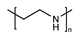
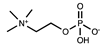
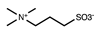
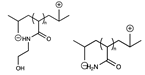
Abstract
Hydrophilic interaction liquid chromatography (HILIC) today is a well-known and largely applied technique to analyse polar compounds such as pharmaceuticals, metabolites, proteins, peptides, amino acids, oligonucleotides, and carbohydrates. Due to the large number of stationary phases employed for HILIC applications, this review aims to help the reader in choosing a proper stationary phase, which often represents the critical point for the success of a separation. A great offer is present for achiral applications in contrast to the chiral phases developed for HILIC enantioseparations. In the last case, up-to-date solutions are presented.
1. Introduction
Effective separation of small polar, acidic, or basic analytes is often demanding in reversed-phase liquid chromatography (RPLC) since these compounds are often not sufficiently retained [1,2,3]. In this perspective, hydrophilic interaction chromatography (HILIC)—as named by Alpert at the beginning of the 1990s [1]—made it possible to analyse different classes of molecules (such as biomarkers, nucleotides, nucleosides, carbohydrates, amino acids, and peptides) effortlessly. The stationary phases used in HILIC are polar, like the classic stationary phases used in normal-phase liquid chromatography (NPLC) (i.e., silica, amino, or cyano) [4,5,6,7,8]. In contrast, the eluents used are similar to those of RPLC (e.g., acetonitrile ACN and water).
The separation process on the polar columns in aqueous-organic mobile phases is assumed to arise from the partition between the aqueous layer in proximity to the solid surface and a highly organic bulk mobile phase [1,9,10]. However, the separation mechanism is based on more than this. As it is possible to observe from the separation of different compounds on HILIC columns, the absorption on the polar centres present in the stationary phase also plays a fundamental role [2]. Thus, in HILIC, the separation mechanism strongly depends on the stationary and mobile phase composition, which influences polar, hydrophobic, or ion exchange interactions that contribute to compound retention reaching a mixed-retention mechanism [8,11]. Indeed, always keeping in mind the structure of the stationary phase, by using acetonitrile-rich eluent, polar interactions control the retention of analytes; on the other hand, in highly aqueous eluent, hydrophobic interaction has a significant role, and the column may show an essentially reversed-phase behaviour. Various moderately or strongly polar stationary phases have been developed in the last years to provide appropriate separation in the HILIC mode. Moreover, ion exchangers, mixed-mode, or zwitterionic bonded materials, showing a combined HILIC–ion interaction retention mechanism, have become increasingly used to expand the scope of HILIC in the separations of complex samples [9].
Any water-miscible aprotic solvent may be used for the HILIC mobile phase. Alcohols can be utilised, too. However, they are rarely used due to their similarity to water, with which they compete both in the surface solvation process and in forming strong hydrogen bonds with the stationary phase [12]. Therefore, in the choice of the organic solvent to be used in the mobile-phase HILIC, it is beneficial to consider the eluotropic series, which in HILIC can be summarised as follows: acetone < isopropanol ~ propanol < acetonitrile < ethanol < dioxane < dimethylformamide ~ methanol < water. HILIC separations are carried out in two elution modes: (i) isocratic mode, with a high proportion of organic solvent, and (ii) gradient mode. Usually, the gradient starts with a high proportion of organic solvent and ends with a high percentage of the aqueous solvent.
In addition, to allow the analysis of compounds generally not retained in RPLC, HILIC has further advantages over RPLC and NPLC. For example, the excellent solubility in the aqueous mobile phase is used in HILIC of polar samples (such as drug formulation, often in the salt form [13]) compared to that obtainable operating NPLC mobile phases. Furthermore, using expensive ion-pair agents is not necessary for HILIC, and it can be suitably coupled with mass spectrometry (MS), especially in the electrospray ionisation (ESI) mode [5,14].
Moreover, HILIC is often considered an orthogonal technique to RPLC, and Alpert was the first to observe that the order of elution of compounds on a hydrophilic stationary phase is reversed concerning that obtained from an apolar stationary phase [1]. Orthogonality is the statistical independence of one dimension to the other [15,16,17]. In an orthogonal separation, the retention mechanism of the column in the first dimension differs from that employed in the second one; this will increase the use of separation space (from mono-dimensional to multidimensional) offered by the system. In conclusion, coupling two orthogonal columns enhances the overall peak capacity of the system, allowing the baseline separation required for identification and quantification [18,19,20,21].
Although the number of commercially available columns designed for HILIC is increasing, there is still the need for a versatile stationary phase similar to C18 in RPLC.
Diol, amino, and amide are commonly used in HILIC and are usually prepared by chemically modifying the surface of the silica gel, such as the C18 phases used for RPLC.
The chemically bonded diol phases demonstrate high polarity and hydrogen bonding properties. They do not contain ionisable groups other than the unreacted silanols, which means they are appropriate for the HILIC mode [21], whereas bonded amino–silica columns are relatively often used in the HILIC mode. Here, the basic analytes are generally strongly retained on the silica gel by the hydrogen bond and ion exchange interactions with the silanol groups. At the same time, the acidic compounds show greater affinity, sometimes leading to irreversible adsorption [4]. On the other hand, the amide-based stationary phase is less reactive and lacks basicity. Retention on these columns is thus less sensitive to the pH of mobile phases and less susceptible to irreversible chemisorption. Moreover, amide silica shows good recovery and stability [12].
During the last years, the evolution in HILIC was striking, and the number of stationary phases rapidly escalated until a recent slowdown. Despite the advantages of the HILIC technique that have been previously described, it presents problems that reduce its use in routine. In particular, hydrolytic decomposition is a crucial problem in HILIC. The main culprit is the retention mechanism, based on the adsorption of a water film on the polar surface of the stationary phase. The proximity of water to the surface of the stationary phase promotes the hydrolysis of the functional-bound groups and silica dissolution [22,23]. This causes the so-called “column bleeding” (a term borrowed from gas chromatography). The bleeding phenomenon goes almost unnoticed if UV detectors are used. Still, it is evident when using evaporative detectors such as charged aerosol detectors (CAD), evaporative light scattering detectors (ELSD), or a mass spectrometer (MS) [24], causing a significant drift during gradients and an increase in noise, often making it impossible to analytes in trace [25]. Furthermore, the presence of column bleeding indicates suffering at the level of the column, which affects analytical performance.
Different strategies can be used to solve column bleeding, such as increasing the anchor points of the functional group on the silica surface [26]. Given the potential of the HILIC technique, it is necessary to know the numerous HILIC stationary phases available to choose the best one for the required analysis. Therefore, the purpose of this review is to provide a survey of the stationary phases most used in HILIC. The reader will find two sections: in the first, the stationary phases for achiral applications are grouped into commercially and not-commercially available; in the second, the chiral stationary phases for enantioseparations are presented. For each stationary phase, relevant applications are reported, focusing the production on the last 15 years.
2. Achiral Stationary Phases in HILIC
The typical HILIC stationary phases consist of classic silica or derivatised silica gel with different polar functional groups. Furthermore, stationary phases based on polymers can also be used. Over the past 15 years, HILIC chromatography has progressed in discovering new stationary phases involving mixed or multiple interacting stationary phases. For clearness, the following HILIC stationary phases are divided into commercial and non-commercial [27]. Moreover, in this section, we present some chiral stationary phases (such as cyclofructanes and cyclodextrins). However, they are commonly used for achiral applications.
2.1. Commercial Stationary Phases
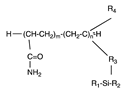
Although this review aims to give an overview of the most recent HILIC stationary phases, it is essential to describe the classic stationary phases to provide a context that led to the development of new structures. Thus, starting from the simplest structure of the stationary phases used in HILIC, it is worth mentioning the bare silica-based one. The first silica-based stationary phases were prepared by precipitation from silicate solutions (type A). They were acidic because of the presence of some metals that activate silanol groups, causing strong retention and asymmetric peaks. On the other hand, type B silica contains small amounts of metals. It is more stable at intermediate and high pH values (up to pH 9). It generally provides better separations, especially for basic samples [28]. Because silanol groups are ionised at higher pH values, cation exchange plays a vital role in retention, especially for positively charged basic compounds [29]. Lastly, a new type of silica called type-C has emerged. This silica has its surface modified with silicon hydride groups (i.e., Si-H); therefore, the percentage of silanols is lower than the type-B silica, making it less polar and susceptible to ionisation, decreasing the interaction with basic analytes [30]. Another widely used stationary phase in HILIC is amino silica; one of its first uses dates to 1975, in which Linden and collaborators separated mixtures of saccharides [27]. Aminopropyl-based columns are versatile and can be used in RPLC, NPLC, ion chromatography, and HILIC. However, as basic analytes are retained on silica gel by hydrogen bonding and ion exchange interactions with silanol groups, acidic compounds show high affinities to amino-silica columns, which can sometimes lead to irreversible adsorption [4]. The stationary phases based on silica and amino are certainly among the first and most used in HILIC; however, among the commercial stationary phases, chronologically, another widely used class is those characterised by the absence of net charge, also defined as neutral. To this class belong those stationary phases whose functional groups are hydroxyl (e.g., diol, pentanediol, polyhydroxy, cyclodextrins, and cyclofructans) or amide (e.g., amide, aspartamide, polyacrylamide, urea) groups.
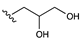
Diol phases exhibit high polarity and hydrogen bonding capabilities and do not comprise ionisable groups different from the non-functionalized silanols on the silica surface. These stationary phases were used to separate high- and low-molecular-weight analytes (such as protein and phenolic acids) [31,32]. Moreover, the columns based on the diol stationary phase allow to observe the anomerisation of sugars. This phenomenon cannot be studied using amino columns due to the basic nature of the stationary phase surface, which not allows to observe the anomerisation process. Furthermore, it is possible to observe the formation of glycosamines on the silica surface due to the reaction between the amino groups and the reducing sugars [33]. Even diol columns, although to a lesser extent than amino columns, can suffer from bleeding, especially in acidic conditions. Crosslinked stationary phases such as the Luna HILIC 200 are much more stable to hydrolysis; this solution maintains characteristics of polyethylene glycol and diol stationary phases and has an increased capacity for hydrophobic interactions and, therefore, has a mixed RP/HILIC behaviour. A phase analogous to diol is pentanediol; the latter has five hydroxyl groups; it was developed because it was thought to be more polar than diol. However, the same retentions have been observed on the diol and pentanediol columns [34,35]. Polyhydroxy phases, on the other hand, show stronger retention against polar compounds concerning the diol counterpart [36]. The last class of stationary phases characterised by the presence of hydroxyl groups are that of cyclodextrins and cyclofructans. These are cyclic structures based on saccharides and possess different free hydroxyl groups on the external rim and a hydrophobic cavity. Cyclodextrins exhibit enantiorecognitive properties and are often used as chiral additives in the mobile phase as chiral selectors anchored on silica and in capillary electrophoresis. Their ability to discriminate enantiomers is expressed mainly in RP and polar organic mode, while in HILIC, they are used for achiral separations of monosaccharides and oligosaccharides [37]. In this case, since retention increases with increasing saccharide units, the interaction takes place on the surface of the cyclodextrin and does not involve the cavity [38].
The last class of stationary phases with neutral groups is the amide one, characterised by amide or carbamoyl groups. In contrast to the amino stationary phases, the polar groups on these phases are not basic and less reactive. This makes these columns longer-lived than amino ones because irreversible adsorption on the stationary phase surface is less likely [12]. Moreover, because of their neutral nature, amide-based columns do not require highly ionic mobile phases, which is helpful for interfacing with a mass spectrometer because otherwise, a high concentration of salt in the mobile phase could worsen the sample ionisation process. The scope of amide-type stationary phases is extensive, and they are mainly used in the separation of peptides [39]. It has been observed in a recent work that there are some differences in selectivity between the different stationary phases present on the market (e.g., XBridge Amide, Sunshell Amide) [40]. Among all the stationary amide-type phases, the most successful one is carbamoyl-silica HILIC TSK-gel Amide-80 for separating oligosaccharides, derivatives of sugars, peptides, and amino acids, showing higher retention than the same separations on bare silica columns [8,41]. Figure 1 shows the separation of several organic acids in HILIC conditions on various columns. It is possible to observe that the analytes are more strongly less retained on amino stationary phases and less retained on bare silica stationary phase; on the other hand, the retention on TSK-gel Amide-80 and ZIC-HILIC is similar.
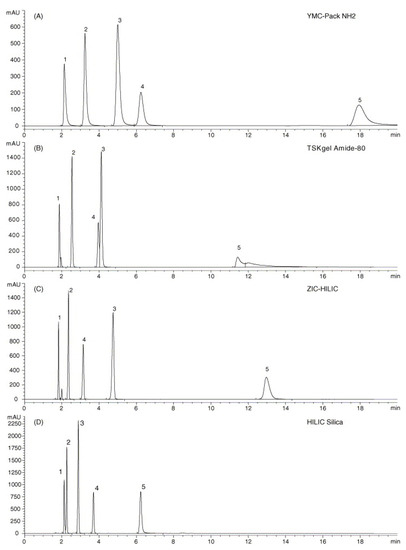
Figure 1.
Separation of acidic compounds (1: salicylamide, 2: salicylic acid, 3: 4-aminosalicylic acid, 4: acetylsalicylic acid, and 5: 3,4-dihydroxyphenylacetic acid) on (A) YMC-Pack NH2; (B) TSKgel Amide-80; (C) ZIC-HILIC; and (D) HILIC silica columns. Reproduced with permission [8].
Indeed, since the groups bounded on silica to obtain HILIC stationary phases are polar, there are much more charged stationary phases, which can be distinguished into anionic, cationic, and zwitterionic. Among the anionic stationary phases, we remember the bare silica one we discussed above, and we can also add those phases with aspartic acid, carboxylic acid, and sulfonic acid. The aspartic acid and carboxylic acid phases are weak cation exchangers, while the sulfonyl phase is a strong cation exchanger due to sulfonate groups [1]. Many stationary phases consist of groups that are positively charged in the slightly acidic pHs of the mobile phases used in HILIC. In addition to the aminopropyl phases, tertiary and quaternary amines, polyethyleneimine, imidazole, and pyridine stationary phases belong to this category.
The leader of this class is the aminopropyl column; however, as mentioned above, the use of columns packed with this phase is not the best choice when dealing with analytes with reactive groups (e.g., aldehydes); in fact, one can observe the formation of Schiff bases and the irreversible adsorption of analytes on the surface of the phase, significantly decreasing the life of the column and analytical performance. Moreover, to overcome this problem, tertiary and quaternary amine groups have been incorporated into the stationary phase, for example, in HILICpak VG-50 and VT-50 columns [9,42]. Indeed, the tertiary and quaternary amine cannot form Schiff bases with carbonyl compounds, which may result in an improved lifetime concerning aminopropyl silica. However, these phases show different retention and selectivity than the aminopropyl phase; therefore, the method transfer is not immediate and needs optimisation. The polymeric version of the amino type phases is based on polyethyleneimine, a polymer characterised by the presence of primary, secondary, and tertiary amines that covers the silica spheres [43].
Zwitterionic stationary phases were launched in the early 2000s [44,45]. This stationary phase is characterised by cationic and anionic moieties; it is explicitly used in HILIC applications [46]. The first compounds used to obtain stationary zwitterionic phases are sulfobetaine and phosphocholine. Stationary phases based on sulfobetaine include an anionic sulfonic acid group and a quaternary ammonium group divided by a small alkyl spacer. The bound sulfobetaine allows water adsorption by hydrogen bonding and the adsorbed water layer, thus essentially controlling the retention mechanism and ion-exchange interactions. This stationary phase is commercially available with the tradenames ZIC-HILIC (if supported on a silica gel) and ZICpHILIC (on a polymeric support). In the case of sulfobetaine and phosphocholine, the anionic/cationic group ratio is 1:1 in most recent zwitterionic stationary phases, as the iHILIC-Fusion (+) column carries net positive charges due to additional quaternary amine groups on the ligand [36]. In 2015, Diez and co-workers developed an interesting separation of polar aminoglycoside antibiotics using zwitterionic columns (i.e., Obelisc R and ZIC-HILIC) (Figure 2) [47]. Aminoglycosides are highly polar compounds; moreover, because of several amino groups, they have a weak basic nature. Compared with the other examined HILIC columns (such as BEH-amide and Amino), zwitterionic stationary phases offered satisfactory separation of the 14 polar aminoglycosides. It should be noted that in the case of zwitterion columns, it is necessary to use cations in the mobile phase to compete with aminoglycosides at the negative ionic heads.
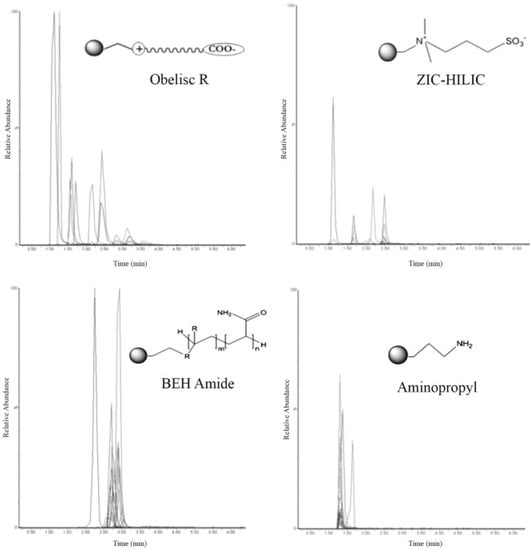
Figure 2.
Fourteen aminoglycosides standard solution separated on the four selected stationary phases with optimised conditions. Reproduced with permission [47].
The principal structures of the stationary phases described above are depicted in Table 1.

Table 1.
Commercial stationary phases (in alphabetical order).
2.2. Non-Commercial Stationary Phases
Non-commercial stationary phases are categorised as neutral, anionic, cationic, and zwitterionic as in the previous section.
Polymers dominate neutral stationary phase composition. Bleeding, the outcome of hydrolytic instability of stationary phases in water, is one of the limitations of the polar stationary phases used in HILIC. Thus, much effort has been made in academic research to decrease this phenomenon, and often using polymer-based stationary phases is the right way to go. A novel stationary phase was developed and synthesised utilising silica gel coated with polyvinyl alcohol (PVA). In this way, a 20-fold reduction in bleeding was achieved by using multiple layers of PVA crosslinked with glutaraldehyde onto silica gel. In a similar approach, the known commercial column TSKgel Amide 80 uses polyacrylamide as a polymer, as highlighted in the previous section. In a study, Li et al. grafted polyacrylamide through free radical polymerisation onto hydrolysed poly (glycidyl methacrylate-divinylbenzene) (GMA-DVB) microspheres, producing a stationary phase with favourable hydrophilicity. This is made possible by the presence of amide and hydroxyl groups on the polymer surface, enhancing the polarity and stability of the stationary phase [48]. Following the trend of the polar polymer, hyperbranched polyglycerol was surface polymerised onto silica. In particular, by repeating the polymerisation step, it was possible to modify the carbon load of the stationary phase, which exhibited a broad polar analyte separation capability [49]. Moreover, a simple and eco-friendly one-pot approach was used to generate a stationary phase using hydrothermally crosslinked polyvinylpyrrolidone (PVP) immobilised on silica. Both HILIC and RP chromatography demonstrated the excellent characteristics of this stationary phase. Additionally, this stationary phase showed good stability even under pH conditions as low as 3.5 or as high as 9.5 [50]. Lastly, starting with a short- and long-chain system of double-alkylated L-lysine, a urea-containing stationary phase was designed. According to the findings, the separation capacity was influenced by the length of the L-lysine chain. Short alkyl chains were found to have a greater ability to separate polar compounds in the L-lysine-derived urea-containing phase in addition to the other chromatographic parameters such as efficiency, resolution, and asymmetry [51].
Among the neutral stationary phases, in addition to the polymeric ones just described, we can count small-molecules-based stationary phases. In particular, in two papers from 2010 [49,50], sugars were anchored on silica through Cu(I)-catalysed Azide-Alkyne Click Chemistry reaction (CuAAC). Moni et al. anchored ethynyl C-galactoside and propargyl O-lactoside to silica using as spacers both (3-glycidoxypropyl) trimethoxysilane and 4-(chloromethyl)-phenyltrimethoxysilane suitably reacted with sodium azide to obtain the corresponding azides [52]. Using the more hydrophobic 4-(chloromethyl)-phenyltrimethoxysilane allowed the evaluation of the contribution of the spacer in the HILIC mechanism. It should be noted that the stationary phases obtained have been helpful in Dynamic-HILIC experiments for the study of sugar anomerisation using ELSD as a detector. The stationary phase was stable enough for baseline drift to be recorded [52]. In the same year, Fu et al. anchored on silica 1-propargyl-O-maltose. Analyses performed on columns packed with this stationary phase were highly reproducible and reliable. Moreover, Fu et al. noted that the presence of the triazole ring in this stationary phase also allowed a weak anion exchange (WAX) mechanism [53]. Conclusively, Ferreira et al. created a novel stationary phase by starting with the pentafluorophenyl degree and adding an amide moiety to make it more polar [54]. The outcome is a highly adaptable stationary phase that may be applied in two different modes of liquid chromatography: reversed-phase liquid chromatography and liquid chromatography with hydrophilic interaction. Under the conditions of hydrophilic interaction liquid chromatography, it demonstrates a significant separation of a mixture of nucleosides and antihypertensive medications. It also allowed the separation of pesticides and benzodiazepines under reversed-phase conditions [54].
Different ligands (hydrophobic, polar, or charged groups) are involved in the non-commercial stationary phase and responsible for various interactions. When the ligand exhibits a charge, electrostatic interactions are involved in separation. However, it is possible to classify every polar stationary phase with charged functional groups as a combined HILIC/IEX mode. It may be questioned if it is required to distinguish the IEX mode from HILIC when charged phases are used under HILIC settings because electrostatic interactions constitute a component of the retention mechanisms in HILIC when the analytes are charged [55].
Among the anionic stationary phases, we detected some distinctive stationary phases. Recent work suggests a novel method to produce mixed-mode stationary phases based on dicarboxylic cellulose by utilising dialdehyde cellulose. As a result, a HILIC/IEX column and a HILIC/IEX/Chiral separation columns were developed, both of which had excellent chromatographic performance [56].
Moreover, another interesting anionic stationary phase involved using deep eutectic solvents (DESs), a novel class of environmentally friendly solvents. In 2019, they were used to create the stationary phase made of poly (itaconic acid)-grafted on thiol-silica. This stationary phase was studied from several points of view, including buffer concentration, mobile phase, pH, and column temperature. The results showed good separations of polar compounds [57].
Finally, we have a chitooligosaccharides sulphonate derivative among the anionic stationary phases. The separation mechanism of the stationary phase was described using two empirical models: partitioning and adsorption. The result is a significant separation of polar molecules, including melamine, salicylic acid, and water-soluble vitamins in HILIC [58].
It is possible to develop a stationary phase for three chromatographic modes (RPLC/IEX and HILIC) by including charged or ionisable groups in the RPLC/HILIC mixed-mode stationary phases. To execute mixed-mode high-performance liquid chromatography, a new stationary phase was created by repeatedly grafting 1,4-butanediol diglycidyl ether (BDDE) and dopamine (DA) on the silica surface. In the RPLC mode, successful separations of alkylbenzenes polycyclic aromatic hydrocarbons (PAHs) and hydrophobic positional isomers were achieved. While some acid and basic analytes were employed to assess the IEX mode, nucleobases, nucleosides, and flavonoid separations were accomplished under the HILIC mode [59]. Using three functional monomers, i.e., lauryl methacrylate (LMA), hydroxyethyl methacrylate (HEMA), and dimethylaminoethyl methacrylate (DMAEMA), that have undergone surface initiated-atom transfer radical polymerisation (SI-ATRP) on the surface of silica, another study demonstrated a simple method for the synthesis of a tri-modal stationary phase. It offered many interactions, including hydrophobic, hydrophilic, and electrostatic interactions, and demonstrated good separation abilities to the tested solutes compared to conventional single-mode columns [60]. A mixed-mode stationary phase of embedded poly(ethyleneimine) was created using an epoxide ring-opening process. The chromatographic performance was investigated and showed an ability to discriminate positional isomers [61]. Another stationary phase that uses L-phenylalanine attached to the surface of activated silica was created using poly(ethyleneimine). The novelty of this stationary phase is that it exhibits chiral resolution capacity, which was demonstrated by the separation of warfarin as a model analyte, and in addition, good resolution capabilities in HILIC/RPLC and IEX modes were confirmed using diverse analyte mixtures [62].
Among the several cationic stationary phases, one has silica particles enclosed in a co-polymer of poly-(vinyl alcohol) cationic cellulose (PVA-CC-Sil). A remarkable feature of the synthesis of this stationary phase is that it is made without organic solvents. Moreover, it showed outstanding stability, a wide pH tolerance range, and excellent separation efficiency, even for saccharides with moderate or high levels of polymerisation [63].
The zwitterionic stationary phases are the most abundant, probably because they are the most versatile; it is sufficient to modulate the pH of the mobile phase to manage the elution of charged substances. Both small peptides and amino acids have been employed as ligands in HILIC for zwitterionic stationary phases. Amino acids are a low-cost, highly available source of small polar molecules that can synthesise new stationary phases. They are also the quintessential zwitterionic molecules thanks to the presence of both an amino group and a carboxyl group. In most cases, amino acids are anchored on silica through “click” reactions.
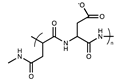
For example, cysteine was used in the click “thiol-ene” reaction to design stationary phases. In 2012, Shen et al. prepared a stationary phase starting from vinyl silica gel and cysteine in the presence of α,α,′-Azoisobutyronitrile (AIBN) as a radical initiator at 65 °C [64,65]. With this procedure, the cysteine loading on the stationary phase surface was 1.52 μmol/m2. Regarding the separation mechanism, the findings showed that the partitioning mechanism rather than the electrostatic one dominates the separation of polar analytes. In 2022, Ciogli et al. proposed the synthesis of a cysteine-based stationary phase, anchoring this amino acid on vinyl silica gel through a photo-induced mechanism [66]. Thanks to this procedure, the loading of cysteine on vinyl silica gel corresponded to 3.96 μmol/m2. This stationary phase has a mixed HILIC/RP/IEX-behaviour, and large coating of the silica surface with vinyl fragments gives a certain degree of hydrophobicity to the stationary phase expressed in an RP mechanism when mobile phases with higher water content are used. In Figure 3 is reported the resolution of a mixture of free amino acids, and two elution conditions were tested: (i) RP mode (methanol/20 mM ammonium acetate in water 80/20, v/v) and (ii) HILIC (methanol/acetonitrile/20 mM ammonium acetate in water 60/20/20, v/v/v). From the histogram in Figure 3, with the presence of ACN, the retention increases consequently, demonstrating a prevalent HILIC separation mechanism. The HILIC/IEX mechanism is due to the portion of anchored cysteine, which, being polar and with the free zwitterionic portion, also works as a weak anionic or cationic exchanger, depending on the working pH. Moreover, this stationary phase was successfully employed for studying anomerisation of xylose and glucose [67].
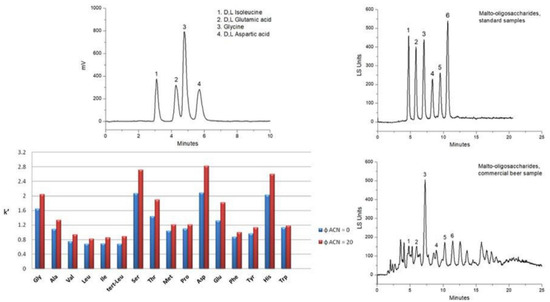
Figure 3.
(Top left) Separation of isoleucine, glutamic acid, glycine, and aspartic acid on VSG_HD-1-Photo-Cys-column (5 µm, 150 × 4.6 mm ID). (Bottom left) Retention factor of other amino acids. Blue bar, RP condition; red bar, HILIC condition. (Top and bottom right) Separation of malto-oligosaccharides (standard samples, top; in a commercial beer sample, bottom). Reproduced with permission [66].
In 2020, a polymeric zwitterionic cysteine-based stationary phase was presented. The poly(glycidyl methacrylate-divinylbenzene) (GMA-DVB) was used, and the epoxide groups on the microsphere surface were hydrolysed to obtain diol groups. Then cysteine was clicked onto the microsphere via “thiol-ene” chemistry. GMA-DVB microspheres have a diol and zwitterionic group (carboxylate and amine group connected with cysteine) on their surface, which contributes to their strong hydrophilicity and supports their efficiency in polar analyte separation. Additionally, this stationary phase exhibited remarkable stability even with alkaline mobile phases, minimal bleed, and strong ionic analyte separation [68]. Beyond cysteine, other amino acids could be used. For example, we report the case of a stationary phase based on tyrosine. The results show that when used to separate phenolic compounds, amino acids, alkaloids, and nucleobases, the click-tyrosine stationary phase has good HILIC properties. Additionally, this stationary phase was examined under several conditions, including pH, buffer concentration, and solvent composition [69]. Moreover, a stationary phase was created with calyx-[4]-arene bonds and tetra-proline modification. Because of their distinct host-guest recognition capabilities, calixarenes and their derivates have been widely used in chromatographic research. Comparing this stationary phase to other calixarene phases, it exhibits superior selectivity [70].
L-isoleucine and 4-phenylbutylamine were used as starting materials to create a stationary phase. Numerous interactions, such as hydrophobic, π-π, hydrogen-bonding, dipole-dipole, and ion-dipole interactions, are involved in the separation. The outcome is a successful separation of the aromatic compounds, with various polarities using RP and HILIC. In the HILIC, nucleosides and nucleotides were separated. Several moderately, weakly polar and non-polar chemicals are evaluated and successfully separated under RP circumstances to examine further the impact of this multifunctional stationary phase under the RP condition. This stationary phase demonstrates HILIC and RPLC performance compared to conventional C18 and commercial HILIC columns, expanding the range of analyte separation [71]. By modifying 4-hydroxy-d-phenyl glycine with methacrylic anhydride, another stationary phase was created. It was subsequently immobilised on silica using thiol-initiated surface polymerisation. This column exhibits high selectivity for both hydrophobic and hydrophilic analytes. This stationary phase has better selectivity against polycyclic aromatic hydrocarbons than it does against alkylbenzenes when compared to a commercial C18 column. This is most likely because of interactions between the amino acid phenylglycine and electron-rich aromatic rings. Additionally, compared to commercial C18 and HILIC, this stationary phase exhibits greater selectivity even for polar chemicals sulfonamides and organic acids [72].
Di- and tripeptides have also been employed as ligands for the stationary phases in HILIC in addition to amino acids. A stationary phase was created using glutathione and copper-free “thiol-ene” click chemistry, showing that it could support cation exchange and hydrophilic interactions. Clearly distinguishing between peptides and carbohydrates with good selectivity, this brand-new stationary phase could be used favourably for glycomic and proteomic applications [73,74].
Regarding small dipeptides, a two-stage procedure was used to anchor glycine and alanine dipeptides on a silica surface directly. The first step provided that the silica was modified using aminopropyltrimethoxysilane. The prepared support was bonded with the first FMOC-amino acid (with DCC as activating agent), followed by deprotection and, finally, further derivatisation with amino acid. The stationary phases obtained were tested by separating a mixture of nucleosides [75].
As the stationary phases containing amino acids and peptides are a significant part of those prepared for HILIC separations, we report a 2019 study where several stationary phases containing amino acids were produced and compared to assess the differences in selectivity [76]. While under RP HPLC conditions, the following features were revealed: hydrophobicity, hydrophobic selectivity, shape selectivity, hydrogen bonding, and electrostatic interactions at pH values 2.7 and 7.6. In the case of the HILIC mode, the characterisation was performed regarding the degree of hydrophilicity and selectivity [76].
Other zwitterionic stationary phases are designed from the synthetic ligand. For example, starting with 2-bromoethanesulfonate, a sulfonate-pyridinium phase was created by quaternising the pyridyl side chains of the polymer anchored to the surface of porous silica. Toluene and benzoic acid were used to test this stationary phase, and the findings showed that they performed well in both HILIC and ion exchange chromatography. This stationary phase exhibited a significantly separative capability of benzoic acid derivates over a broad pH range compared to a typical sulfobetaine-based zwitterion-grafted porous silica. The interactions between the analytes and the zwitterionic groups on the polymer chains were thought to cause this selectivity [77].
Utilising a combination of two functional monomers, a stationary phase known as HILIC/IEX was created using the atom transfer radical polymerisation process. The ratio of mixed monomers made it simple to control the charge and polarity of the stationary phase and separation selectivity. Additionally, this stationary phase had properties that responded to temperature. The columns demonstrate the potential for nucleoside and ß-agonist separation [78].
All the stationary phases described in this section are shown in Table 2.

Table 2.
Non-commercial stationary phases (in alphabetical order).
Table 2.
Non-commercial stationary phases (in alphabetical order).
| Phase Type | Phase Name | Functional Groups | Reference |
|---|---|---|---|
| Neutral | Alkylurea | 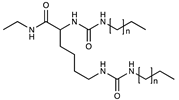 | [50] |
| Pentafluorobenzamide | 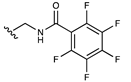 | [53] | |
| Polyacrylamide | 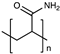 | [47] | |
| Polyvinylpyrrolidone | 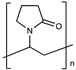 | [49] | |
| Polyglycerol | 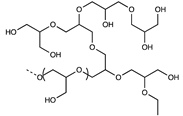 | [48] | |
| Anionic | Dicarboxylic cellulose | 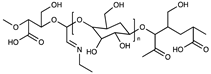 | [55] |
| Poly(itaconic acid) | 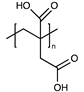 | [56] | |
| Sulfonated chitooligosaccharide | 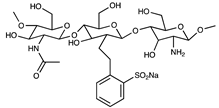 | [57] | |
| Cationic | Butanediol/dopamine dendrimer | 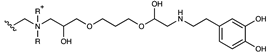 | [58] |
| Dodecyl/hydroxyethyl/ quaternary amine | 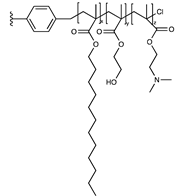 | [59] | |
| Polyethyleneimine embedded | 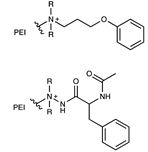 | [60,61] | |
| PVA-cationic cellulose | 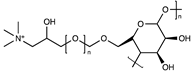 | [62] | |
| Zwitterionic | Amino acids | 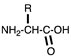 | [63,64,65,66,67,68,69] |
| Peptides (Glutathione, glycylalanine) | 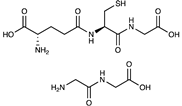 | [70,71,72,73] | |
| Sulfonate/pyridinium | 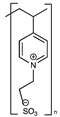 | [74] | |
| Carboxylate or sulfonate/tertiary amine | 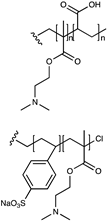 | [75] |
3. Chiral Stationary Phases in HILIC
By inspection of tables in previous sections, such a wide choice of stationary phases for achiral applications guarantees a solution even for the most complicated analytical issue but still in an achiral system. In contrast, the use of chiral stationary phases in HILIC conditions have yielded relatively recent and poorly represented results. Enantiomers are well-separated in HILIC conditions by using stationary phases having zwitterionic behaviours or, more recently, by employing polysaccharide-base stationary phases. In the first case, the macrocyclic glycopeptide antibiotics covalently bonded on silica have been found more useful for peptide- or protein-based stationary phases [79]. Commercially available teicoplanin, vancomycin, and ristocetin A stationary phases are well-known in the enantioseparations of several classes of racemates, including N-protected amino acids, alfa-aryloxy acids, beta-blocker, and anti-inflammatory drugs mainly in reversed-phase (RP) and polar organic mode (POM) elution conditions. Further, suitable elution modes are normal phase (NP), hydrophilic interaction (HILIC), both weak anion and weak cation exchanger (WAX and WCX), as well as supercritical fluid (SFC) [80,81,82,83,84]. It is important to note that a careful tune of pH, ionic strength, buffer composition, and concentration affect the selectivity of these phases to simultaneously separate analytes with very different hydrophobicity/hydrophilicity and charge state in a more complex system, defined as multimodal elution. In addition, the second generation of stationary phases based only on teicoplanin, and vancomycin were developed to the extent their enantio-resolution power for negatively charged compounds (i.e., N-protected amino acids, unretained or poorly retained with the first versions of CSPs, and inorganic anions) and in HILIC conditions. To achieve it, the bonding chemistry was modified, introducing a permanent zwitterionic structure of the chiral selector. Overall, these phases have practical relevance in separating chiral APIs (active pharmaceutical ingredients) in salt form [82]. A separation of enantiomers and their counterion will be pursued in one single chromatographic run. For example, enantiomers of carglumic acid, an orphan drug, and their sodium ion were resolved on the first generation of teicoplanin CSP by HILIC/WCX (Figure 4 top). At the same time, the separation of D, L-propionyl carnitine hydrochloride was achieved on the second generation of teicoplanin phases (Figure 4 bottom).
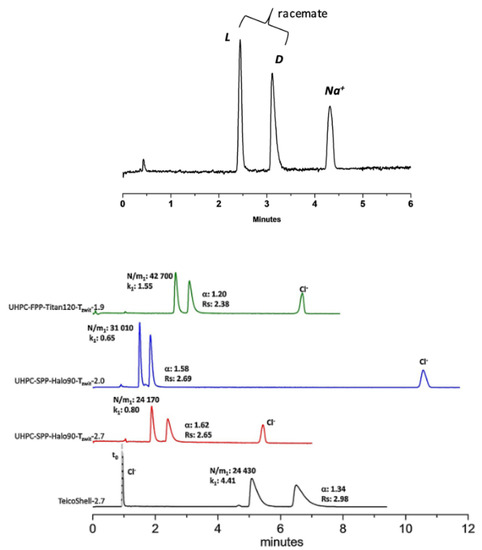
Figure 4.
(Top) Direct gradient separation of D, L-Carglumic acid sodium salt on UHPC-Titan120-TCOOH-1.9 column. (Bottom) Separation of Propionyl-D, L-carnitine hydro-chloride on UHPC-Titan120-TZWIT-1.9 column. Comparison with commercially available column TeicoShell-2.7 and TZWIT SPP columns (packed with SPP 2.7 µm, 2.0 µm). Reproduced with permission [82].
Exciting results in the separation of enantiomers in HILIC conditions concern the use of widespread polysaccharide-based immobilised stationary phases (Chiralpak and Lux series). Starting from 2012, with the separation of (R, S) amlodipine, in the literature, there are more and more examples of racemates resolved using the most classic of the mobile phases for HILIC: acetonitrile/water. A detailed review of all these examples is given in [85]. From these data, the potentials of these stationary phases in HILIC appear to be multiple and various, as very recently reported in [86,87]. In both studies, analytical methods have involved mass spectrometry detection without the appearance of a bleeding effect, representing the main drawback of zwitterionic-like stationary phases. Medina-Hernández and co-workers explored the potential of five commercial cellulose-based chiral stationary phases with 54 structurally unrelated basic and acid compounds in both RP and HILIC conditions [87]. Both modalities resulted in the equivalency of, except for the enantiomers of promethazine, all the compounds that were baseline resolved in HILIC and RPLC.
4. Conclusions
The fact that there is no universal stationary phase for HILIC, such as C18 for RPLC, is reflected in many commercial stationary phases. It is up to the operators to choose wisely the type of column, and it is essential to know the chemical properties of the investigated analytes to evaluate the use of neutral, anionic, cationic, or zwitterionic stationary phases. In addition to the charge of the analyte, the side reactions with the groups of selectors must also be considered. For example, in the analysis of saccharides, when the amino stationary phase was employed, the unwanted formation of the Schiff bases between the amino group of the stationary phase and the aldehyde of sugar can occur, proving the irreversible adsorption of analytes.
The biggest limitation of HILIC stationary phases is related to their stability. In fact, due to the water film on the surface of the stationary phase, they undergo hydrolytic decomposition. For this reason, several scholars in the academic world have developed new solutions and new stationary phases. However, despite the scientific ferment in the synthesis and design of new stationary HILIC achiral phases, the same interest has yet to be generated regarding chiral stationary phases. We hope that this sector will develop further; since most drugs are polar and chiral, the development of new chiral methods for HILIC could be necessary for the pharmaceutical industry and beyond without forgetting the possibility of HILIC being used in LC-MS applications.
Author Contributions
Conceptualization, G.M. and A.C.; data curation, M.A.G. and A.F.; writing—original draft preparation, M.A.G. and G.M.; writing—review and editing, G.M. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alpert, A.J. Hydrophilic-Interaction Chromatography for the Separation of Peptides, Nucleic Acids and Other Polar Compounds. J. Chromatogr. A 1990, 499, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Janás, P. Recent Advances in Stationary Phases and Understanding of Retention in Hydrophilic Interaction Chromatography. A Review. Anal. Chim. Acta 2017, 967, 12–32. [Google Scholar] [CrossRef]
- Nováková, L.; Havlíková, L.; Vlčková, H. Hydrophilic Interaction Chromatography of Polar and Ionizable Compounds by UHPLC. TrAC Trends Anal. Chem. 2014, 63, 55–64. [Google Scholar] [CrossRef]
- Oyler, A.R.; Armstrong, B.L.; Cha, J.Y.; Zhou, M.X.; Yang, Q.; Robinson, R.I.; Dunphy, R.; Burinsky, D.J. Hydrophilic Interaction Chromatography on Amino-Silica Phases Complements Reversed-Phase High-Performance Liquid Chromatography and Capillary Electrophoresis for Peptide Analysis. J. Chromatogr. A 1996, 724, 378–383. [Google Scholar] [CrossRef]
- Garbis, S.D.; Melse-Boonstra, A.; West, C.E.; Van Breemen, R.B. Determination of Folates in Human Plasma Using Hydrophilic Interaction Chromatography—Tandem Mass Spectrometry. Anal. Chem. 2001, 73, 5358–5364. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.A. Hydrophilic Interaction Chromatography Using Amino and Silica Columns for the Determination of Polar Pharmaceuticals and Impurities. J. Chromatogr. A 2001, 913, 113–122. [Google Scholar] [CrossRef]
- Li, R.; Huang, J. Chromatographic Behavior of Epirubicin and Its Analogues on High-Purity Silica in Hydrophilic Interaction Chromatography. J. Chromatogr. A 2004, 1041, 163–169. [Google Scholar] [CrossRef]
- Guo, Y.; Gaiki, S. Retention Behavior of Small Polar Compounds on Polar Stationary Phases in Hydrophilic Interaction Chromatography. J. Chromatogr. A 2005, 1074, 71–80. [Google Scholar] [CrossRef]
- Jandera, P. Stationary and Mobile Phases in Hydrophilic Interaction Chromatography: A Review. Anal. Chim. Acta 2011, 692, 1–25. [Google Scholar] [CrossRef]
- Buszewski, B.; Noga, S. Hydrophilic Interaction Liquid Chromatography (HILIC)-a Powerful Separation Technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef]
- Jandera, P.; Hájek, T.; Škeříková, V.; Soukup, J. Dual Hydrophilic Interaction-RP Retention Mechanism on Polar Columns: Structural Correlations and Implementation for 2-D Separations on a Single Column. J. Sep. Sci. 2010, 33, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Hemström, P.; Irgum, K. Hydrophilic Interaction Chromatography. J. Sep. Sci. 2006, 29, 1784–1821. [Google Scholar] [CrossRef]
- Erkmen, C.; Gebrehiwot, W.H.; Uslu, B. Hydrophilic Interaction Liquid Chromatography (HILIC): Latest Applications in the Pharmaceutical Researches. Curr. Pharm. Anal. 2020, 17, 316–345. [Google Scholar] [CrossRef]
- Gargano, A.F.G.; Roca, L.S.; Fellers, R.T.; Bocxe, M.; Domínguez-Vega, E.; Somsen, G.W. Capillary HILIC-MS: A New Tool for Sensitive Top-Down Proteomics. Anal. Chem. 2018, 90, 6601–6609. [Google Scholar] [CrossRef]
- Stevenson, P.G.; Mnatsakanyan, M.; Francis, A.R.; Shalliker, R.A. A Discussion on the Process of Defining 2-D Separation Selectivity. J. Sep. Sci. 2010, 33, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Gilar, M.; Olivova, P.; Daly, A.E.; Gebler, J.C. Orthogonality of Separation in Two-Dimensional Liquid Chromatography. Anal. Chem. 2005, 77, 6426–6434. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Patterson, D.G.; Lee, M.L. Geometric Approach to Factor Analysis for the Estimation of Orthogonality and Practical Peak Capacity in Comprehensive Two-Dimensional Separations. Anal. Chem. 1995, 67, 3840–3845. [Google Scholar] [CrossRef]
- Brandão, P.F.; Duarte, A.C.; Duarte, R.M.B.O. Comprehensive Multidimensional Liquid Chromatography for Advancing Environmental and Natural Products Research. TrAC Trends Anal. Chem. 2019, 116, 186–197. [Google Scholar] [CrossRef]
- Dugo, P.; Cacciola, F.; Kumm, T.; Dugo, G.; Mondello, L. Comprehensive Multidimensional Liquid Chromatography: Theory and Applications. J. Chromatogr. A 2008, 1184, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, J.N.; Horváth, K.; Guiochon, G. Approaches to Comprehensive Multidimensional Liquid Chromatography Systems. J. Chromatogr. A 2009, 1216, 1363–1371. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Rasmussen, H.T. Orthogonal Method Development Using Hydrophilic Interaction Chromatography and Reversed-Phase High-Performance Liquid Chromatography for the Determination of Pharmaceuticals and Impurities. J. Chromatogr. A 2005, 1083, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Yang, Z.; Zhang, F.; Yang, B.; Dasgupta, P.K. Low-Bleed Silica-Based Stationary Phase for Hydrophilic Interaction Liquid Chromatography. Anal. Chem. 2018, 90, 8750–8755. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Peng, Y.; Zhang, F.; Yang, B.; Liang, X. Preparation of a Low Bleeding Polar Stationary Phase for Hydrophilic Interaction Liquid Chromatography. Talanta 2018, 182, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Richards, M.A.; Zha, Y.; Francis, R.; Lozano, R.; Ruan, J. Determination of Inorganic Pharmaceutical Counterions Using Hydrophilic Interaction Chromatography Coupled with a Corona® CAD Detector. J. Pharm. Biomed. Anal. 2009, 50, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Park, J.H.; Lee, J.; Kwon, S.W. Comparison of Two Aerosol-Based Detectors for the Analysis of Gabapentin in Pharmaceutical Formulations by Hydrophilic Interaction Chromatography. Talanta 2011, 85, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wahab, M.F.; Breitbach, Z.S.; Armstrong, D.W. Carboxylated Cyclofructan 6 as a Hydrolytically Stable High Efficiency Stationary Phase for Hydrophilic Interaction Liquid Chromatography and Mixed Mode Separations. Anal. Methods 2016, 8, 6038–6045. [Google Scholar] [CrossRef]
- Linden, J.C.; Lawhead, C.L. Liquid Chromatography of Saccharides. J. Chromatogr. A 1975, 105, 125–133. [Google Scholar] [CrossRef]
- McCalley, D.V. Is Hydrophilic Interaction Chromatography with Silica Columns a Viable Alternative to Reversed-Phase Liquid Chromatography for the Analysis of Ionisable Compounds? J. Chromatogr. A 2007, 1171, 46–55. [Google Scholar] [CrossRef]
- Pack, B.W.; Risley, D.S. Evaluation of a Monolithic Silica Column Operated in the Hydrophilic Interaction Chromatography Mode with Evaporative Light Scattering Detection for the Separation and Detection of Counter-Ions. J. Chromatogr. A 2005, 1073, 269–275. [Google Scholar] [CrossRef]
- Pesek, J.J.; Matyska, M.T. Silica Hydride: A Separation Material Every Analyst Should Know About. Molecules 2021, 26, 7505. [Google Scholar] [CrossRef]
- Regnier, F.E.; Noel, R. Glycerolpropylsilane Bonded Phases in the Steric Exclusion Chromatography of Biological Macromolecules. J. Chromatogr. Sci. 1976, 14, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Hájek, T. Utilization of Dual Retention Mechanism on Columns with Bonded PEG and Diol Stationary Phases for Adjusting the Separation Selectivity of Phenolic and Flavone Natural Antioxidants. J. Sep. Sci. 2009, 32, 3603–3619. [Google Scholar] [CrossRef] [PubMed]
- Pazourek, J. Monitoring of Mutarotation of Monosaccharides by Hydrophilic Interaction Chromatography. J. Sep. Sci. 2010, 33, 974–981. [Google Scholar] [CrossRef]
- Jandera, P.; Hájek, T. A New Definition of the Stationary Phase Volume in Mixed-mode Chromatographic Columns in Hydrophilic Liquid Chromatography. Molecules 2021, 26, 4819. [Google Scholar] [CrossRef]
- Molnarova, K.; Kozlík, P. Comparison of Different HILIC Stationary Phases in the Separation of Hemopexin and Immunoglobulin G Glycopeptides and Their Isomers. Molecules 2020, 25, 4655. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bhalodia, N.; Fattal, B. Evaluating Relative Retention of Polar Stationary Phases in Hydrophilic Interaction Chromatography. Separations 2019, 6, 42. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Jin, H.L. Evaluation of the Liquid Chromatographic of monosaccharides, disaccharides, trisaccharides, tetrasaccharides, deoxysaccharides and sugar alcohols with stable cyclodextrin bonded phase columns. J. Chromatogr. A 1989, 462, 219–232. [Google Scholar] [CrossRef]
- Berthod, A.; Chang, S.S.C.; Kullman, J.P.S.; Armstrong, D.W. Practice and Mechanism of HPLC Oligosaccharide Separation with a Cyclodextrin Bonded Phase. Talanta 1998, 47, 1001–1012. [Google Scholar] [CrossRef]
- Yoshida, T. Peptide Separation in Normal Phase Liquid Chromatography. Sci. Instrum. Div. 1997, 69, 3038–3043. [Google Scholar] [CrossRef]
- Ikegami, T.; Taniguchi, A.; Okada, T.; Horie, K.; Arase, S.; Ikegami, Y. Functionalization Using Polymer or Silane? A Practical Test Method to Characterize Hydrophilic Interaction Chromatography Phases in Terms of Their Functionalization Method. J. Chromatogr. A 2021, 1638, 461850. [Google Scholar] [CrossRef]
- Karlsson, G.; Winge, S.; Sandberg, H. Separation of Monosaccharides by Hydrophilic Interaction Chromatography with Evaporative Light Scattering Detection. J. Chromatogr. A 2005, 1092, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Iturrospe, E.; Da Silva, K.M.; Andújar, B.T.; Cuykx, M.; Boeckmans, J.; Vanhaecke, T.; Covaci, A.; van Nuijs, A.L.N. An Exploratory Approach for an Oriented Development of an Untargeted Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry Platform for Polar Metabolites in Biological Matrices. J. Chromatogr. A 2021, 1637, 461807. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Hou, Y.; Zhang, F.; Shen, G.; Yang, B. A Hyperbranched Polyethylenimine Functionalized Stationary Phase for Hydrophilic Interaction Liquid Chromatography. Anal. Bioanal. Chem. 2016, 408, 3633–3638. [Google Scholar] [CrossRef]
- Jiang, W.; Irgum, K. Covalently Bonded Polymeric Zwitterionic Stationary Phase for Simultaneous Separation of Inorganic Cations and Anions. Anal. Chem. 1999, 71, 333–344. [Google Scholar] [CrossRef]
- Jiang, W.; Fischer, G.; Girmay, Y.; Irgum, K. Zwitterionic Stationary Phase with Covalently Bonded Phosphorylcholine Type Polymer Grafts and Its Applicability to Separation of Peptides in the Hydrophilic Interaction Liquid Chromatography Mode. J. Chromatogr. A 2006, 1127, 82–91. [Google Scholar] [CrossRef]
- Guo, Y. A Survey of Polar Stationary Phases for Hydrophilic Interaction Chromatography and Recent Progress in Understanding Retention and Selectivity. Biomed. Chromatogr. 2022, 36, e5332. [Google Scholar] [CrossRef]
- Díez, C.; Guillarme, D.; Spörri, A.S.; Cognard, E.; Ortelli, D.; Edder, P.; Rudaz, S. Aminoglycoside Analysis in Food of Animal Origin with a Zwitterionic Stationary Phase and Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chim. Acta 2015, 882, 127–139. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Zhang, F.; Geng, H.; Yang, B. A Hydrolytically Stable Amide Polar Stationary Phase for Hydrophilic Interaction Chromatography. Talanta 2021, 231, 122340. [Google Scholar] [CrossRef]
- Geng, H.; Jing, J.; Zhang, F.; Zhang, F.; Yang, B. A Polar Stationary Phase Obtained by Surface-Initiated Polymerization of Hyperbranched Polyglycerol onto Silica. Talanta 2020, 209, 120525. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Zhou, X.; Wang, L.; Wan, L.; Wu, R. One-Pot Hydrothermal Cross-Linking Preparation of Poly(Vinylpyrrolidone) Immobilized Silica Stationary Phase for Hydrophilic Interaction Chromatography. J. Chromatogr. A 2020, 1633, 461656. [Google Scholar] [CrossRef]
- Mallik, A.K.; Guragain, S.; Rahman, M.M.; Takafuji, M.; Ihara, H. L-Lysine-Derived Highly Selective Stationary Phases for Hydrophilic Interaction Chromatography: Effect of Chain Length on Selectivity, Efficiency, Resolution, and Asymmetry. Sep. Sci. Plus 2019, 2, 42–50. [Google Scholar] [CrossRef]
- Moni, L.; Ciogli, A.; D’Acquarica, I.; Dondoni, A.; Gasparrini, F.; Marra, A. Synthesis of Sugar-Based Silica Gels by Copper-Catalysed Azide-Alkyne Cycloaddition via a Single-Step Azido-Activated Silica Intermediate and the Use of the Gels in Hydrophilic Interaction Chromatography. Chem.A Eur. J. 2010, 16, 5712–5722. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Guo, Z.; Liang, T.; Zhang, X.; Xu, Q.; Liang, X. Chemically Bonded Maltose via Click Chemistry as Stationary Phase for HILIC. Anal. Methods 2010, 2, 217–224. [Google Scholar] [CrossRef]
- de Ferreira, C.C.; Gama, M.R.; da Silva, G.S.; Almeida, W.P.; Collins, C.H.; Jardim, I.C.S.F. Synthesis and Evaluation of a Pentafluorobenzamide Stationary Phase for HPLC Separations in the Reversed Phase and Hydrophilic Interaction Modes. J. Sep. Sci. 2018, 41, 3855–3862. [Google Scholar] [CrossRef] [PubMed]
- McCalley, D.V. Understanding and Manipulating the Separation in Hydrophilic Interaction Liquid Chromatography. J. Chromatogr. A 2017, 1523, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Luo, G.; Li, Z.; Li, H.; Zhao, L.; Qiu, H. A New Strategy for the Preparation of Mixed-Mode Chromatographic Stationary Phases Based on Modified Dialdehyde Cellulose. J. Chromatogr. A 2020, 1618, 460885. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, T.; Zhang, H.; Chen, J.; Li, Z.; Qiu, H. Poly(Itaconic Acid)-Grafted Silica Stationary Phase Prepared in Deep Eutectic Solvents and Its Unique Performance in Hydrophilic Interaction Chromatography. Talanta 2019, 191, 265–271. [Google Scholar] [CrossRef]
- Yan, K.; Yang, H.; Huang, S.; Bai, Z. A Sulfonated Chitooligosaccharide Modified Silica Material for Hydrophilic Interaction Liquid Chromatography and Its Chromatographic Evaluation. Anal. Methods 2018, 10, 1258–1265. [Google Scholar] [CrossRef]
- Zhou, D.; Zeng, J.; Fu, Q.; Gao, D.; Zhang, K.; Ren, X.; Zhou, K.; Xia, Z.; Wang, L. Preparation and Evaluation of a Reversed-Phase/Hydrophilic Interaction/Ion-Exchange Mixed-Mode Chromatographic Stationary Phase Functionalized with Dopamine-Based Dendrimers. J. Chromatogr. A 2018, 1571, 165–175. [Google Scholar] [CrossRef]
- Bo, C.; Jia, Z.; Dai, X.; Wei, Y. Facile Preparation of Polymer-Brush Reverse-Phase/Hydrophilic Interaction/Ion-Exchange Tri-Mode Chromatographic Stationary Phases by Controlled Polymerization of Three Functional Monomers. J. Chromatogr. A 2020, 1619, 460966. [Google Scholar] [CrossRef]
- Ren, X.; Hu, C.; Gao, D.; Fu, Q.; Zhang, K.; Zu, F.; Zeng, J.; Wang, L.; Xia, Z. Preparation of a Poly(Ethyleneimine) Embedded Phenyl Stationary Phase for Mixed-Mode Liquid Chromatography. Anal. Chim. Acta 2018, 1042, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Luo, Q.; Ren, X.; Zheng, Y.; Gao, D.; Fu, Q.; Zu, F.; Xia, Z.; Wang, L. Preparation and Performance of a Poly(Ethyleneimine) Embedded N-Acetyl-L-Phenylalanine Mixed-Mode Stationary Phase for HPLC. Microchem. J. 2020, 157, 105021. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, F.; Pan, X.; Hou, Y.; Yang, B. Poly(Vinyl Alcohol)-Cationic Cellulose Copolymer Encapsulated SiO2 Stationary Phase for Hydrophilic Interaction Liquid Chromatography. RSC Adv. 2017, 7, 21336–21341. [Google Scholar] [CrossRef]
- Shen, A.; Guo, Z.; Cai, X.; Xue, X.; Liang, X. Preparation and Chromatographic Evaluation of a Cysteine-Bonded Zwitterionic Hydrophilic Interaction Liquid Chromatography Stationary Phase. J. Chromatogr. A 2012, 1228, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Guo, Z.; Yu, L.; Cao, L.; Liang, X. A Novel Zwitterionic HILIC Stationary Phase Based on “Thiol-Ene” Click Chemistry between Cysteine and Vinyl Silica. Chem. Commun. 2011, 47, 4550–4552. [Google Scholar] [CrossRef] [PubMed]
- Ciogli, A.; Buonsenso, F.; Proietti, N.; Mazzoccanti, G.; Manetto, S.; Calcaterra, A.; De Angelis, M.; Gasparrini, F. Preparation of a High-Density Vinyl Silica Gel to Anchor Cysteine via Photo-Click Reaction and Its Applications in Hydrophilic Interaction Chromatography. J. Chromatogr. A 2022, 1675, 463173. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, A.; Manetto, S.; Buonsenso, F.; Francioso, A.; Pierini, M.; Villani, C. Separation of Monosaccharide Anomers on Photo-Click Cysteine-Based Stationary Phase: The α/β Interconversion Process Studied by Dynamic Hydrophilic Liquid Chromatography. Separations 2022, 9, 203. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Zhang, F.; Geng, H.; Yang, B. A Polymer-Based Zwitterionic Stationary Phase for Hydrophilic Interaction Chromatography. Talanta 2020, 216, 120927. [Google Scholar] [CrossRef]
- Farhadpour, M.; Maghari, S.; Rezadoost, H.; Bagheri, M.; Ghassempour, A. A Click Tyrosine Zwitterionic Stationary Phases for Hydrophilic Interaction Liquid Chromatography. J. Chromatogr. A 2020, 1621, 14–16. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Zhang, Y.; Lan, C.; Miao, Y.; Deng, Z.; Ba, X.; Zhao, W.; Zhang, S. Tetra-Proline Modified Calix[4]Arene Bonded Silica Gel: A Novel Stationary Phase for Hydrophilic Interaction Liquid Chromatography. Talanta 2019, 193, 56–63. [Google Scholar] [CrossRef]
- Aral, H.; Çelik, K.S.; Altındağ, R.; Aral, T. Synthesis, Characterization, and Application of a Novel Multifunctional Stationary Phase for Hydrophilic Interaction/Reversed Phase Mixed-Mode Chromatography. Talanta 2017, 174, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, X.; Peng, J.; He, Y.; Chen, Y.; Chen, F.; Li, S. Preparation and Evaluation of Surface-Bonded Phenylglycine Zwitterionic Stationary Phase. Anal. Bioanal. Chem. 2018, 410, 5941–5950. [Google Scholar] [CrossRef]
- Shen, A.; Li, X.; Dong, X.; Wei, J.; Guo, Z.; Liang, X. Glutathione-Based Zwitterionic Stationary Phase for Hydrophilic Interaction/Cation-Exchange Mixed-Mode Chromatography. J. Chromatogr. A 2013, 1314, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Liu, Y.; Shen, A.; Xiao, Y.; Yu, L.; Liang, X. Preparation of Glutathione-Functionalized Zwitterionic Silica Material for Efficient Enrichment of Sialylated N-Glycopeptides. Anal. Bioanal. Chem. 2019, 411, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Skoczylas, M.; Bocian, S.; Buszewski, B. Dipeptide-Bonded Stationary Phases for Hydrophilic Interaction Liquid Chromatography. RSC Adv. 2016, 6, 96389–96397. [Google Scholar] [CrossRef]
- Buszewski, B.; Skoczylas, M. Multi-Parametric Characterization of Amino Acid- and Peptide-Silica Stationary Phases. Chromatographia 2019, 82, 153–166. [Google Scholar] [CrossRef]
- Takafuji, M.; Shahruzzaman, M.; Sasahara, K.; Ihara, H. Preparation and Characterization of a Novel Hydrophilic Interaction/Ion Exchange Mixed-Mode Chromatographic Stationary Phase with Pyridinium-Based Zwitterionic Polymer-Grafted Porous Silica. J. Sep. Sci. 2018, 41, 3957–3965. [Google Scholar] [CrossRef]
- Bo, C.; Wang, X.; Wang, C.; Wei, Y. Preparation of Hydrophilic Interaction/Ion-Exchange Mixed-Mode Chromatographic Stationary Phase with Adjustable Selectivity by Controlling Different Ratios of the Co-Monomers. J. Chromatogr. A 2017, 1487, 201–210. [Google Scholar] [CrossRef]
- Bocian, S.; Skoczylas, M.; Buszewski, B. Amino Acids, Peptides, and Proteins as Chemically Bonded Stationary Phases—A Review. J. Sep. Sci. 2016, 39, 83–92. [Google Scholar] [CrossRef]
- Ismail, O.H.; Ciogli, A.; Villani, C.; De Martino, M.; Pierini, M.; Cavazzini, A.; Bell, D.S.; Gasparrini, F. Ultra-Fast High-Efficiency Enantioseparations by Means of a Teicoplanin-Based Chiral Stationary Phase Made on Sub-2μm Totally Porous Silica Particles of Narrow Size Distribution. J. Chromatogr. A 2016, 1427, 55–68. [Google Scholar] [CrossRef]
- Ismail, O.H.; Antonelli, M.; Ciogli, A.; Villani, C.; Cavazzini, A.; Catani, M.; Felletti, S.; Bell, D.S.; Gasparrini, F. Future Perspectives in High Efficient and Ultrafast Chiral Liquid Chromatography through Zwitterionic Teicoplanin-Based 2-Μm Superficially Porous Particles. J. Chromatogr. A 2017, 1520, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.H.; Antonelli, M.; Ciogli, A.; De Martino, M.; Catani, M.; Villani, C.; Cavazzini, A.; Ye, M.; Bell, D.S.; Gasparrini, F. Direct Analysis of Chiral Active Pharmaceutical Ingredients and Their Counterions by Ultra High Performance Liquid Chromatography with Macrocyclic Glycopeptide-Based Chiral Stationary Phases. J. Chromatogr. A 2018, 1576, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccanti, G.; Manetto, S.; Ricci, A.; Cabri, W.; Orlandin, A.; Catani, M.; Felletti, S.; Cavazzini, A.; Ye, M.; Ritchie, H.; et al. High–Throughput Enantioseparation of Nα–Fluorenylmethoxycarbonyl Proteinogenic Amino Acids through Fast Chiral Chromatography on Zwitterionic-Teicoplanin Stationary Phases. J. Chromatogr. A 2020, 1624, 461235. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.H.; Catani, M.; Mazzoccanti, G.; Felletti, S.; Manetto, S.; De Luca, C.; Ye, M.; Cavazzini, A.; Gasparrini, F. Boosting the Enantioresolution of Zwitterionic-Teicoplanin Chiral Stationary Phases by Moving to Wide-Pore Core-Shell Particles. J. Chromatogr. A 2022, 1676, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Costi, R.; Di Santo, R.; La Torre, F.; Pierini, M.; Siani, G. Perturbing Effects of Chiral Stationary Phase on Enantiomerization Second-Order Rate Constants Determined by Enantioselective Dynamic High-Performance Liquid Chromatography: A Practical Tool to Quantify the Accessible Acid and Basic Catalytic Sites Bonded. Anal. Chem. 2009, 81, 3560–3570. [Google Scholar] [CrossRef] [PubMed]
- Kasa, S.K.M.; Venkatanarayana, M.; Chennuru, L.N.; Rao, B.C.S.; Vemparala, M.; Chaman, A.F.; Talluri, M.V.N.K. Chiral LC Method Development: Stereo-Selective Separation, Characterization, and Determination of Cabotegravir and Related RS, RR, and SS Isomeric Impurities on Coated Cellulose-Based Chiral Stationary Phase by HILIC-LC and LC-MS. J. Pharm. Biomed. Anal. 2023, 222, 115062. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Baeza, M.; Escuder-Gilabert, L.; Martín-Biosca, Y.; Sagrado, S.; Medina-Hernández, M.J. Comparative Study on Retention Behaviour and Enantioresolution of Basic and Neutral Structurally Unrelated Compounds with Cellulose-Based Chiral Stationary Phases in Reversed Phase Liquid Chromatography-Mass Spectrometry Conditions. J. Chromatogr. A 2022, 1673, 463073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).