An Evaluation of Immobilized Poly-(S)-N-(1-phenylethyl)acrylamide Chiral Stationary Phases
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
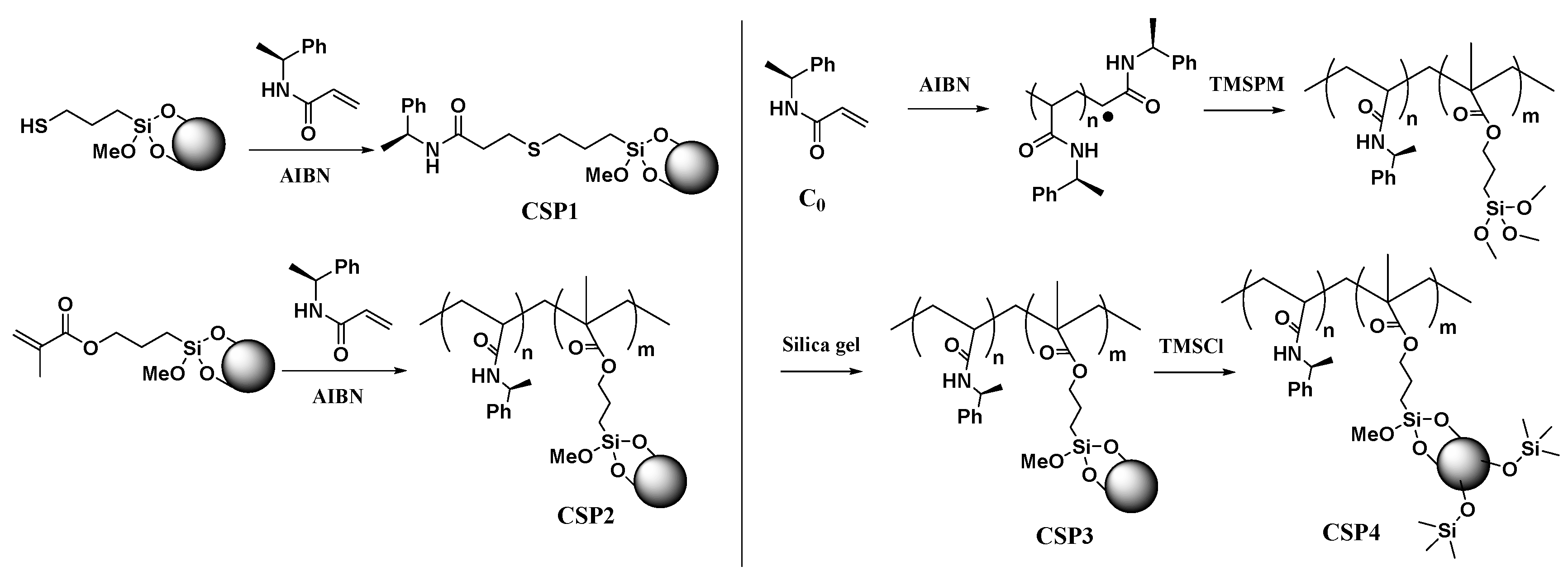
2.2. Preparation of Chiral Stationary Phases
2.3. Chromatographic Measurements
3. Results
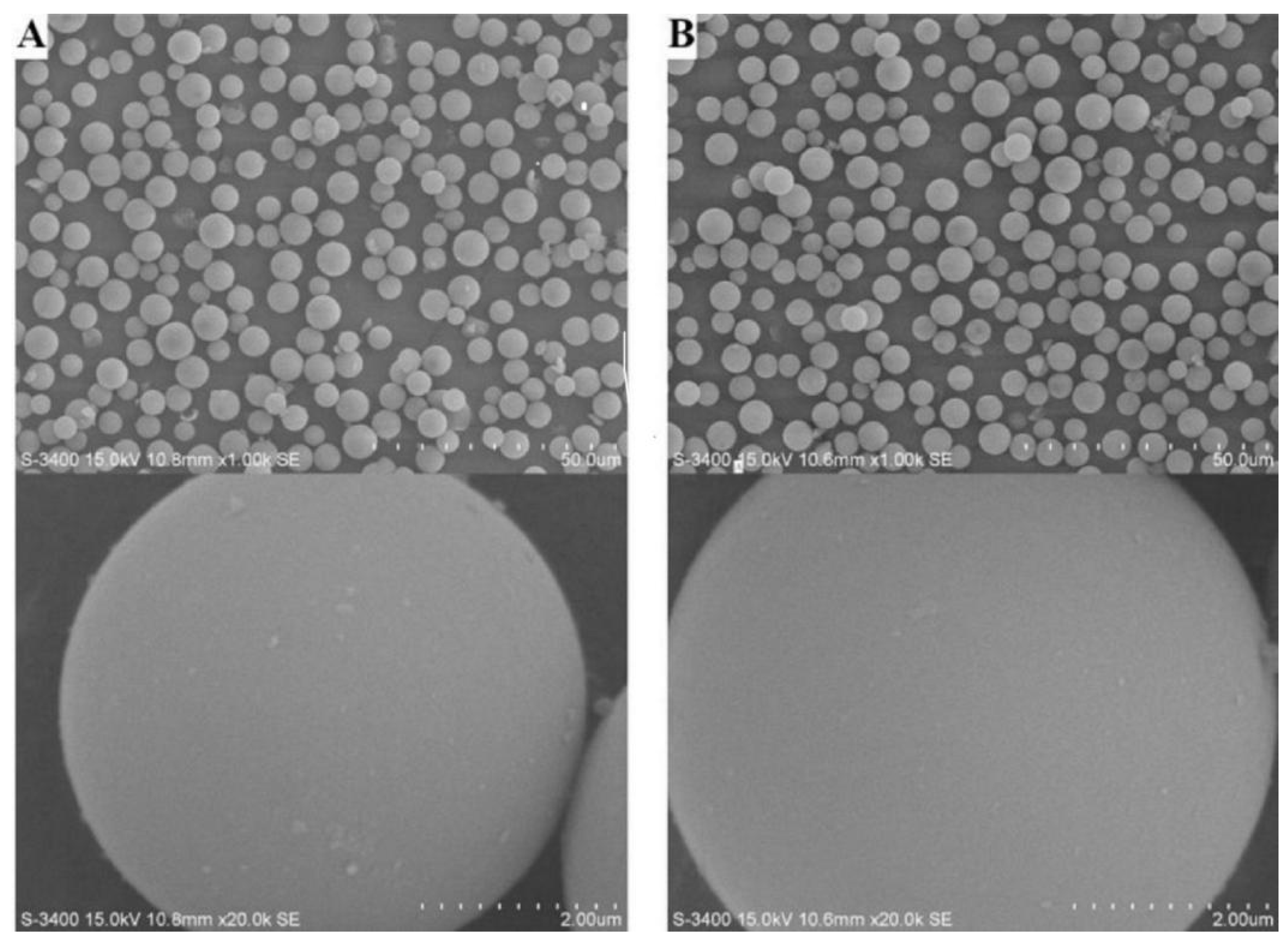
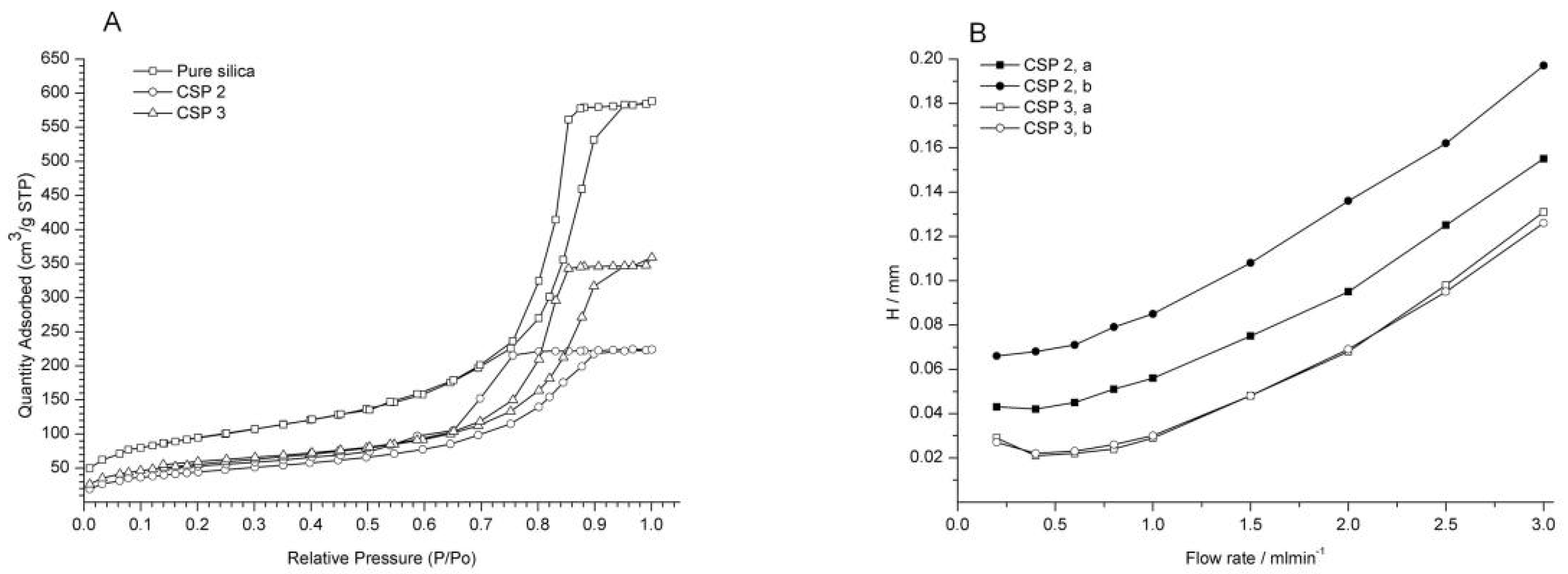
3.1. Preparation and Characterization of CSP1~CSP4
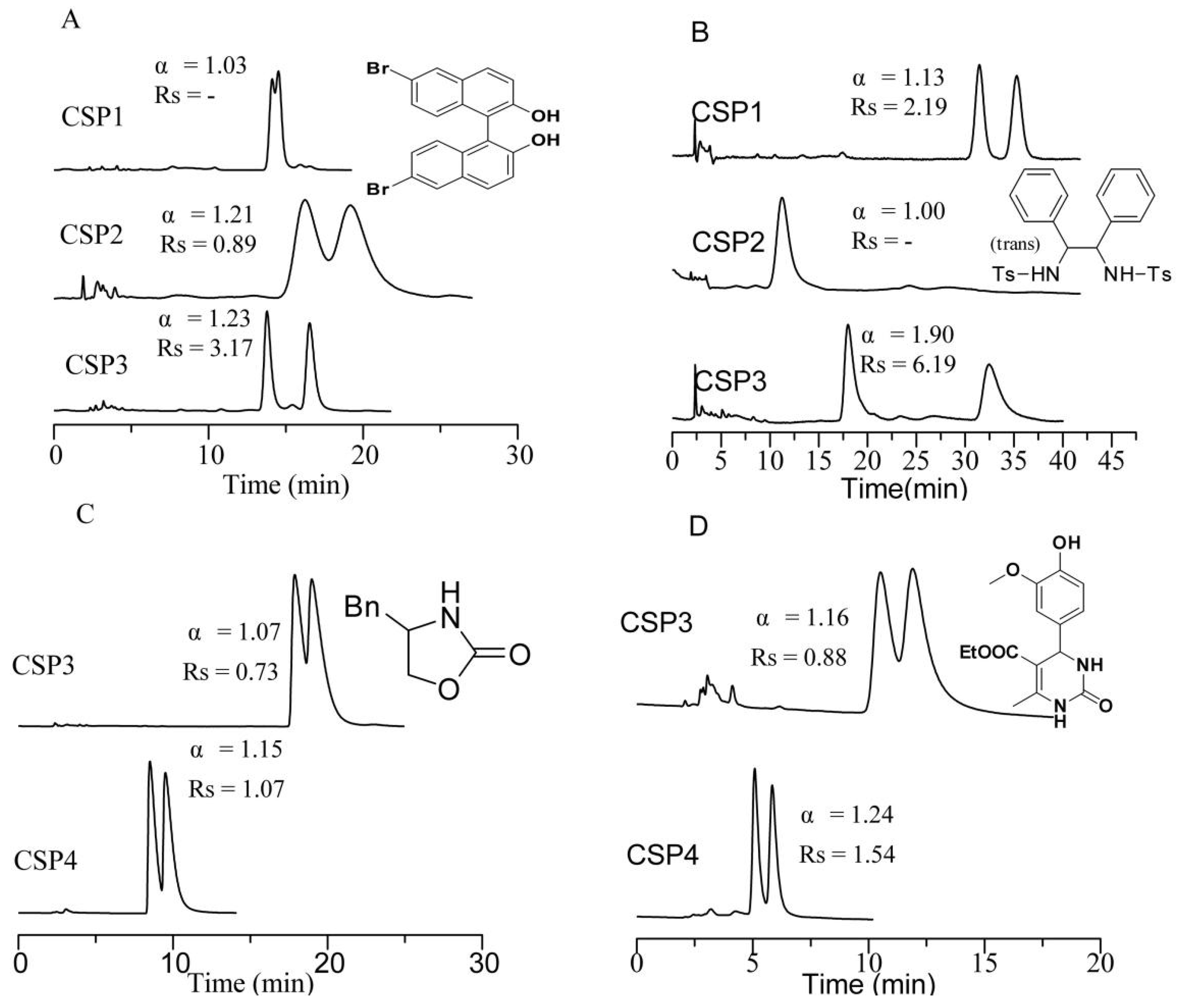
3.2. Chromatographic Separation Results
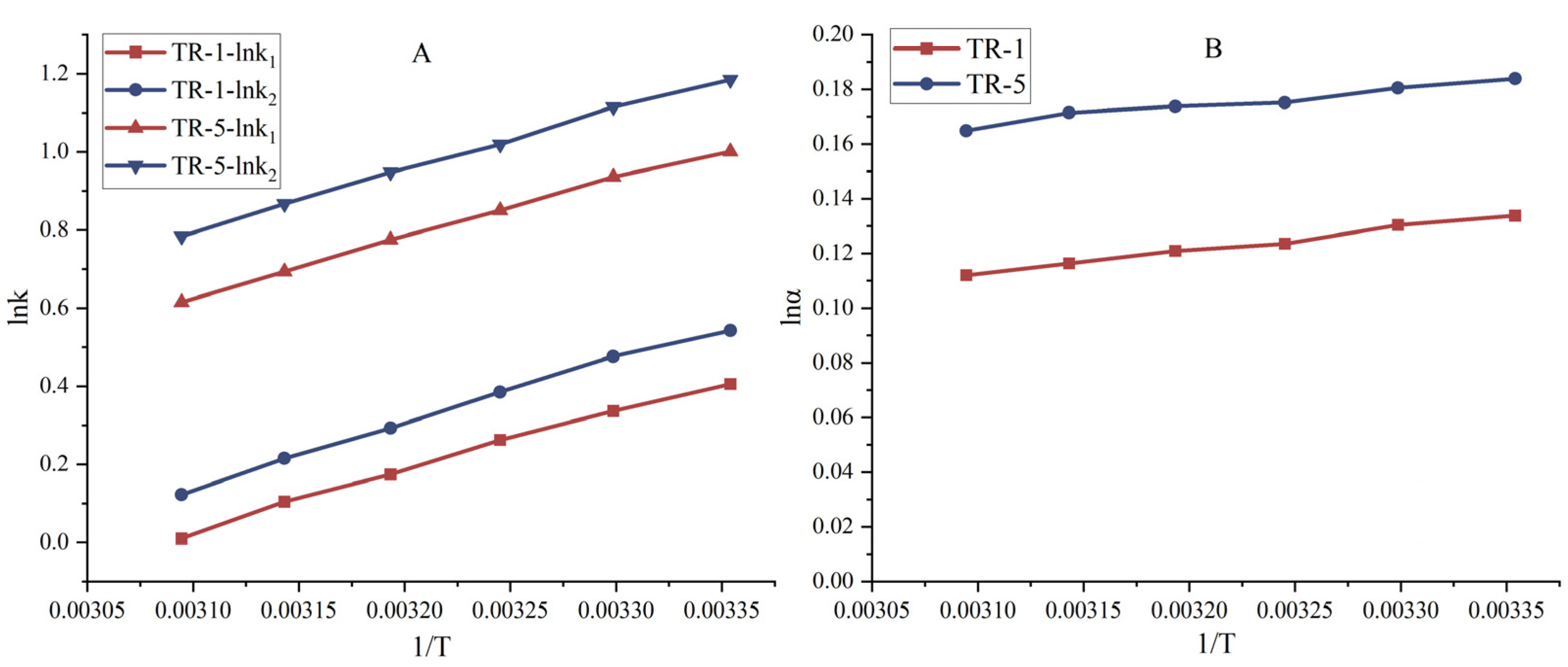
3.3. The Effect of Temperature on Separations
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bajtai, A.; Nemeti, G.; Le, T.M.; Szakonyi, Z.; Peter, A.; Ilisz, I. Enantiomeric separation of newly synthesized amino, thio, and oxy derivatives of monoterpene lactones, amides, and ester applying polysaccharide-based chiral stationary phases in normal-phase mode. J. Chromatogr. A 2022, 1672, 463050. [Google Scholar] [CrossRef] [PubMed]
- Geryk, R.; Kalikova, K.; Vozka, J.; Plecita, D.; Schmid, M.G.; Tesarova, E. Enantioselective potential of chiral stationary phases based on immobilized polysaccharides in reversed phase mode. J. Chromatogr. A 2014, 1363, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ikai, T.; Okamoto, Y. Synthesis and chiral recognition of novel amylose derivatives containing regioselectively benzoate and phenylcarbamate groups. J. Chromatogr. A 2010, 1217, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Zheng, X.; Azaria, S.; Beeram, S.; Li, Z.; Hage, D.S. Chromatographic Studies of Protein-Based Chiral Separations. Separations 2016, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Haginaka, J.; Yamashita, T.; Tsujino, H.; Arisawa, M. Revisiting Chiral Recognition Mechanism on Chicken Alpha 1-Acid Glycoprotein: Location of Chiral Binding Sites and Insight into Chiral Binding Mechanism. Separations 2021, 8, 73. [Google Scholar] [CrossRef]
- Xu, S.J.; Wang, Y.Y.; Tang, Y.X.; Ji, Y.B. A protein-based mixed selector chiral monolithic stationary phase in capillary electrochromatography. New J. Chem. 2018, 42, 13520–13528. [Google Scholar] [CrossRef]
- Okamoto, Y. Chiral Polymers for Resolution of Enantiomers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1731–1739. [Google Scholar] [CrossRef]
- Doi, Y.; Kiniwa, H.; Nishikaji, T. Chromatographic optical resolution of hydantoins by poly(N5-benzyl-L-glutamine) covalently bound to polystyrene resin. J. Chromatogr. A 1987, 396, 395–398. [Google Scholar] [CrossRef]
- Blaschke, G.; Donow, F. Trennwirkung optisch aktiven Poly[N-((S)-1-phenyläthyl)acrylamids] in Abhängigkeit vom Polymerisationsverfahren. Chem. Ber. 1975, 108, 1188–1197. [Google Scholar] [CrossRef]
- Okamoto, Y.; Honda, S.; Okamoto, I.; Yuki, H. Novel packing material for optical resolution: (+)-poly(triphenylmethyl methacrylate) coated on macroporous silica gel. J. Am. Chem. Soc. 1981, 103, 6971–6973. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kakimoto, M.; Imai, Y. Chiral Recognition Abilities of New Optically Active Polyurethanes Derived from Chiral 1,3-Diols and Diisocyanates. Polym. J. 1993, 25, 969–975. [Google Scholar] [CrossRef]
- Yashima, E.; Huang, S.; Okamoto, Y. An Optically Active Stereoregular Polyphenylacetylene Derivative as a Novel Chiral Stationary Phase for HPLC. J. Chem. Soc. Chem. Commun. 1994, 7, 1811–1812. [Google Scholar] [CrossRef]
- Blaschke, G.; Donow, F. Polymere 1-Phenyläthylamin-Derivate als optisch aktive Adsorbentien. Chem. Ber. 1975, 108, 2792–2798. [Google Scholar] [CrossRef]
- Yuki, H.; Okamoto, Y.; Okamoto, I. Resolution of racemic compounds by optically active poly(triphenylmethyl methacrylate). J. Am. Chem. Soc. 1980, 102, 6356–6358. [Google Scholar] [CrossRef]
- Cavazzini, A.; Pasti, L.; Massi, A.; Marchetti, N.; Dondi, F. Recent applications in chiral high performance liquid chromatography: A review. Anal. Chim. Acta 2011, 706, 205–222. [Google Scholar] [CrossRef]
- Yamamoto, C.; Okamoto, Y. Optically active polymers for chiral separation. Bull. Chem. Soc. Jpn. 2004, 77, 227–257. [Google Scholar] [CrossRef]
- Yang, J.H.; Choi, S.H. Comparison study of a chiral stationary phase based on cellulose derivatives prepared by "grafting from" and "grafting to" methods. J. Appl. Polym. Sci. 2013, 127, 4122–4128. [Google Scholar] [CrossRef]
- Gasparrini, F.; Misiti, D.; Rompietti, R.; Villani, C. New hybrid polymeric liquid chromatography chiral stationary phase prepared by surface-initiated polymerization. J. Chromatogr. A 2005, 1064, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lu, W.; Che, Y.; Shen, L.B.; Jiang, L.M.; Shen, Z.Q. Synthesis and Characterization of Macroporous Silica Modified with Optically Active Poly N-(oxazolinylphenyl)acrylamide Derivatives for Potential Application as Chiral Stationary Phases. J. Appl. Polym. Sci. 2010, 115, 999–1007. [Google Scholar] [CrossRef]
- Cai, J.F.; Cheng, L.P.; Zhao, J.C.; Fu, Q.; Jin, Y.; Ke, Y.X.; Liang, X.M. A polyacrylamide-based silica stationary phase for the separation of carbohydrates using alcohols as the weak eluent in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2017, 1524, 153–159. [Google Scholar] [CrossRef]
- Blaschke, G.; Broker, W.; Fraenkel, W. Enantiorneric Resolution by HPLC on Silica-Gel-Bound, Optically Active Polyarnides. Angew. Chem. Int. Ed. Engl. 1986, 25, 830–831. [Google Scholar] [CrossRef]
- Katz, S.M. Permanent Hysteresis in Physical Adsorption. A Theoretical Discussion. J. Phys. Chem. 1949, 53, 1166–1186. [Google Scholar] [CrossRef]
- McBain, J.W. An Explanation of Hysteresis in the Hydration and Dehydration of Gels. J. Am. Chem. Soc. 1935, 57, 699–700. [Google Scholar] [CrossRef]
- Liu, Y.; Berthod, A.; Mitchell, C.R.; Xiao, T.L.; Zhang, B.; Armstrong, D.W. Super/subcritical fluid chromatography chiral separations with macrocyclic glycopeptide stationary phases. J. Chromatogr. A 2002, 978, 185–204. [Google Scholar] [CrossRef] [PubMed]
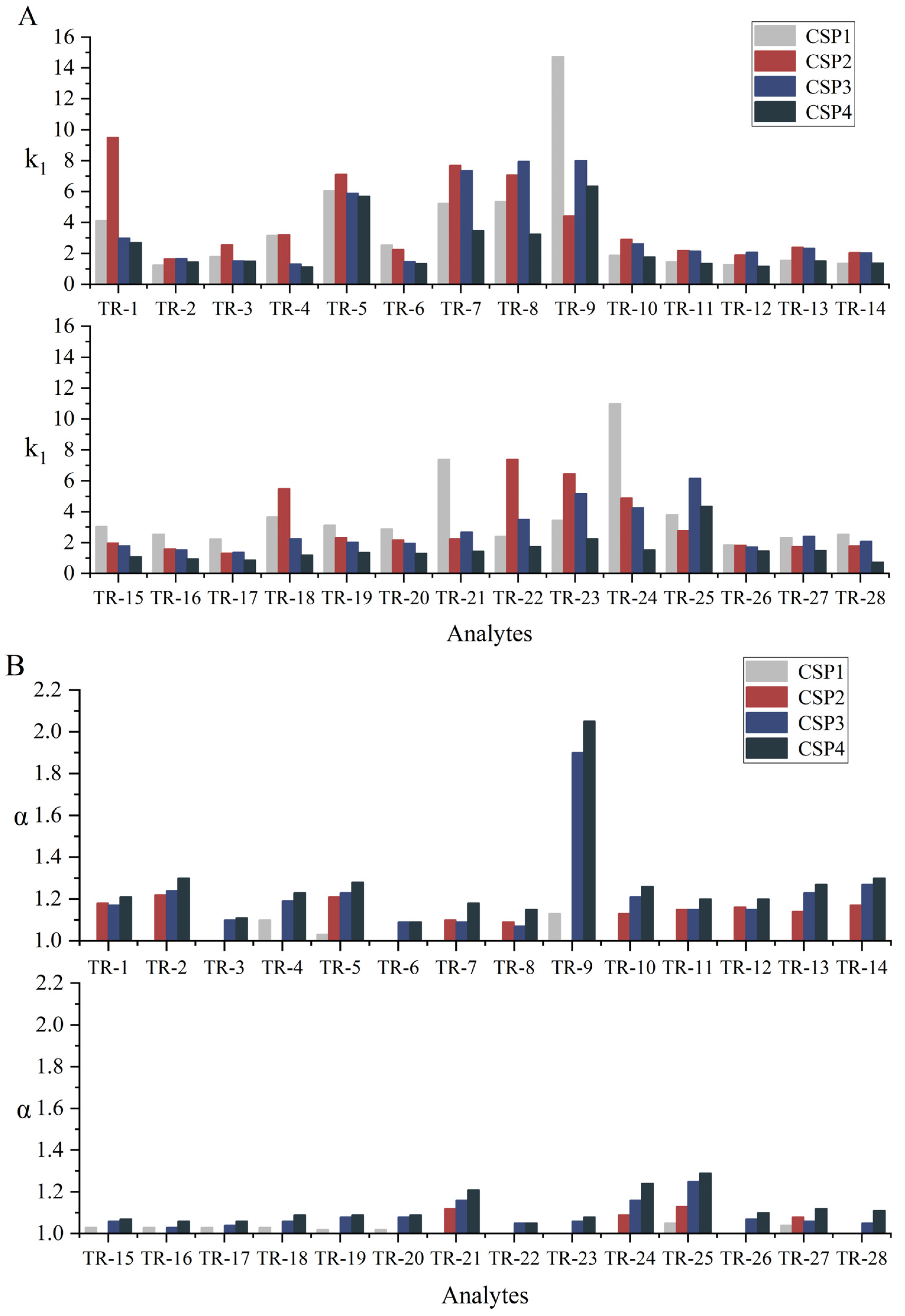
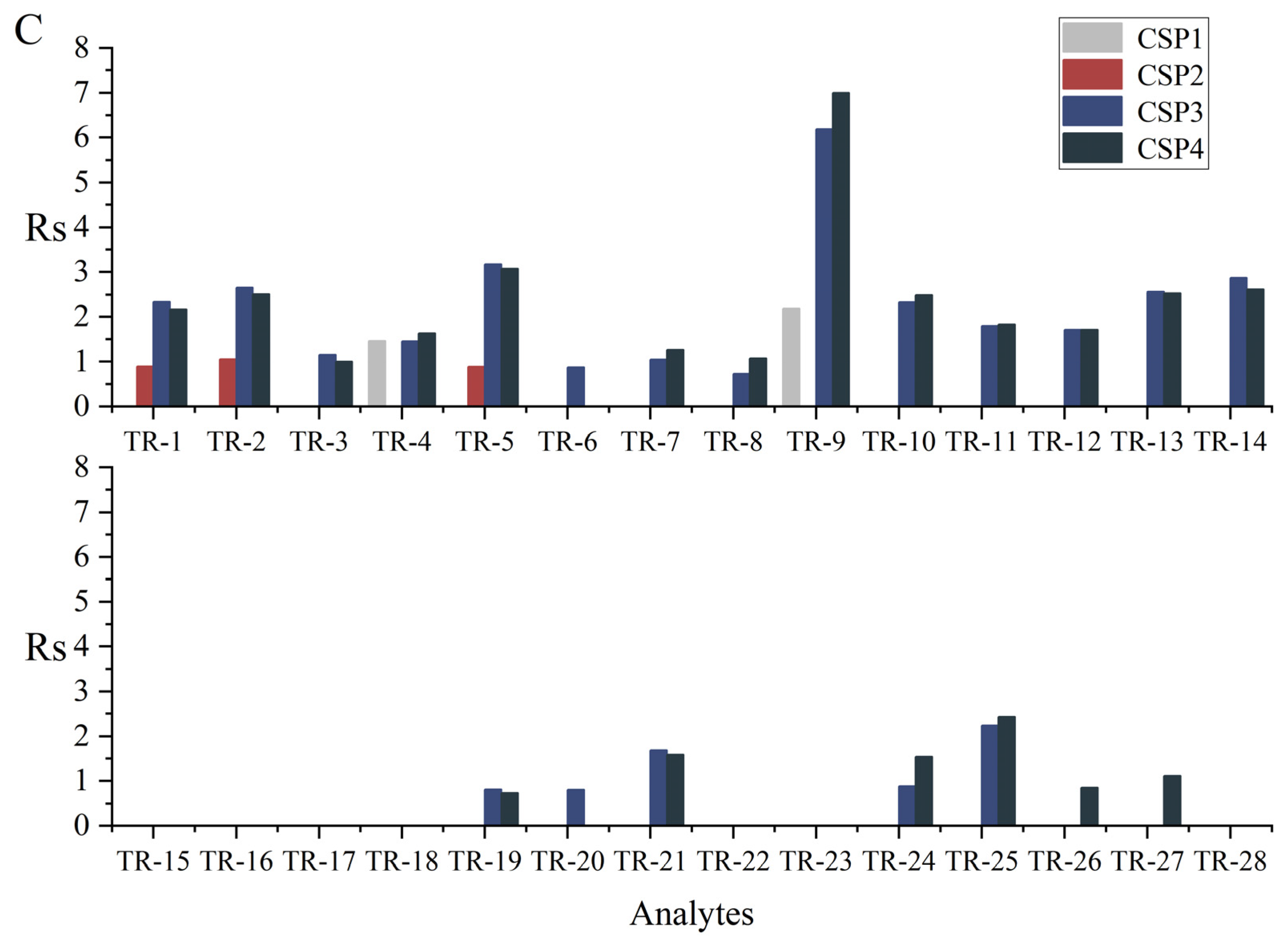
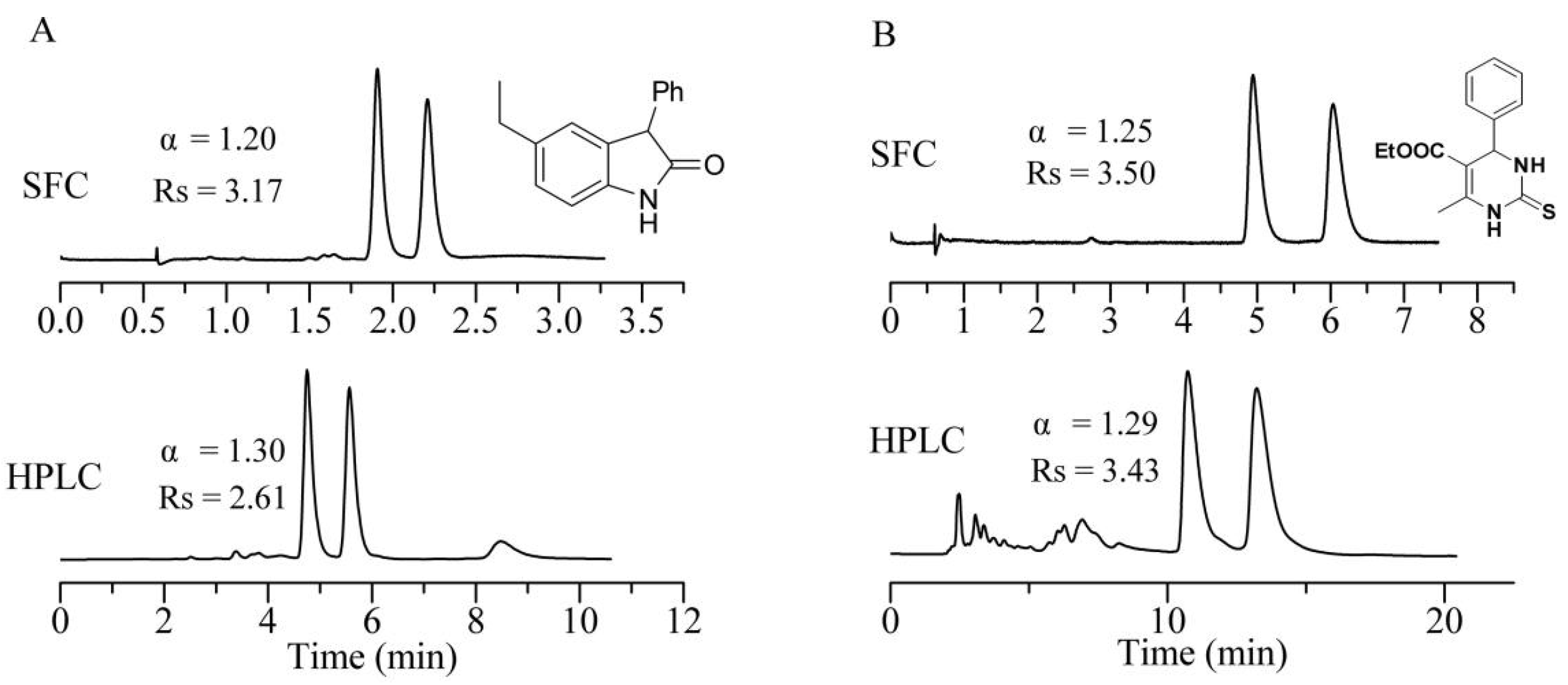
| Analyte | CSP1 11 | CSP2 | CSP3 | CSP4 | Analyte | CSP1 11 | CSP2 | CSP3 | CSP4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPLC | HPLC | HPLC | HPLC | SFC | HPLC | HPLC | HPLC | HPLC | SFC | ||||
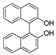 | k1 | 4.12 | 9.51 | 2.99 | 2.70 | 8.05 | 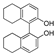 | k1 | 1.25 | 1.66 | 1.67 | 1.45 | 3.21 |
| α | - | 1.18 | 1.17 | 1.21 | 1.18 | α | - | 1.22 | 1.24 | 1.30 | 1.18 | ||
| Rs | - | 0.90 | 2.34 | 2.17 | 2.79 | Rs | - | 1.05 | 2.65 | 2.51 | 2.50 | ||
| TR-1 | Mp | B | B | B | B | J | TR-2 | Mp | B | B | A | A | J |
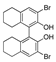 | k1 | 1.81 | 2.55 | 1.52 | 1.51 | 7.08 | 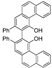 | k1 | 3.17 | 3.21 | 1.32 | 1.13 | 11.05 |
| α | - | - | 1.10 | 1.11 | 1.08 | α | 1.10 | - | 1.19 | 1.23 | 1.13 | ||
| Rs | - | - | 1.15 | 1.00 | 1.35 | Rs | 1.46 | - | 1.45 | 1.63 | 2.06 | ||
| TR-3 | Mp | B | B | A | A | J | TR-4 | Mp | B | C | A | A | K |
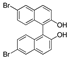 | k1 | 6.06 | 7.12 | 5.89 | 5.70 | 16.70 | 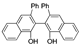 | k1 | 2.53 | 2.25 | 1.48 | 1.34 | 7.85 |
| α | 1.03 | 1.21 | 1.23 | 1.28 | 1.22 | α | - | - | 1.09 | 1.09 | 1.08 | ||
| Rs | - | 0.89 | 3.17 | 3.07 | 3.42 | Rs | - | - | 0.88 | - | 1.41 | ||
| TR-5 | Mp | B | C | B | B | K | TR-6 | Mp | B | C | A | A | J |
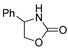 | k1 | 5.25 | 7.71 | 7.36 | 3.46 | 2.44 | 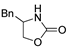 | k1 | 5.35 | 7.08 | 7.94 | 3.25 | 2.52 |
| α | - | 1.10 | 1.09 | 1.18 | 1.14 | α | - | 1.09 | 1.07 | 1.15 | 1.11 | ||
| Rs | - | - | 1.04 | 1.26 | 1.64 | Rs | - | - | 0.73 | 1.07 | 1.30 | ||
| TR-7 | Mp | B | C | B | B | J | TR-8 | Mp | B | C | B | B | J |
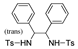 | k1 | 14.73 | 4.44 | 8.00 | 6.36 | 14.13 | 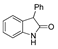 | k1 | 1.89 | 2.91 | 2.61 | 1.78 | 2.22 |
| α | 1.13 | - | 1.90 | 2.05 | 1.58 | α | - | 1.13 | 1.21 | 1.26 | 1.20 | ||
| Rs | 2.19 | - | 6.19 | 7.00 | 7.39 | Rs | - | - | 2.33 | 2.49 | 3.37 | ||
| TR-9 | Mp | B | C | B | B | J | TR-10 | Mp | B | C | B | B | K |
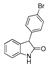 | k1 | 1.47 | 2.19 | 2.14 | 1.36 | 4.13 | 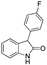 | k1 | 1.28 | 1.90 | 2.06 | 1.18 | 2.34 |
| α | - | 1.15 | 1.15 | 1.20 | 1.16 | α | - | 1.16 | 1.15 | 1.20 | 1.18 | ||
| Rs | - | - | 1.80 | 1.83 | 2.53 | Rs | - | - | 1.71 | 1.71 | 2.36 | ||
| TR-11 | Mp | B | C | B | B | J | TR12 | Mp | B | C | B | B | J |
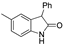 | k1 | 1.57 | 2.40 | 2.33 | 1.52 | 4.06 | 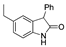 | k1 | 1.37 | 2.04 | 2.03 | 1.38 | 2.18 |
| α | - | 1.14 | 1.23 | 1.27 | 1.19 | α | - | 1.17 | 1.27 | 1.30 | 1.23 | ||
| Rs | - | - | 2.56 | 2.53 | 2.77 | Rs | - | - | 2.87 | 2.61 | 2.76 | ||
| TR-13 | Mp | B | C | B | B | J | TR-14 | Mp | B | C | B | B | K |
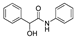 | k1 | 3.05 | 1.99 | 1.80 | 1.09 | 3.10 | 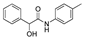 | k1 | 2.56 | 1.60 | 1.54 | 0.95 | 3.12 |
| α | 1.03 | - | 1.06 | 1.07 | 1.07 | α | 1.03 | - | 1.03 | 1.06 | 1.07 | ||
| Rs | - | - | - | - | 0.83 | Rs | - | - | - | - | 0.82 | ||
| TR-15 | Mp | B | C | B | B | J | TR-16 | Mp | B | C | B | B | J |
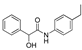 | k1 | 2.25 | 1.33 | 1.38 | 0.87 | 3.22 | 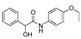 | k1 | 3.66 | 5.50 | 2.26 | 1.20 | 3.85 |
| α | 1.03 | - | 1.04 | 1.06 | 1.07 | α | 1.03 | - | 1.06 | 1.09 | 1.08 | ||
| Rs | - | - | - | - | 0.88 | Rs | - | - | - | - | 1.08 | ||
| TR-17 | Mp | B | C | B | B | J | TR-18 | Mp | B | B | B | B | J |
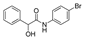 | k1 | 3.13 | 2.33 | 2.03 | 1.36 | 5.10 | 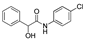 | k1 | 2.89 | 2.18 | 1.98 | 1.31 | 4.24 |
| α | 1.02 | - | 1.08 | 1.09 | 1.10 | α | 1.02 | - | 1.08 | 1.09 | 1.09 | ||
| Rs | - | - | 0.81 | 0.73 | 1.47 | Rs | - | - | 0.80 | - | 1.42 | ||
| TR-19 | Mp | B | C | B | B | J | TR-20 | Mp | B | C | B | B | J |
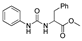 | k1 | 7.39 | 2.27 | 2.68 | 1.44 | 3.13 | 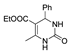 | k1 | 2.42 | 7.39 | 3.50 | 1.75 | 4.03 |
| α | - | 1.12 | 1.16 | 1.21 | 1.16 | α | - | - | 1.05 | 1.05 | 1.04 | ||
| Rs | - | - | 1.69 | 1.59 | 1.54 | Rs | - | - | - | - | - | ||
| TR-21 | Mp | B | C | B | B | J | TR-22 | Mp | B | C | D | D | K |
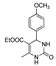 | k1 | 3.46 | 6.46 | 5.17 | 2.27 | 4.77 | 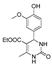 | k1 | 11.00 | 4.89 | 4.26 | 1.54 | 23.33 |
| α | - | - | 1.06 | 1.08 | 1.04 | α | - | 1.09 | 1.16 | 1.24 | 1.20 | ||
| Rs | - | - | - | - | - | Rs | - | - | 0.88 | 1.54 | 2.87 | ||
| TR-23 | Mp | B | E | D | D | K | TR-24 | Mp | B | F | G | G | J |
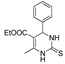 | k1 | 3.81 | 2.78 | 6.15 | 4.36 | 7.24 | 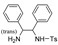 | k1 | 1.86 | 1.83 | 1.72 | 1.46 | |
| α | 1.05 | 1.13 | 1.25 | 1.29 | 1.25 | α | - | - | 1.07 | 1.10 | |||
| Rs | - | - | 2.24 | 2.43 | 3.50 | Rs | - | - | - | 0.85 | |||
| TR-25 | Mp | B | F | B | B | J | TR-26 | Mp | I | I | I | H | |
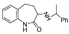 | k1 | 2.33 | 1.75 | 2.42 | 1.50 | 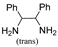 | k1 | 2.55 | 1.80 | 2.09 | 0.73 | ||
| α | 1.04 | 1.08 | 1.06 | 1.12 | α | - | - | 1.05 | 1.11 | ||||
| Rs | - | - | - | 1.11 | Rs | - | - | - | - | ||||
| TR-27 | Mp | I | I | I | H | TR-28 | Mp | H | H | H | H | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, G.; Miao, Y.; Zhao, J.; Chen, X.; Ke, Y. An Evaluation of Immobilized Poly-(S)-N-(1-phenylethyl)acrylamide Chiral Stationary Phases. Separations 2023, 10, 11. https://doi.org/10.3390/separations10010011
Lu G, Miao Y, Zhao J, Chen X, Ke Y. An Evaluation of Immobilized Poly-(S)-N-(1-phenylethyl)acrylamide Chiral Stationary Phases. Separations. 2023; 10(1):11. https://doi.org/10.3390/separations10010011
Chicago/Turabian StyleLu, Guangying, Yiyuan Miao, Jianchao Zhao, Xin Chen, and Yanxiong Ke. 2023. "An Evaluation of Immobilized Poly-(S)-N-(1-phenylethyl)acrylamide Chiral Stationary Phases" Separations 10, no. 1: 11. https://doi.org/10.3390/separations10010011
APA StyleLu, G., Miao, Y., Zhao, J., Chen, X., & Ke, Y. (2023). An Evaluation of Immobilized Poly-(S)-N-(1-phenylethyl)acrylamide Chiral Stationary Phases. Separations, 10(1), 11. https://doi.org/10.3390/separations10010011





