Clear Cell Acanthoma with Malignant Cytologic Features: A Case Report and Review of the Literature
Abstract
1. Introduction
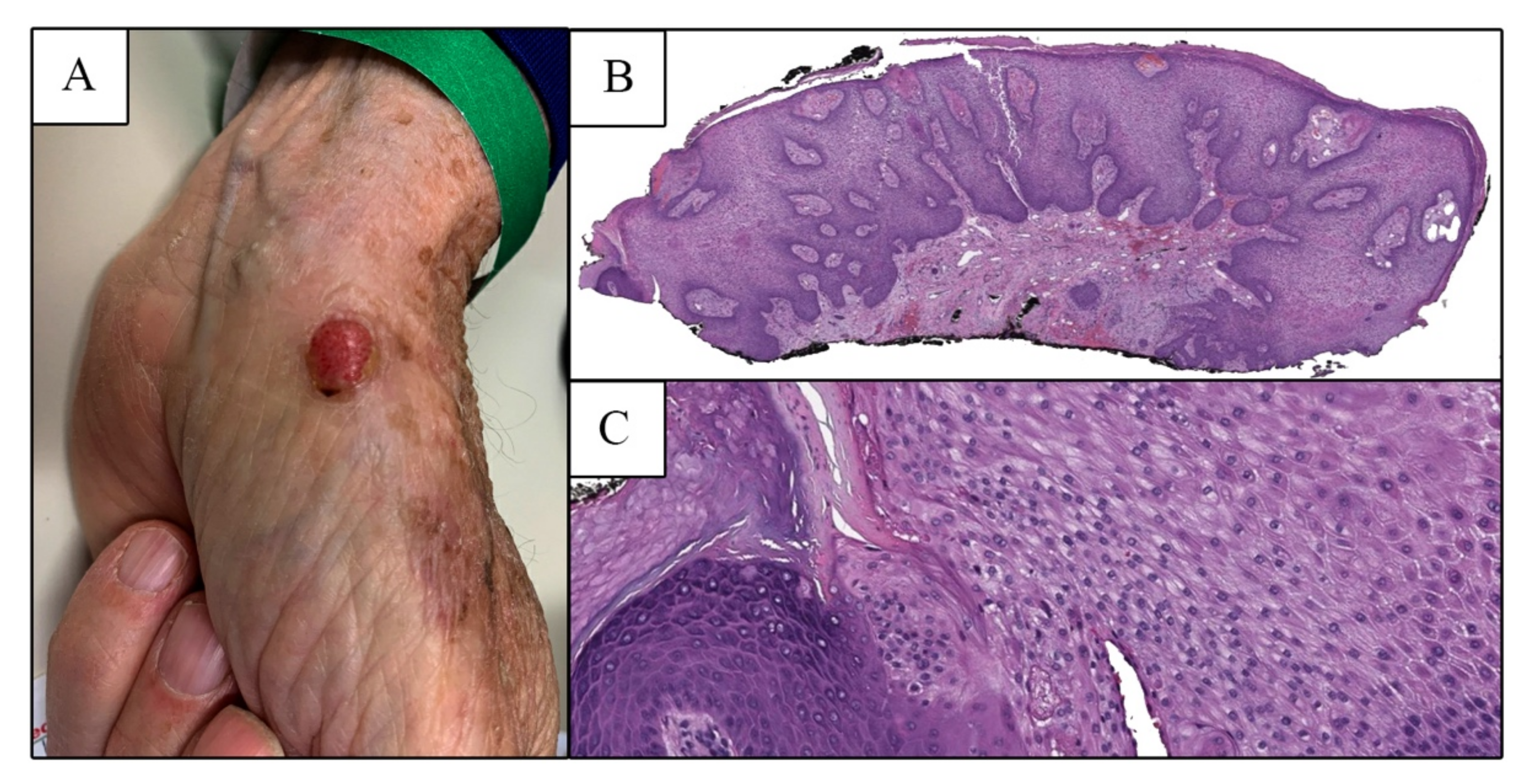
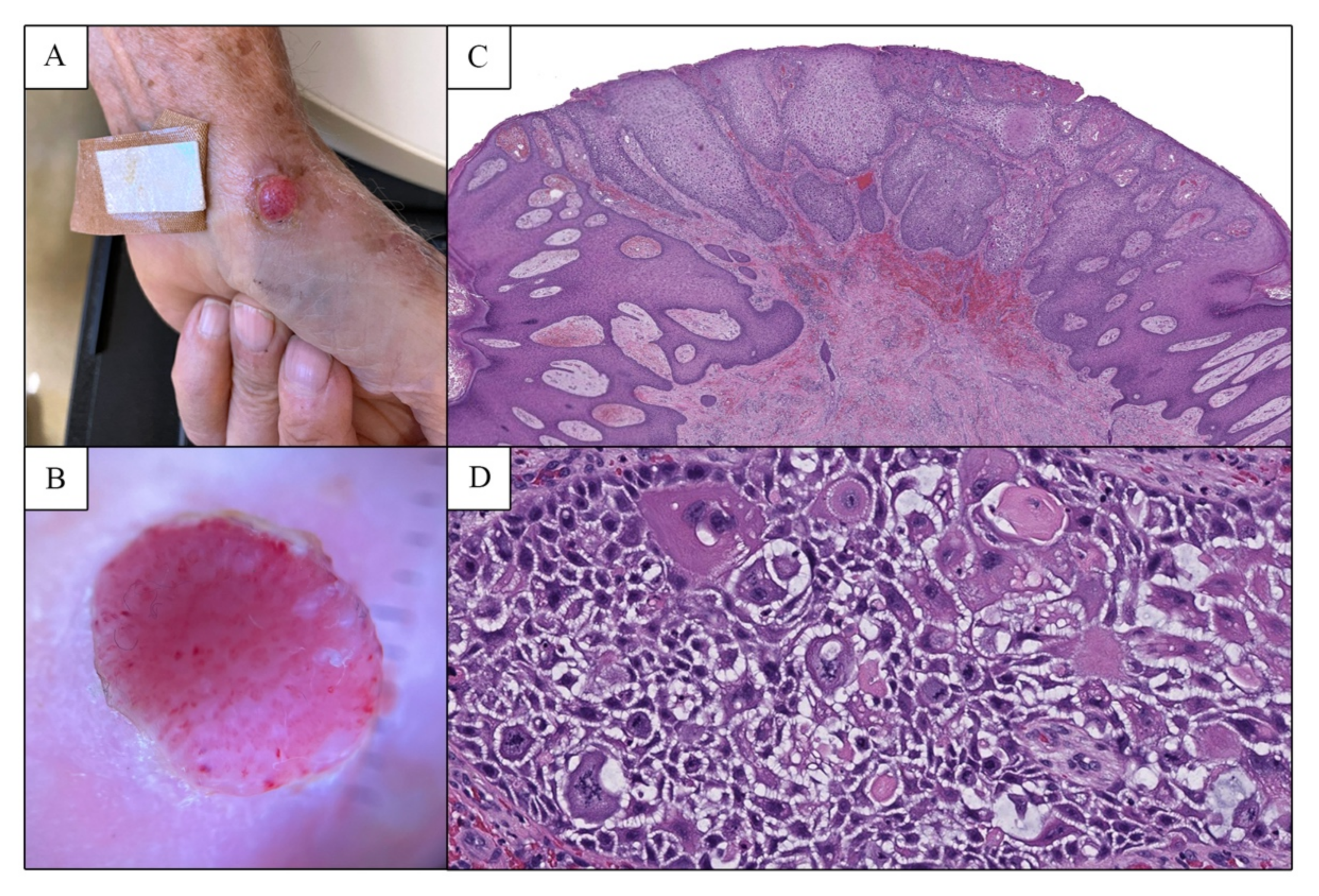
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Degos, R.; Delort, J.; Civatte, J.; Poiares Baptista, A. Epidermal tumor with an unusual appearance: Clear cell acanthoma. Ann. Dermatol. Syphiligr. 1962, 89, 361–371. [Google Scholar]
- Degos, R.; Civatte, J. Clear-cell acanthoma: Experience of 8 years. Br. J. Dermatol. 1970, 83, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Hayakawa, K.; Watanabe, Y.; Nishikawa, T. Lectin-binding sites in clear cell acanthoma. J. Cutan. Pathol. 1990, 17, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.G.H.; Neumann, E. Some evidence concerning the sweat duct origin of clear cell acanthoma. Acta Dermatol. Venereol. 1973, 53, 511–514. [Google Scholar]
- Ohnishi, T.; Watanabe, S. Immunohistochemical characterization of keratin expression in clear cell acanthoma. Br. J. Dermatol. 1995, 133, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Zedek, D.; Langel, D.; White, W. Clear-cell acanthoma versus acanthosis: A psoriasiform reaction pattern lacking tricholemmal differentiation. Am. J. Dermatopathol. 2007, 29, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Duperrat, B.; Vanbremeersch, F.; David, V.; Mascaro, J.M.; Bedard, A. Giant form of the acanthoma of Degos. Bull. Soc. Fr. Dermatol. Syphiligr. 1966, 73, 884–886. [Google Scholar] [PubMed]
- Petzelbauer, P.; Konrad, K. Polypous clear cell acanthoma. Am. J. Dermatopathol. 1990, 12, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Langer, K.; Wuketich, S.; Konrad, K. Pigmented clear cell acanthoma. Am. J. Dermatopathol. 1994, 16, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, M.H.; Rothem, A.; Halevy, S. Atypical clear cell acanthoma. Int. J. Dermatol. 1991, 30, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lee, L.Y.; Kuo, T.T. Malignant clear cell acanthoma: Report of a rare case of clear cell acanthoma-like tumor with malignant features. Am. J. Dermatopathol. 2016, 38, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Arida, M.; English, C.; Mully, T. Giant clear cell acanthoma with keratoacanthoma-like changes: A case report. Dermatol. Online J. 2006, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.E.; Ratz, J.L. Squamous cell carcinoma in situ arising within clear cell acanthoma. Dermatol. Surg. 1997, 23, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Shirai, A.; Saeki, H.; Matsuzaki, H.; Ito, K.; Nakagawa, H. Multiple clear cell acanthoma associated with multiple Bowen’s disease. Int. J. Dermatol. 2014, 53, e386–e388. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, M.H.; Fernando, S.; Shapiro, L. Clear cell acanthoma: Clinicopathologic analysis of 37 new cases. Am. J. Clin. Pathol. 1973, 59, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T. Clear cell carcinoma of the skin A variant of the squamous cell carcinoma that simulates sebaceous carcinoma. Am. J. Surg. Pathol. 1980, 4, 573–584. [Google Scholar] [CrossRef]
- Rinker, M.H.; Fenske, N.A.; Scalf, L.A.; Glass, L.F. Histologic Variants of Squamous Cell Carcinoma of the Skin. Cancer Control 2001, 8, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.O.; Adisa, A.O.; Olajide, M.A.; Olusanya, A.A. Clear cell variant of squamous cell carcinoma of skin: A report of a case. J. Oral Maxillofac. Pathol. 2013, 17, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Al-Arashi, M.Y.H.; Byers, H.R. Cutaneous clear cell squamous cell carcinoma in situ: Clinical, histological and immunohistochemical characterization. J. Cutan. Pathol. 2007, 34, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.R.; Patterson, J.W. Selected Pseudoneoplastic Lesions of the Skin. Arch. Pathol. Lab. Med. 2010, 134, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.; Zidar, N.; Cardesa, A.; Nadal, A. Benign and Potentially Malignant Lesions of the Squamous Epithelium and Squamous Cell Carcinoma. In Pathology of the Head and Neck, 2nd ed.; Cardesa, A., Slootweg, P.J., Gale, N., Frachi, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–48. ISBN 978-3-662-49672-5. [Google Scholar]
| Case | Clinical Information | Atypical Histopathologic Features | Interpretation | Follow-Up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Age/Sex | Location | Size * | Description | Duration | Initial Biopsy of CCA | Pleomorphism | Increased NC Ratio | Prominent Nucleoli | Atypical Mitotic Figures | |||
| Grunwald et al., 1991 [10] | 74/M | Forehead | 1 cm | Dome-shaped, scaly | 2 years | N/A | + | + | + | + | Atypical CCA | No recurrence or metastases at 4–5 years † |
| 71/F | Forehead | 1.5 cm | Slightly elevated, pink | 3 years | N/A | + | − | + | + | Atypical CCA | ||
| Parsons and Ratz, 1997 [13] | 60/F | Right leg | 2.6 cm | Fungating erythematous tumor | 2 years | + | + | NR | NR | NR | SCCIS arising within CCA | Mohs surgery, NED at 6 months |
| Arida et al., 2006 [12] | 45/M | Left groin | 3 cm | Reddish polypoid lesion | Many years | N/A | − ‡ | − | − § | NR | Malignant degeneration within CCA | Excision †, follow-up NR |
| Lin et al., 2016 [11] | 92/F | Temple | 1.5 cm | Erythematous moist nodule | 3 months | N/A | + | + | − | NR ¶ | Malignant CCA | Biopsy and cryotherapy, NED at 6 months |
| Current case | 78/M | Left palm | 1 cm | Erythematous friable papule | weeks | + | + | + | + | + | Atypical CCA | Complete excision, NED at 9 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melson, G.; Saliba, E.; Patel, S.; Eisen, R.; Brem, C.E. Clear Cell Acanthoma with Malignant Cytologic Features: A Case Report and Review of the Literature. Dermatopathology 2022, 9, 355-360. https://doi.org/10.3390/dermatopathology9040041
Melson G, Saliba E, Patel S, Eisen R, Brem CE. Clear Cell Acanthoma with Malignant Cytologic Features: A Case Report and Review of the Literature. Dermatopathology. 2022; 9(4):355-360. https://doi.org/10.3390/dermatopathology9040041
Chicago/Turabian StyleMelson, Gabriella, Elie Saliba, Shreya Patel, Richard Eisen, and Candice E. Brem. 2022. "Clear Cell Acanthoma with Malignant Cytologic Features: A Case Report and Review of the Literature" Dermatopathology 9, no. 4: 355-360. https://doi.org/10.3390/dermatopathology9040041
APA StyleMelson, G., Saliba, E., Patel, S., Eisen, R., & Brem, C. E. (2022). Clear Cell Acanthoma with Malignant Cytologic Features: A Case Report and Review of the Literature. Dermatopathology, 9(4), 355-360. https://doi.org/10.3390/dermatopathology9040041






