Study on Enhancement of Denitrification Performance of Alcaligenes faecalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Instruments
2.1.1. Preparation of Bacterial Suspensions
2.1.2. Culture Medium
2.1.3. Simulated Nitrogen-Containing Wastewater
2.1.4. Experimental Instruments
2.2. Experimental Design
2.3. Analytical Methods
3. Results and Discussion
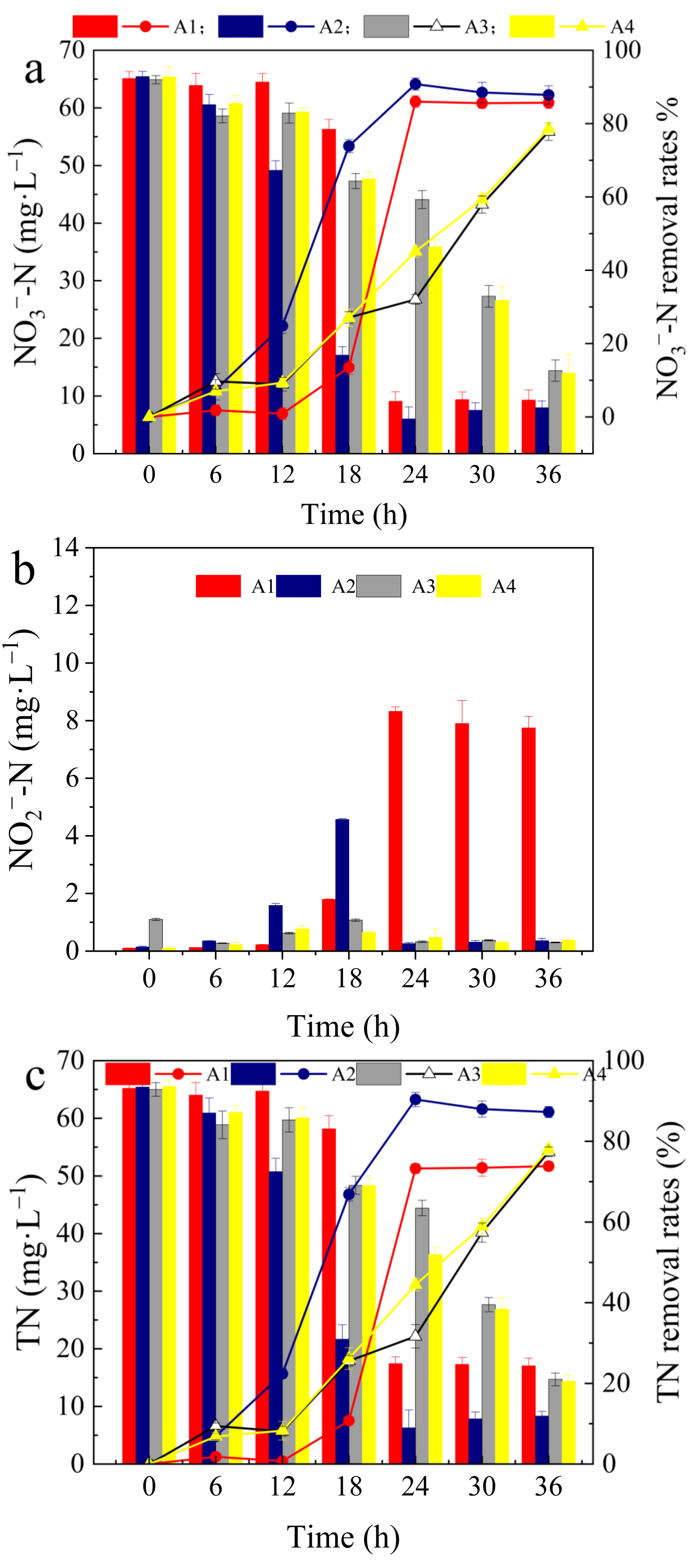
3.1. Effects of Different Aeration Modes on Denitrification Performance
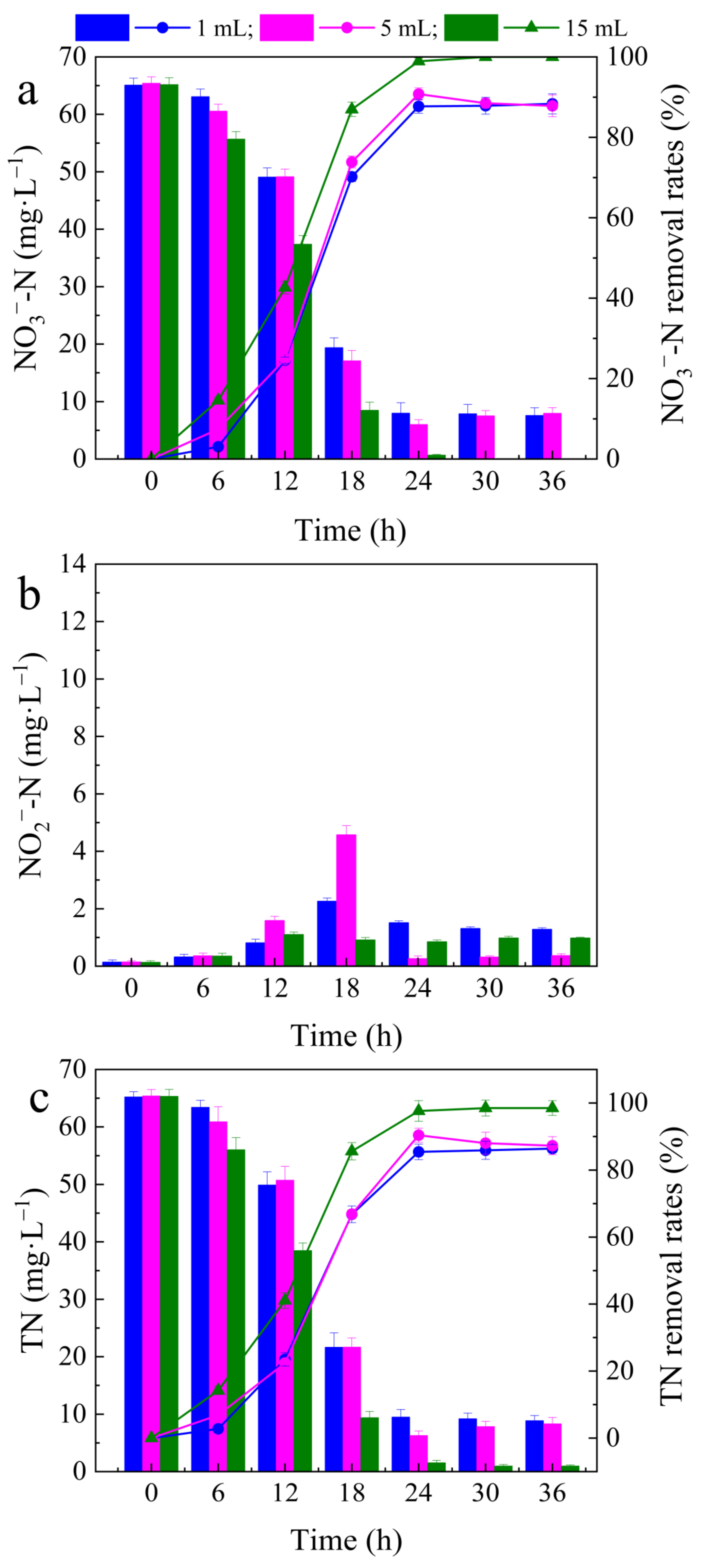
3.2. Effects of Different Microbial Dosages on Denitrification Performance
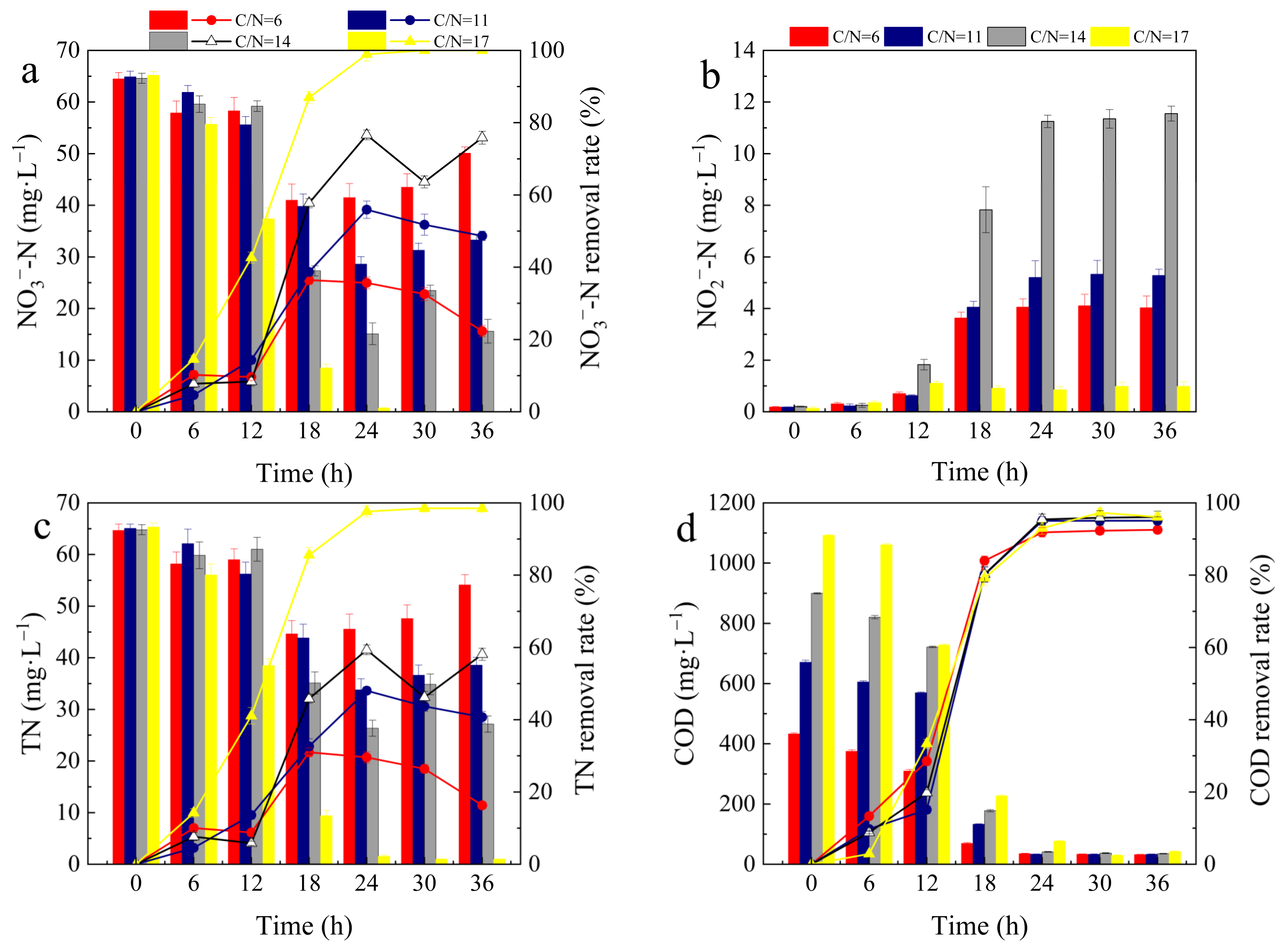
3.3. Effect of Different C/N Ratios on Denitrification Performance
3.4. Mechanism of Nitrogen Removal by Aerobic Denitrifying Bacteria
4. Conclusions
- (1)
- The optimal aeration mode for denitrification by Alcaligenes faecalis is intermittent aeration for 10 min, at 30 min intervals, achieving NO3−–N and TN removal rates of 87.82% and 87.18%, respectively;
- (2)
- When the bacterial suspension dosage was 15 mL, NO3−–N could be completely removed by denitrification, resulting in low levels of NO2−–N accumulation and a good denitrification performance by Alcaligenes faecalis, achieving NO3−–N and TN removal rates of 100% and 98.50%, respectively;
- (3)
- Under the determined optimal aeration conditions and bacterial suspension dosage, the denitrification performance of Alcaligenes faecalis gradually increased in accordance with the C/N ratio, achieving high rates of NO3−–N, TN and COD removal of 100%, 98.50% and 96.13%, respectively, at an optimal C/N ratio of 17;
- (4)
- In the future, we will continue to explore the operating conditions of different aerobic denitrifying microorganisms and compare the performance of different aerobic denitrifying microorganisms to find aerobic denitrifying microorganisms suitable for the treatment of nitrogenous wastewater with different physicochemical parameters. Meanwhile, we will continue to explore the combination of aerobic denitrifying microorganisms with other wastewater treatment facilities, such as constructed wetlands, in order to find a highly efficient and green method to purify nitrogenous wastewater.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Woolway, R.I.; Kraemer, B.M.; Lenters, J.D.; Merchant, C.J.; O’Reilly, C.M.; Sharma, S. Global lake responses to climate change. Nat. Rev. Earth Environ. 2020, 1, 388–403. [Google Scholar] [CrossRef]
- Jiang, Q.; He, J.; Wu, J.; Hu, X.; Ye, G.; Christakos, G. Assessing the severe eutrophication status and spatial trend in the coastal waters of Zhejiang province (China). Limnol. Oceanogr. 2019, 64, 3–17. [Google Scholar] [CrossRef]
- Liao, M.; Yu, G.; Guo, Y. Eutrophication in Poyang Lake (Eastern China) over the last 300 years in response to changes in climate and lake biomass. PLoS ONE 2017, 12, e0169319. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Deng, W.; Zhang, Y.; Tao, K.; Yuan, J.; Liu, J.; Li, Z.; Lek, S.; Wang, Q.; Hugueny, B. Eutrophication induces functional homogenization and traits filtering in Chinese lacustrine fish communities. Sci. Total Environ. 2023, 857, 159651. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, H.; Li, Y.; Sun, W.; Song, C.; Wang, L.; Du, G.; Qiao, S.; Sun, J.; Nuamah, L.A. Dredging effects on nutrient release of the sediment in the long-term operational free water surface constructed wetland. J. Environ. Manag. 2022, 322, 116160. [Google Scholar] [CrossRef]
- Yi, M.; Wang, C.; Wang, M.; Ma, X.; Wang, H.; Liu, Z.; Cao, J.; Gao, F.; Ke, X.; Lu, M. Immobilized denitrifying bacteria on modified oyster shell as biofilter carriers enhance nitrogen removal. J. Environ. Chem. Eng. 2023, 11, 109214. [Google Scholar] [CrossRef]
- Elkarrach k Merzouki, M.; Atia, F.; Laidi, O.; Benlemlih, M. Aerobic denitrification using Bacillus pumilus, Arthrobacter sp., and Streptomyces lusitanus: Novel aerobic denitrifying bacteria. Bioresour. Technol. Rep. 2021, 14, 100663. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Zhang, S.; Liu, F.; Peng, J.; Peng, Y.; Wu, J. Nitrogen removal characteristics of a novel heterotrophic nitrification and aerobic denitrification bacteria, Alcaligenes faecalis strain WT14. J. Environ. Manag. 2021, 282, 111961. [Google Scholar] [CrossRef]
- Aguilar, L.; Gallegos, Á.; Martín Pérez, L.; Arias, C.A.; Rubio, R.; Haulani, L.; Raurich, J.G.; Pallarés, M.; de Pablo, J.; Morató, J. Effect of intermittent induced aeration on nitrogen removal and denitrifying-bacterial community structure in Cork and gravel vertical flow pilot-scale treatment wetlands. J. Environ. Sci. Health Part A 2021, 56, 1121–1130. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Li, S.; Chen, S.; Huang, T.; Li, N.; Yang, S.; Wang, Y.; Kou, L.; Zhang, X. Nitrogen removal by mix-cultured aerobic denitrifying bacteria isolated by ultrasound: Performance, co-occurrence pattern and wastewater treatment. Chem. Eng. J. 2019, 372, 26–36. [Google Scholar] [CrossRef]
- Sun, Y.; Su, J.; Ali, A.; Zhang, S.; Zheng, Z.; Min, Y. Effect of fungal pellets on denitrifying bacteria at low carbon to nitrogen ratio: Nitrate removal, extracellular polymeric substances, and potential functions. Sci. Total Environ. 2022, 847, 157591. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Luo, J.; Zhuang, X.; Zhang, D.; Huang, Z.; Zhang, D.; Huang, Z. Diversity of culturable aerobic denitrifying bacteria in the sediment, water and biofilms in Liangshui River of Beijing, China. Sci. Rep. 2017, 7, 10032. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Wang, T.; Wan, Q.; Cao, R.; Li, K.; Wang, J.; Huang, T. Enhanced nitrogen removal of aerobic denitrifier using extracellular algal organic matter as carbon source: Application to actual reservoir water. Bioprocess Biosyst. Eng. 2020, 43, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lv, Y.; Liu, Y.; Ren, R. Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a novel metal resistant bacterium Cupriavidus sp. S1. Bioresour. Technol. 2016, 220, 142–150. [Google Scholar] [CrossRef]
- Ren, J.; Wei, C.; Ma, H.; Dai, M.; Fan, J.; Liu, Y.; Wu, Y.; Han, R. The Nitrogen-Removal Efficiency of a Novel High-Efficiency Salt-Tolerant Aerobic Denitrifier, Halomonas Alkaliphile HRL-9, Isolated from a Seawater Biofilter. Int. J. Environ. Res. Public Health 2019, 16, 4451. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Li, J.; Zhang, J.; Zheng, Z.; Wang, Z.; Guo, W.; Zhang, Y.; Luo, R. Isolation and identification of a novel aerobic denitrifying bacterium with quorum quenching activity and the effect of environmental factors on its performance. Environ. Technol. Innov. 2022, 28, 102913. [Google Scholar] [CrossRef]
- Wang, F.; Liu, W.; Liu, W.; Xiao, L.; Ai, S.; Sun, X.; Bian, D. Simultaneous removal of organic matter and nitrogen by heterotrophic nitrification–aerobic denitrification bacteria in an air-lift multi-stage circulating integrated bioreactor. Bioresour. Technol. 2022, 363, 127888. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Chen, J.; Lv, Y.; Chen, R.; Xu, J.; Li, D.; He, X.; Hou, J. Formation of microorganism-derived dissolved organic nitrogen in intermittent aeration constructed wetland and its stimulating effect on phytoplankton production: Implications for nitrogen mitigation. Water Res. 2023, 230, 119563. [Google Scholar] [CrossRef]
- Bian, X.; Wu, Y.; Li, J.; Yin, M.; Li, D.; Pei, H.; Chang, S.; Guo, W. Effect of dissolved oxygen on high C/N wastewater treatment in moving bed biofilm reactors based on heterotrophic nitrification and aerobic denitrification: Nitrogen removal performance and potential mechanisms. Bioresour. Technol. 2022, 365, 128147. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Y.; Zhao, X.; Kumar, J.L.G. High rate nitrogen removal in an alum sludge-based intermittent aeration constructed wetland. Environ. Sci. Technol. 2012, 46, 4583–4590. [Google Scholar] [CrossRef]
- Wu, H.; Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S. Decentralized domestic wastewater treatment using intermittently aerated vertical flow constructed wetlands: Impact of influent strengths. Bioresour. Technol. 2015, 176, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.R.; Burr, M.D.; Camper, A.K.; Moss, J.J.; Stein, O.R. Seasonality, C:N ratio and plant species influence on denitrification and plant nitrogen uptake in treatment wetlands. Ecol. Eng. 2023, 191, 106946. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Huang, J.; Huang, M.; Wang, T.; Bao, S.; Tang, W.; Fang, T. Bacteria-supported iron scraps for the removal of nitrate from low carbon-to-nitrogen ratio wastewater. RSC Adv. 2019, 9, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, Y.; Yue, J.; Song, Z.; Li, M.; Wang, N.; Liu, J.; Guo, H.; Li, B. Metagenomic analysis revealed the mechanism of extracellular polymeric substances on enhanced nitrogen removal in coupled MABR systems with low C/N ratio containing salinity. J. Environ. Chem. Eng. 2023, 11, 109599. [Google Scholar] [CrossRef]
- Robertson, L.; Kuenen, J. Aerobic denitrification—Old wine in new bottles? Antonie Van Leeuwenhoek 1984, 50, 525–544. [Google Scholar] [CrossRef]
| Item | COD/(mg/L) | ρ/(mg/L) | pH | |
|---|---|---|---|---|
| NO3−–N | TN | |||
| Content | 400~1100 | 65 | 65 | 7.0~7.2 |
| No. | Factors | Experimental Condition | NO3−–N (mg/L) | COD (mg/L) |
|---|---|---|---|---|
| 1 | Aeration method | A1 (Continuous aeration) | 65 ± 1 | 1100 ± 50 |
| A2 (Aeration: 10 min/intermittent 30 min) | 65 ± 1 | 1100 ± 50 | ||
| A3 (Aeration: 10 min/intermittent 60 min) | 65 ± 1 | 1100 ± 50 | ||
| A4 (Aeration: 10 min/intermittent 90 min) | 65 ± 1 | 1100 ± 50 | ||
| 2 | Dosage of bacterial suspension/mL | 1 | 65 ± 1 | 1100 ± 50 |
| 5 | 65 ± 1 | 1100 ± 50 | ||
| 15 | 65 ± 1 | 1100 ± 50 | ||
| 3 | C/N | 6 | 65 ± 1 | 400 ± 20 |
| 11 | 65 ± 1 | 700 ± 20 | ||
| 14 | 65 ± 1 | 900 ± 20 | ||
| 17 | 65 ± 1 | 1100 ± 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Hu, S.; Zhang, Y.; Yu, G.; Yang, Y.; Li, Q.; Duan, P. Study on Enhancement of Denitrification Performance of Alcaligenes faecalis. Separations 2023, 10, 597. https://doi.org/10.3390/separations10120597
Luo Z, Hu S, Zhang Y, Yu G, Yang Y, Li Q, Duan P. Study on Enhancement of Denitrification Performance of Alcaligenes faecalis. Separations. 2023; 10(12):597. https://doi.org/10.3390/separations10120597
Chicago/Turabian StyleLuo, Zicheng, Shugen Hu, Yameng Zhang, Guanlong Yu, Yunhe Yang, Qing Li, and Peng Duan. 2023. "Study on Enhancement of Denitrification Performance of Alcaligenes faecalis" Separations 10, no. 12: 597. https://doi.org/10.3390/separations10120597
APA StyleLuo, Z., Hu, S., Zhang, Y., Yu, G., Yang, Y., Li, Q., & Duan, P. (2023). Study on Enhancement of Denitrification Performance of Alcaligenes faecalis. Separations, 10(12), 597. https://doi.org/10.3390/separations10120597






