Drug-Induced vs. Viral Maculopapular Exanthem—Resolving the Dilemma
Abstract
:1. Introduction
2. Clinical Features
3. Hematological and Biochemical Investigations
4. Histopathology
4.1. Spongiosis
4.2. Viral Cytopathic Changes
4.3. Necrotic Keratinocytes and Basal Cell Damage
4.4. Chronic Dermal Inflammatory Infiltrate
4.5. Eosinophilic Dermal Infiltrate
4.6. Lymphocytic Vasculitis
5. Immunohistochemical Techniques
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hunziker, T.; Künzi, U.P.; Braunschweig, S.; Zehnder, D.; Hoigné, R. Comprehensive hospital drug monitoring (CHDM): Adverse skin reactions, a 20-year survey. Allergy 1997, 52, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, G.; Franceschini, F.; Caimmi, S.; Bottau, P.; Liotti, L.; Saretta, F.; Bernardini, R.; Cardinale, F.; Mori, F.; Caffarelli, C. Mild cutaneous reactions to drugs. Acta Biomed. 2019, 90, 36–43. [Google Scholar] [PubMed]
- Doshi, B.R.; Manjunathswamy, B.S. Maculopapular drug eruption versus maculopapular viral exanthem. Indian J. Drugs Dermatol. 2017, 3, 45–47. [Google Scholar] [CrossRef]
- Singh, S.; Khandpur, S.; Arava, S.; Rath, R.; Ramam, M.; Singh, M.; Sharma, V.K.; Kabra, S.K. Assessment of histopathological features of maculopapular viral exanthem and drug-induced exanthem. J. Cutan. Pathol. 2017, 44, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Coskey, R.J.; Paul, L. Ampicillin Sensitivity in Infectious Mononucleosis. Arch. Dermatol. 1969, 100, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Yawalkar, N.; Shrikhande, M.; Hari, Y.; Nievergelt, H.; Braathen, L.R.; Pichler, W.J. Evidence for a role for IL5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J. Allergy Clin. Immunol. 2000, 106, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, X.; Li, M. Peripheral Blood Eosinophil counts predict the prognosis of drug eruptions. J. Investig. Allergol. Clin. Immunol. 2013, 23, 248–255. [Google Scholar] [PubMed]
- Tabak, F.; Murtezaoglu, A.; Tabak, O.; Ozaras, R.; Mete, B.; Kutlubay, Z.; Mert, A.; Öztürk, R. Clinical features and etiology of adult patients with Fever and rash. Ann. Dermatol. 2012, 24, 420–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiatt, K.; Horn, T. Cutaneous Toxicities of Drugs. In Histopathology of the Skin, 10th ed.; Elder, D., Ed.; J. B. Lippincott: Philadelphia, PA, USA, 2009; p. 312. [Google Scholar]
- Brinster, N. Cutaneous adverse reactions to drugs. In McKee’s Pathology of the Skin, 4th ed.; Mckee, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; p. 593. [Google Scholar]
- Ackerman, A.B.; Chongchitnant, N.; Sanchez, J.; Guo, Y.; Bennin, B.; Reichel, M.; Randall, M.B. Histologic Diagnosis of Inflammatory Skin Diseases, 2nd ed.; Williams & Wilkins: Baltimore, ML, USA, 1997; p. 317. [Google Scholar]
- Seitz, C.S.; Rose, C.; Kerstan, A.; Trautmann, A. Drug-induced exanthems: Correlation of allergy testing with histologic diagnosis. J. Am. Acad. Dermatol. 2013, 69, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Naim, M.; Weyers, W.; Metze, D. Histopathologic features of exanthemtous drug eruptions of the macular and papular type. Am. J. Dermatopathol. 2011, 33, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, N.; Valeyrie-Allanore, L.; Bastuji-Garin, S.; Wechsler, J.; De Feraudy, S.; Duong, T.-A.; Delfau-Larue, M.-H.; Chosidow, O.; Wolkenstein, P.; Roujeau, J.-C. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: A morphological and phenotypical study. Br. J. Dermatol. 2015, 173, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Liersch, J.; Omaj, R.; Schaller, J. Histopathological and Immunohistochemical Characteristics of Measles Exanthema: A Study of a Series of 13 Adult Cases and Review of the Literature. Am. J. Dermatopathol. 2019, 41, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Weyers, W.; Metze, D. Histopathology of drug eruptions-general criteria, common patterns, and differential diagnosis. Dermatol. Pract. Concept. 2011, 1, 33–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianotti, R.; Recalcati, S.; Fantini, F.; Riva, C.; Milani, M.; Dainese, E.; Boggio, F. Histopathological study of a broad spectrum of skin dermatoses in patients affected or highly suspected of infection by COVID-19 in the northern part of italy: Analysis of the many faces of the viral-induced skin diseases in previous and new reported cases. Am. J. Dermatopathol. 2020, 42, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Stur, K.; Karlhofer, F.M.; Stingl, G. Soluble fas ligand a discriminating feature between drug Induced skin eruptions and viral exanthems. J. Investig. Dermatol. 2007, 127, 802–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, E.C.; Lee, J.S.; Tan, A.W.; Tang, M.B.Y. Fas-ligand staining in non-drug and drug-induced maculopapular rashes. J. Cutan. Pathol. 2011, 38, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Hari, Y.; Frutig-Schnyder, K.; Hurni, M.; Yawalkar, N.; Zanni, M.P.; Schnyder, B.; Kappeler, A.; Von Greyerz, S.; Braathen, L.R.; Pichler, W.J. T-cell involvement in cutaneous drug eruptions. Clin. Exp. Allergy 2001, 31, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Bellini, V.; Pelliccia, S.; Lisi, P. Drug- and virus- or bacteria-induced exanthems: The role of immunohistochemical staining for cytokines in differential diagnosis. Dermatitis 2013, 24, 85–90. [Google Scholar] [CrossRef] [PubMed]
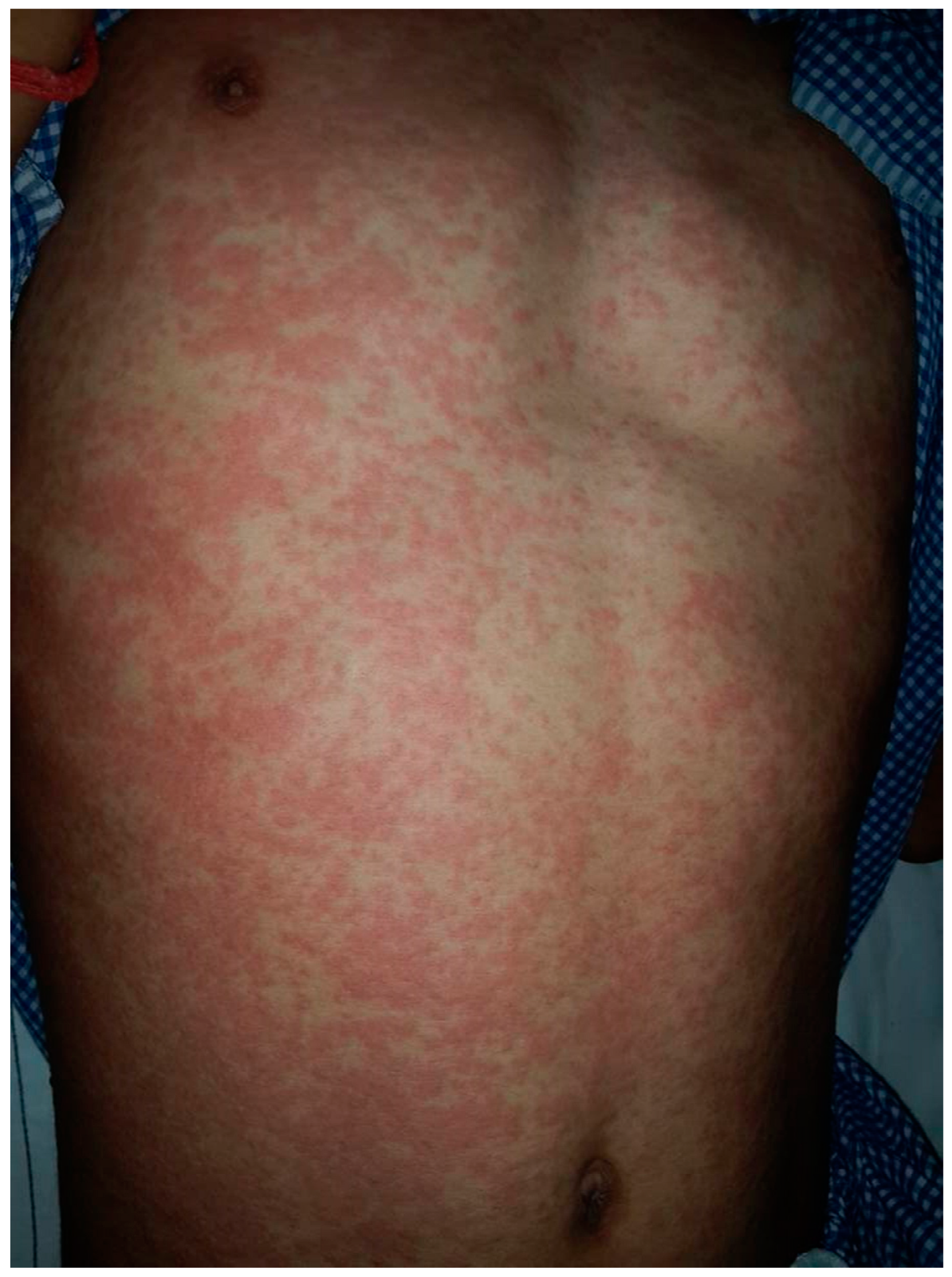
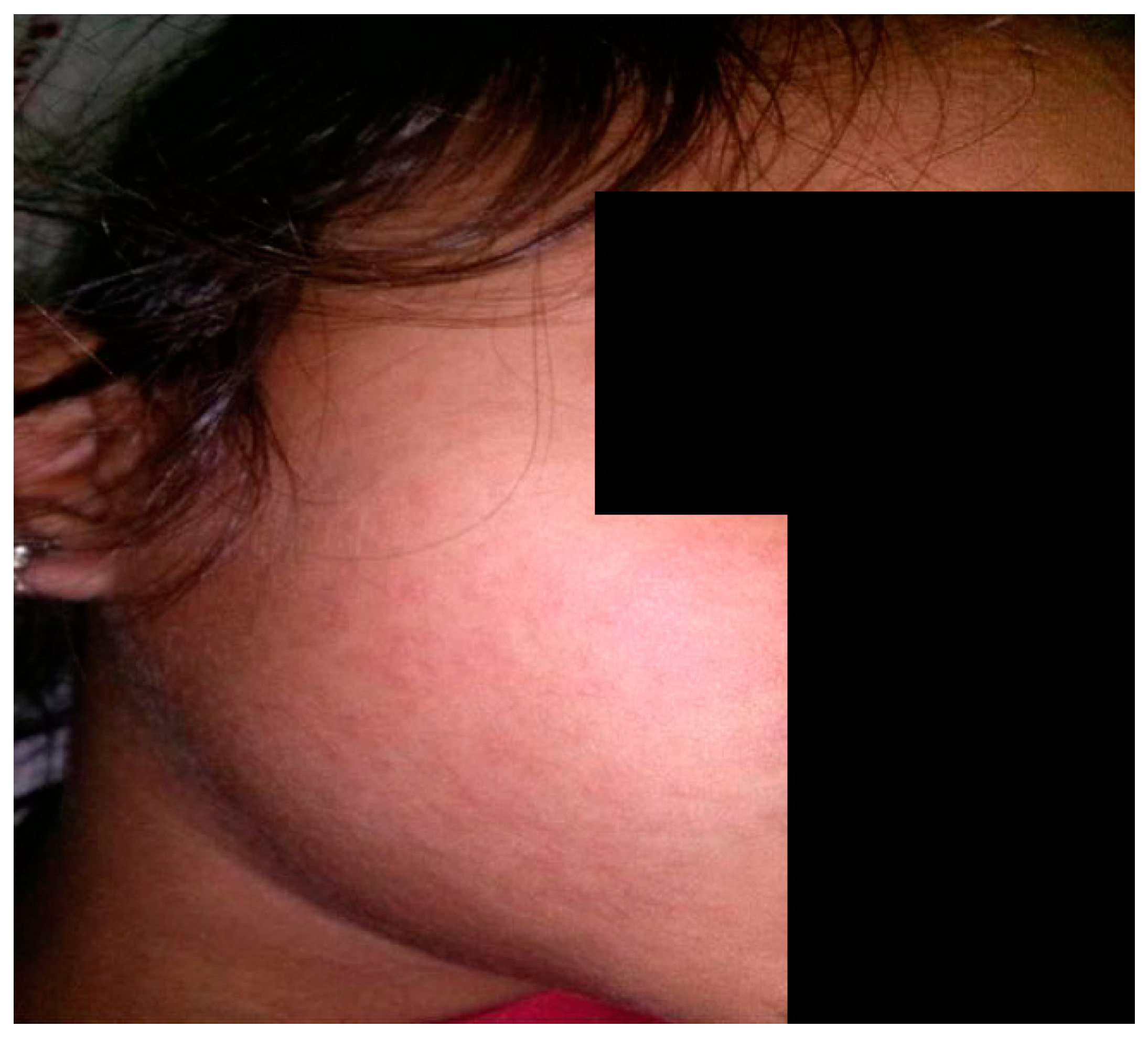
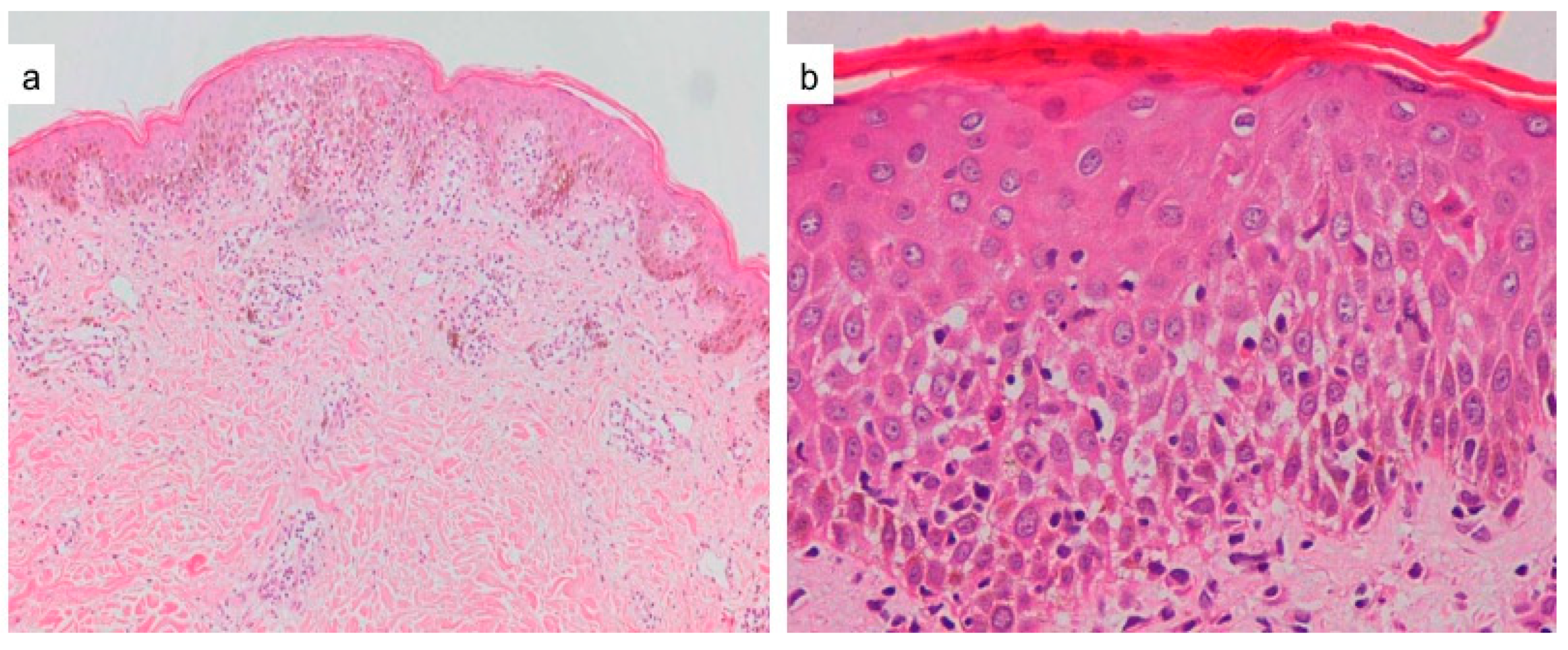
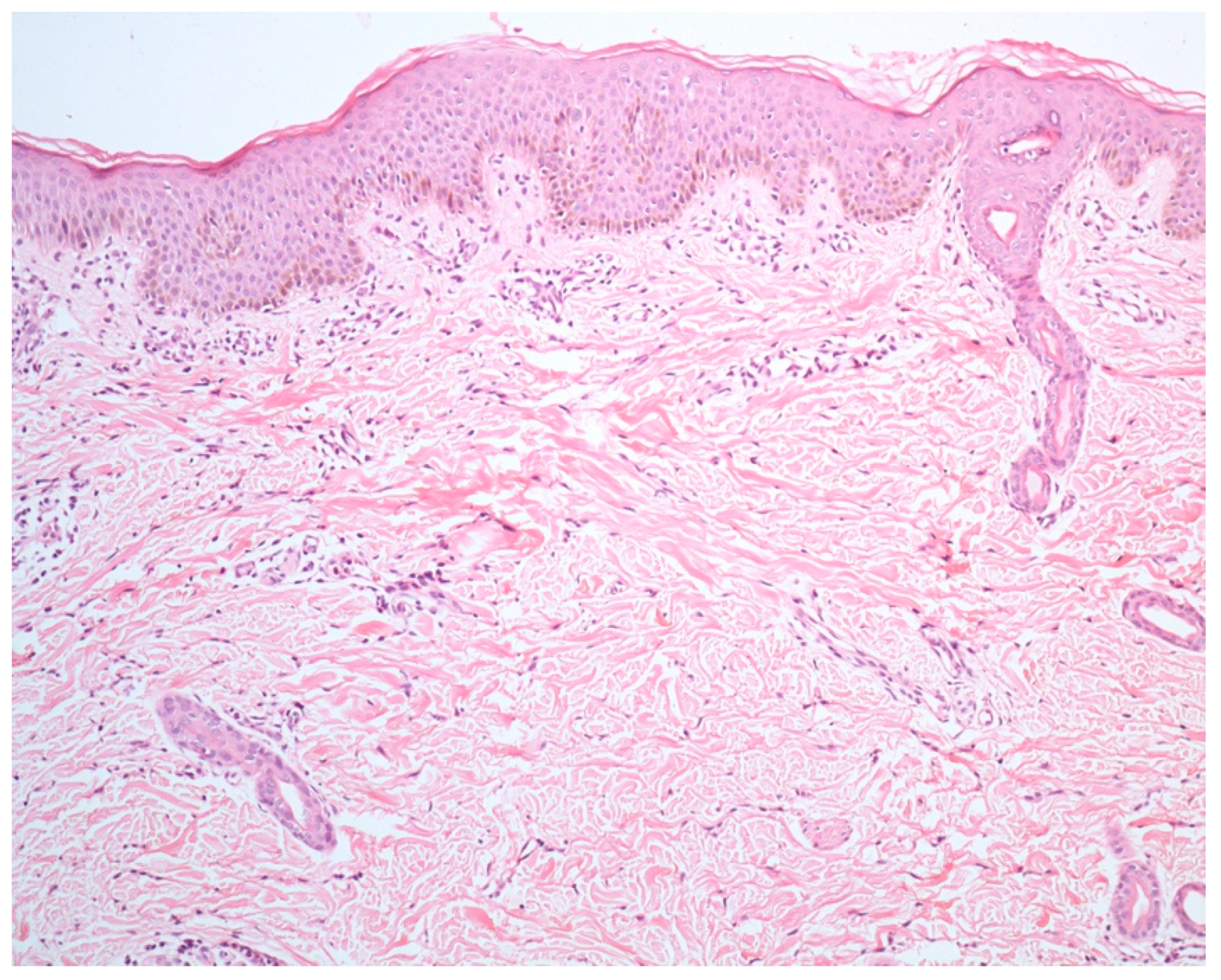
| Maculopapular Eruption | Drug-Induced | Viral-Induced |
|---|---|---|
| Clinical features | ||
| Usually absent | Fever, conjunctivitis, rhinorrhea, myalgia, arthralgia |
| Pruritic, may be confluent on dependant areas ± facial involvement | Usually non-pruritic, with patterned distribution ± enanthems |
| Haphazard distribution. Starts from trunk and proximal extremities | Cephalocaudal spread |
| Within 7–10 days after drug intake, improves on withdrawal, reappears on re-challenge | None |
| Hematological investigations | ||
| Higher median absolute eosinophil count | Lower median absolute eosinophil count |
| Histopathology | ||
| More common(50%), usually moderate-severe degree | Less common(16.8%), usually mild |
| None | Seen in some infections eg. ballooning and multinucleated keratinocytes in measles, keratinocytes with shrunken nuclei in infection by herpesviruses |
| More common (20.8% and 29%) Many necrotic keratinocytes and basal cell damage clue to a drug exanthem | Less common (4% and 8.3%) |
| More common (54–91.3%) | Less common (12.5%) |
| More common (45–62.5%) | Less common (12.5–20%) |
| More common | Less common |
| Immunohistochemical evaluation | ||
| Higher levels, role in recruiting eosinophils | Lower expression |
| Higher levels, expressed by infiltrating cytotoxic T cells | Lower levels |
| Levels disproportionately higher in patients with amoxicillin- induced drug eruption | Levels may be raised |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandpur, S.; Ahuja, R. Drug-Induced vs. Viral Maculopapular Exanthem—Resolving the Dilemma. Dermatopathology 2022, 9, 164-171. https://doi.org/10.3390/dermatopathology9020021
Khandpur S, Ahuja R. Drug-Induced vs. Viral Maculopapular Exanthem—Resolving the Dilemma. Dermatopathology. 2022; 9(2):164-171. https://doi.org/10.3390/dermatopathology9020021
Chicago/Turabian StyleKhandpur, Sujay, and Rhea Ahuja. 2022. "Drug-Induced vs. Viral Maculopapular Exanthem—Resolving the Dilemma" Dermatopathology 9, no. 2: 164-171. https://doi.org/10.3390/dermatopathology9020021
APA StyleKhandpur, S., & Ahuja, R. (2022). Drug-Induced vs. Viral Maculopapular Exanthem—Resolving the Dilemma. Dermatopathology, 9(2), 164-171. https://doi.org/10.3390/dermatopathology9020021





