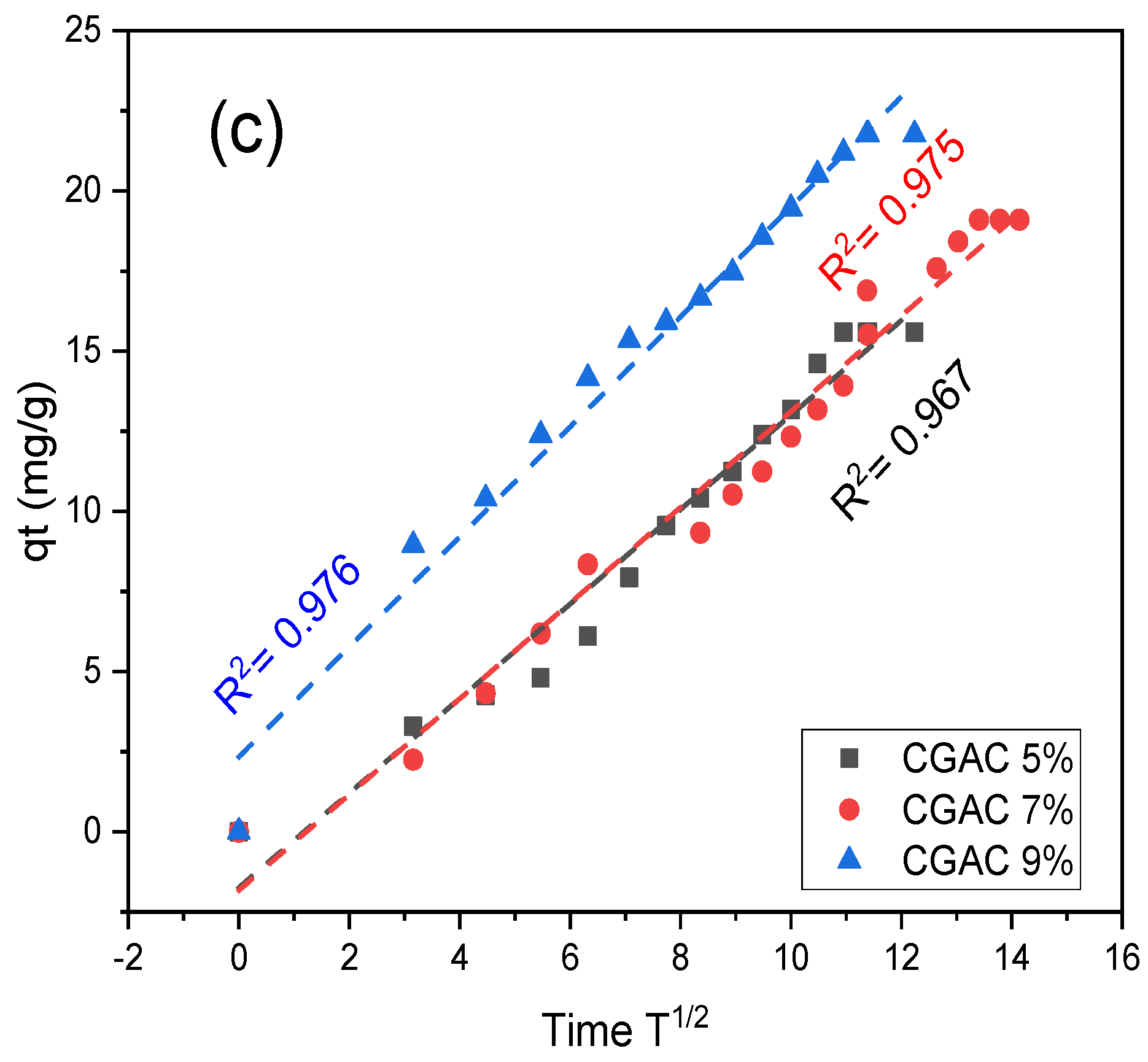
3.4. Adsorption Kinetics
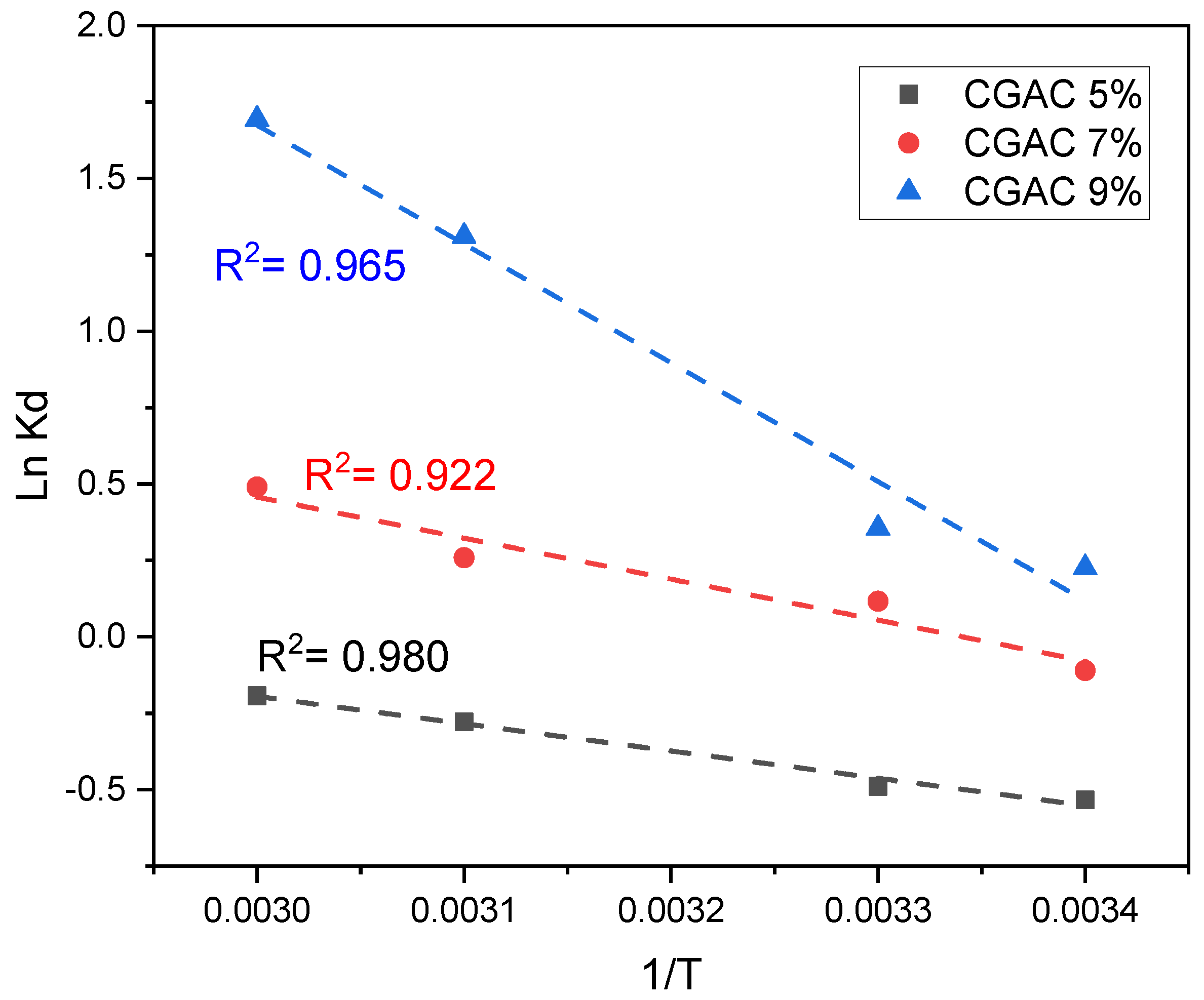
3.5. Adsorption Thermodynamics
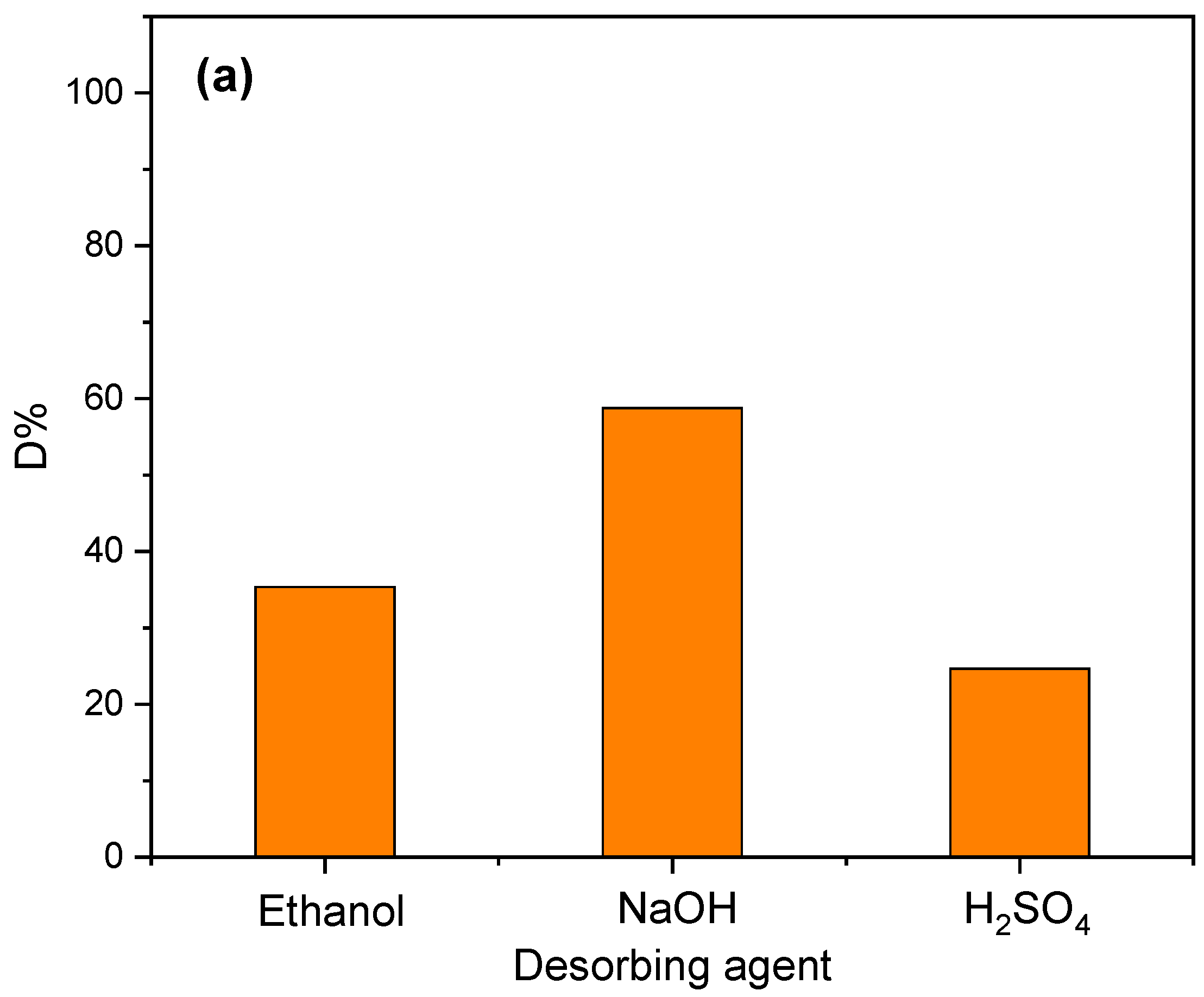
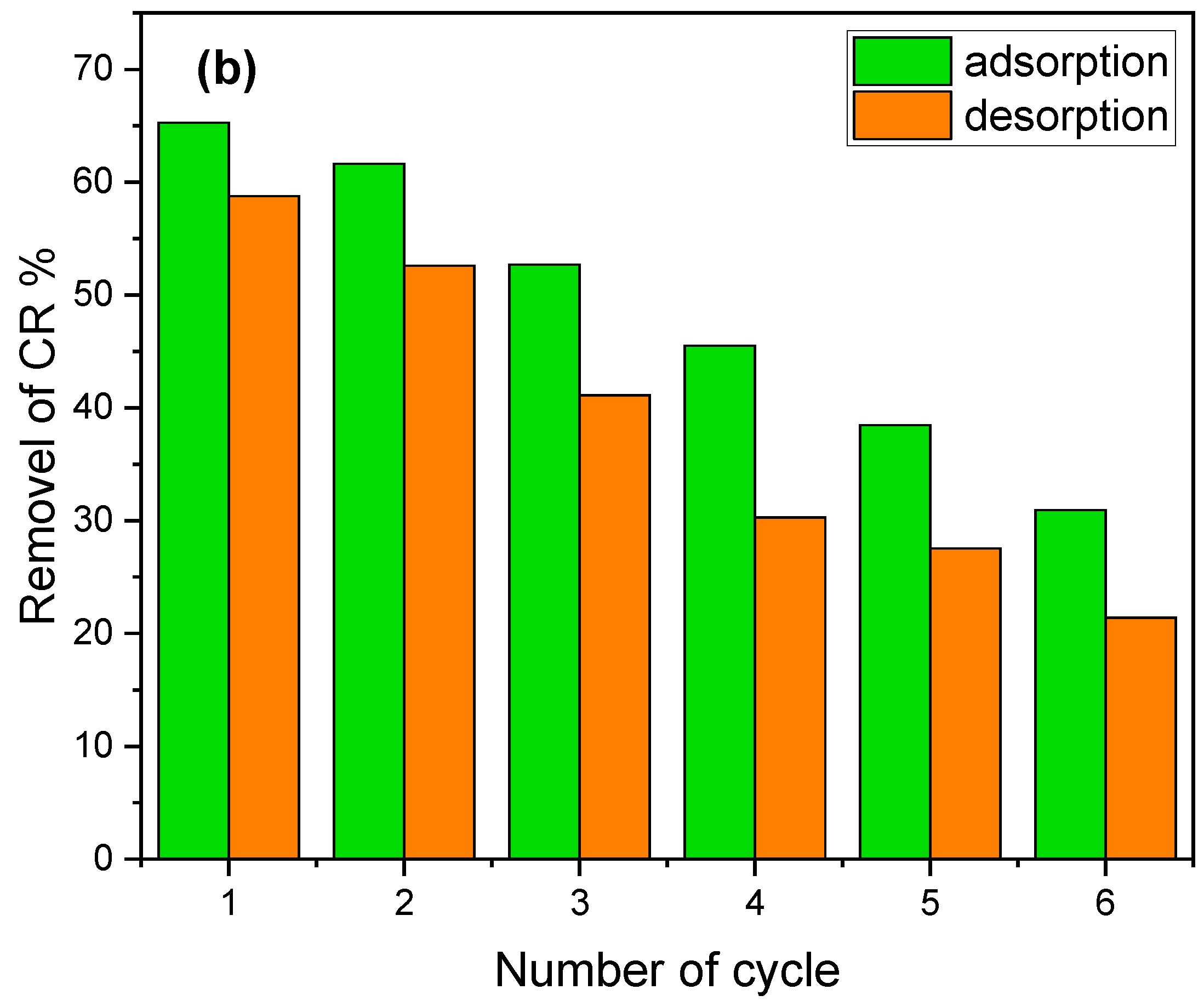
3.6. Desorption and Regeneration
3.7. Mechanism of the CR Adsorption into Chitin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoaib, M.; Ashar, A.; Bhutta, Z.A.; Muzammil, I.; Ali, M.; Kanwal, A. Chapter 15—Biological methods for degradation of textile dyes from textile effluent. In Development in Wastewater Treatment Research and Processes; Shah, M.P., Rodriguez-Couto, S., Kapoor, R.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–353. [Google Scholar]
- Banerjee, D.; Sharma, A.K.; Das, N.S. Nano Materials Induced Removal of Textile Dyes from Waste Water; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022. [Google Scholar]
- Mashkoor, F.; Nasar, A. Magsorbents: Potential candidates in wastewater treatment technology—A review on the removal of methylene blue dye. J. Magn. Magn. Mater. 2020, 500, 166408. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Malachite Green “a Cationic Dye” and Its Removal from Aqueous Solution by Adsorption. Appl. Water Sci. 2017, 7, 3407–3445. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Ighalo, J.O.; Emenike, E.C.; Ogunfowora, L.A.; Igwegbe, C.A. Adsorption of methyl orange: A review on adsorbent performance. Curr. Res. Green Sustain. Chem. 2021, 4, 100179. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Bamigboye, M.O.; Ogunbiyi, O.D.; Akano, M.T. Toxicity and Decontamination Strategies of Congo Red Dye. Groundw. Sustain. Dev. 2022, 19, 100844. [Google Scholar] [CrossRef]
- Nag, S.; Biswas, S. Cellulose-Based Adsorbents for Heavy Metal Removal. In Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water; Springer: Cham, Switzerland, 2021; pp. 113–142. [Google Scholar]
- Selvaraj, V.; Swarna Karthika, T.; Mansiya, C.; Alagar, M. An over Review on Recently Developed Techniques, Mechanisms and Intermediate Involved in the Advanced Azo Dye Degradation for Industrial Applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Shafiqul Alam, M.; Khanom, R.; Arifur Rahman, M. Removal of Congo Red Dye from Industrial Wastewater by Untreated Sawdust. Am. J. Environ. Prot. 2015, 4, 207. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, A. Various methods for removal of dyes from industrial effluents–A review. Indian J. Sci. Technol 2018, 11, 1–21. [Google Scholar] [CrossRef]
- Fan, H.; Zhou, S.; Jiao, W.-Z.; Qi, G.-S.; Liu, Y.-Z. Removal of heavy metal ions by magnetic chitosan nanoparticles prepared continuously via high-gravity reactive precipitation method. Carbohydr. Polym. 2017, 174, 1192–1200. [Google Scholar] [CrossRef]
- Sherlala, A.; Raman, A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004. [Google Scholar] [CrossRef]
- Al-sareji, O.J.; Meiczinger, M.; Somogyi, V.; Al-Juboori, R.A.; Grmasha, R.A.; Stenger-Kovács, C.; Jakab, M.; Hashim, K.S. Removal of Emerging Pollutants from Water Using Enzyme-Immobilized Activated Carbon from Coconut Shell. J. Environ. Chem. Eng. 2023, 11, 109803. [Google Scholar] [CrossRef]
- Sardar, M.; Manna, M.; Maharana, M.; Sen, S. Remediation of Dyes from Industrial Wastewater Using Low-Cost Adsorbents. In Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water; Inamuddin, A.M.I., Lichtfouse, E., Asiri, A.M., Eds.; Springer: Cham, Switzerland, 2021; pp. 377–403. [Google Scholar]
- Rahali, S.; Aissa, M.A.B.; Modwi, A.; Said, R.B.; Belhocine, Y. Application of mesoporous CaO@g-C3N4 nanosorbent materials for high-efficiency removal of Pb (II) from aqueous solution. J. Mol. Liq. 2023, 379, 121594. [Google Scholar] [CrossRef]
- Moumen, A.; Belhocine, Y.; Sbei, N.; Rahali, S.; Ali, F.A.M.; Mechati, F.; Hamdaoui, F.; Seydou, M. Removal of Malachite Green Dye from Aqueous Solution by Catalytic Wet Oxidation Technique Using Ni/Kaolin as Catalyst. Molecules 2022, 27, 7528. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yang, Q.; Yuan, Y.; Chen, L.; Song, Y.; Lian, H. Sustainable co-solvent induced one step extraction of low molecular weight chitin with high purity from raw lobster shell. Carbohydr. Polym. 2019, 205, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Zamora, A.; García-Mena, J.; Cervantes-González, E. Removal of Congo Red from the aqueous phase by chitin and chitosan from waste shrimp, Desalin. Water Treat. 2016, 57, 14674–14685. [Google Scholar] [CrossRef]
- Devi, R.; Dhamodharan, R. Pretreatment in Hot Glycerol for Facile and Green Separation of Chitin from Prawn Shell Waste. ACS Sustain. Chem. Eng. 2017, 6, 846–853. [Google Scholar] [CrossRef]
- Liu, C.; Wang, G.; Sui, W.; An, L.; Si, C. Preparation and Characterization of Chitosan by a Novel Deacetylation Approach Using Glycerol as Green Reaction Solvent. ACS Sustain. Chem. Eng. 2017, 5, 4690–4698. [Google Scholar] [CrossRef]
- Ifuku, S.; Ikuta, A.; Izawa, H.; Morimoto, M.; Saimoto, H. Control of mechanical properties of chitin nanofiber film using glycerol without losing its characteristics. Carbohydr. Polym. 2014, 101, 714–717. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, M.; Lu, X.; Shi, C.; Li, X.; Xin, J.; Yue, G.; Zhang, S. Base-free preparation of low molecular weight chitin from crab shell. Carbohydr. Polym. 2018, 190, 148–155. [Google Scholar] [CrossRef]
- Kaya, M.; Sofi, K.; Sargin, I.; Mujtaba, M. Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr. Polym. 2016, 145, 64–70. [Google Scholar] [CrossRef]
- Boultif, W.; Dehchar, C.; Belhocine, Y.; Zouaoui, E.; Rahali, S.; Zouari, S.E.; Sbei, N.; Seydou, M. Chitosan and Metal Oxide Functionalized Chitosan as Efficient Sensors for Lead (II) Detection in Wastewater. Separations 2023, 10, 479. [Google Scholar] [CrossRef]
- Soon, C.Y.; Tee, Y.B.; Tan, C.H.; Rosnita, A.T.; Khalina, A. Extraction and physicochemical characterization of chitin and chitosan from Zophobas morio larvae in varying sodium hydroxide concentration. Int. J. Biol. Macromol. 2018, 108, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and facile production of chitin from crustacean shells using a natural deep eutectic solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.; Charlet, G. Chitin fractionation and characterization in N, N-dimethylacetamide/lithium chloride solvent system. Carbohydr. Polym. 2002, 50, 363–370. [Google Scholar] [CrossRef]
- Setoguchi, T.; Kato, T.; Yamamoto, K.; Kadokawa, J.-I. Facile production of chitin from crab shells using ionic liquid and citric acid. Int. J. Biol. Macromol. 2012, 50, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Focher, B.; Beltranme, P.L.; Naggi, A.; Torri, G. Alkaline Ndeacetylation of chitin enhanced by flash treatments: Reaction kinetics and structure modifications. Carbohydr. Polym. 1990, 12, 405–418. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Veer, A.; Shirsath, S.R.; Sonawane, S.H. Ultrasound assisted preparation, characterization and adsorption study of ternary chitosan-ZnO-TiO2 nanocomposite: Advantage over conventional method. Ultrason. Sonochem. 2019, 52, 120–130. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in the solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Ho, Y.S. Equilibrium Sorption of Anionic Dye from Aqueous Solution by Palm Kernel Fibre as Sorbent. Dye Pigment. 2007, 74, 60–66. [Google Scholar] [CrossRef]
- Al-Sareji, O.J.; Meiczinger, M.; Al-Juboori, R.A.; Grmasha, R.A.; Andredaki, M.; Somogyi, V.; Idowu, I.A.; Stenger-Kovács, C.; Jakab, M.; Lengyel, E.; et al. Efficient removal of pharmaceutical contaminants from water and wastewater using immobilized laccase on activated carbon derived from pomegranate peels. Sci. Rep. 2023, 13, 11933. [Google Scholar] [CrossRef] [PubMed]
- Ozacar, M. Equilibrium and Kinetic Modelling of Adsorption of Phosphorus on Calcined Alunite. Adsorption 2003, 9, 125–132. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sharma, A. Azadirachta indica leaf powder as an effective biosorbent for dyes: A case study with aqueous Congo Red solutions. J. Environ. Manag. 2004, 71, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Auta, M.; Hameed, B.H. Chitosan–clay composite as highly effective and low cost adsorbent for batch and fixed-bed adsorption of methylene blue. Chem. Eng. J. 2014, 237, 352–361. [Google Scholar] [CrossRef]
- Wang, W.; Bo, S.Q.; Li, S.Q.; Qin, W. Determination of the Mark-Houwink equation for chitosans with different degrees of deacetylation. Int. J. Biol. Macromol. 1991, 13, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Shamshina, J.L.; Barber, P.S.; Gurau, G.; Griggs, C.S.; Rogers, R.D. Pulping of Crustacean Waste using Ionic Liquids: To Extract or Not to Extract? ACS Sustain. Chem. Eng. 2016, 4, 6072–6081. [Google Scholar] [CrossRef]
- Focher, B.; Naggi, A.; Torri, G.; Cosani, A.; Terbojevich, M. Structural differences between chitin polymorphs and their precipitates from solutions-evidence from CP-MAS 13CNMR, FT-IR and FT-Raman spectroscopy. Carbohydr. Polym. 1992, 17, 97–102. [Google Scholar] [CrossRef]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Cárdenas, G.; Carbrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Wang, W.T.; Zhu, J.; Wang, X.L.; Huang, Y.; Wang, Y.Z. Dissolution behavior of chitin in ionic liquids. J. Macromol. Sci. Part B Phys. 2010, 49, 528–541. [Google Scholar] [CrossRef]
- Ifuku, S.; Nomura, R.; Morimoto, M.; Saimoto, H. Preparation of chitin nanofibers from mushrooms. Materials 2011, 4, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Elhussiny, A.; Faisal, M.; D’angelo, G.; Everitt, N.M.; Fahim, I.S. Experimental Investigation of Chitosan Film Reinforced by Chitin Fibers and Chitin Whiskers Extracted from Shrimp Shell Waste. J. Eng. Sci. Technol. 2020, 15, 2730–2745. [Google Scholar]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, P.; Hori, R.; Wada, M. Revisit of α-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 2009, 10, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Mujtaba, M.; Bulut, E.; Akyuz, B.; Zelencova, L.; Sofi, K. Fluctuation in physicochemical properties of chitins extracted from different body parts of honeybee. Carbohydr. Polym. 2015, 132, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Dotto, G.L.; Santos, J.M.N.; Rodrigues, I.L.; Rosa, R.; Pavan, F.A.; Lima, E.C. Adsorption of methylene blue by ultrasonic surface modified chitin. J. Colloid Interface Sci. 2015, 446, 133–140. [Google Scholar] [CrossRef]
- González, J.A.; Villanueva, M.E.; Piehl, L.L.; Copello, G. Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: Adsorption and desorption study. Chem. Eng. J. 2015, 280, 41–48. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem. 2005, 40, 1347–1361. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Li, H.; Zhu, Y.; Cui, B. The structure property and adsorption capacity of new enzyme-treated potato and sweet potato starches. Int. J. Biol. Macromol. 2020, 144, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.V.C.; Nora, F.B.D.; Peres, E.C.; Reis, G.S.; Lima Éder, C.; Oliveira, M.L.; Dotto, G.L. Synthesis and characterization of biopolymers functionalized with APTES (3-aminopropyltriethoxysilane) for the adsorption of sunset yellow dye. J. Environ. Chem. Eng. 2019, 7, 103410. [Google Scholar] [CrossRef]
- Cakmak, M.; Tasar, S.; Selen, V.; Ozer, D.; Ozer, A. Removal of astrazon golden yellow 7GL from colored wastewater using chemically modified clay. J. Central South Univ. 2017, 24, 743–753. [Google Scholar] [CrossRef]
- Onida, B.; Bonelli, B.; Flora, L.; Geobaldo, F.; Arean, C.O.; Garrone, E. Permeability of micelles in surfactant-containing MCM-41 silica as monitored by embedded dye molecules. Chem. Commun. 2001, 21, 2216–2217. [Google Scholar] [CrossRef] [PubMed]
- Lafi, R.; Montasser, I.; Hafiane, A. Adsorption of congo red dye from aqueous solutions by prepared activated carbon with oxygen-containing functional groups and its regeneration. Adsorpt. Sci. Technol. 2018, 37, 160–181. [Google Scholar] [CrossRef]
- Liu, S.; Ding, Y.; Li, P.; Diao, K.; Tan, X.; Lei, F.; Zhan, Y.; Li, Q.; Huang, B.; Huang, Z. Adsorption of the anionic dye Congo red from aqueous solution onto natural zeolites modified with N,N-dimethyl dehydroabietylamine oxide. Chem. Eng. J. 2014, 248, 135–144. [Google Scholar] [CrossRef]
- Purkait, M.K.; Maiti, A.; DasGupta, S.; De, S. Removal of congo red using activated carbon and its regeneration. J. Hazard. Mater. 2007, 145, 287–295. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; Gonzalez-Alvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Markou, G.; Çelekli, A.; Bozkurt, H. Biosorption of methylene blue onto Arthrospira platensis biomass: Kinetic, equilibrium and thermodynamic studies. J. Environ. Chem. Eng. 2015, 3, 670–680. [Google Scholar] [CrossRef]
- Hou, F.; Wang, D.; Ma, X.; Fan, L.; Ding, T.; Ye, X.; Liu, D. Enhanced adsorption of Congo red using chitin suspension after sonoenzymolysis. Ultrason. Sonochem. 2021, 70, 105327. [Google Scholar] [CrossRef]
- Wei, C.; Huang, Y.; Liao, Q.; Xia, A.; Zhu, X.; Zhu, X. Adsorption thermodynamic characteristics of Chlorella vulgaris with organic polymer adsorbent cationic starch: Effect of temperature on adsorption capacity and rate. Bioresour. Technol. 2019, 293, 122056. [Google Scholar] [CrossRef]
- Kumar, R.; Barakat, M.A. Decolourization of hazardous brilliant green from aqueous solution using binary oxidized cactus fruit peel. Chem. Eng. J. 2013, 226, 377–383. [Google Scholar] [CrossRef]


| Samples | Yield (%) | Water (%) | ASH (%) | Protein (%) | Purity % | Intrinsic Viscosity (dL/g) | M (KDa) |
|---|---|---|---|---|---|---|---|
| Shrimp shells | - | 9.90 ± 0.14 | 38.30 ± 1.05 | 27.845 ± 2.29 | - | - | - |
| CGAC 5% | 41.25 ± 0.23 | 3.33 ± 0.61 | 0.354 ± 0.19 | 1.7966 ± 0.54 | 94 | 6.946 | 167 |
| CGAC 7% | 29.64 ± 0.12 | 3.05 ± 0.21 | 0.177 ± 0.34 | 1.7237 ± 0.27 | 95 | 6.160 | 147 |
| CGAC 9% | 28.07 ± 0.03 | 2.62 ± 0.15 | 0.165 ± 0.28 | 0.6464 ± 0.41 | 97 | 4.799 | 113 |
| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | RL | KL | R2 | 1/nf | Kf | R2 | |
| CGAC5% | 34.674 | 0.74–0.26 | 0.035 | 0.927 | 0.591 | 2.299 | 0.949 |
| CGAC7% | 28.483 | 0.52–0.12 | 0.091 | 0.935 | 0.426 | 4.731 | 0.805 |
| CGAC9% | 36.123 | 0.55–0.13 | 0.082 | 0.981 | 0.488 | 4.899 | 0.939 |
| qe (mg/g) exp | Pseudo 1st Order | Pseudo 2nd Order | Intra-Particle Diffusion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe cal (mg/g) | R2 | K1 (min−1) | qe cal(mg/g) | R2 | K2 (min−1) | Kint | C | R2 | ||
| CGAC5% | 15.59 | 18.64 | 0.905 | 0.021 | 29.87 | 0.749 | 2.63 × 10−4 | 1.66 | −3.38 | 0.975 |
| CGAC7% | 19.10 | 23.42 | 0.862 | 0.015 | 32.80 | 0.813 | 2.07 × 10−4 | 1.61 | −3.04 | 0.967 |
| CGAC9% | 21.76 | 20.95 | 0.912 | 0.024 | 26.33 | 0.966 | 1.15 × 10−3 | 1.57 | 3.81 | 0.976 |
| Adsorbent | T (K) | Kd | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol) | R2 |
|---|---|---|---|---|---|---|
| GCAC5% | 293 303 313 323 | 0.586 0.613 0.757 0.824 | 1.301 1.231 0.724 0.519 | 7.42 | 20.64 | 0.980 |
| GCAC7% | 293 303 313 323 | 0.895 1.123 1.296 1.633 | 0.270 −0.292 −0.674 −1.316 | 11.16 | 37.28 | 0.922 |
| GCAC9% | 293 303 313 323 | 1.254 1.427 3.707 5.437 | −0.551 −0.895 −3.407 −4.544 | 32.31 | 110.86 | 0.965 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharbi, F.Z.; Bougdah, N.; Belhocine, Y.; Sbei, N.; Rahali, S.; Damous, M.; Seydou, M. Green and Fast Extraction of Chitin from Waste Shrimp Shells: Characterization and Application in the Removal of Congo Red Dye. Separations 2023, 10, 599. https://doi.org/10.3390/separations10120599
Gharbi FZ, Bougdah N, Belhocine Y, Sbei N, Rahali S, Damous M, Seydou M. Green and Fast Extraction of Chitin from Waste Shrimp Shells: Characterization and Application in the Removal of Congo Red Dye. Separations. 2023; 10(12):599. https://doi.org/10.3390/separations10120599
Chicago/Turabian StyleGharbi, Fatma Zohra, Nabil Bougdah, Youghourta Belhocine, Najoua Sbei, Seyfeddine Rahali, Maamar Damous, and Mahamadou Seydou. 2023. "Green and Fast Extraction of Chitin from Waste Shrimp Shells: Characterization and Application in the Removal of Congo Red Dye" Separations 10, no. 12: 599. https://doi.org/10.3390/separations10120599
APA StyleGharbi, F. Z., Bougdah, N., Belhocine, Y., Sbei, N., Rahali, S., Damous, M., & Seydou, M. (2023). Green and Fast Extraction of Chitin from Waste Shrimp Shells: Characterization and Application in the Removal of Congo Red Dye. Separations, 10(12), 599. https://doi.org/10.3390/separations10120599






