PRAME Expression as Helpful Immunohistochemical Marker in Rhabdoid Melanoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection and Data Assessment
2.2. Immunohistochemistry
3. Results
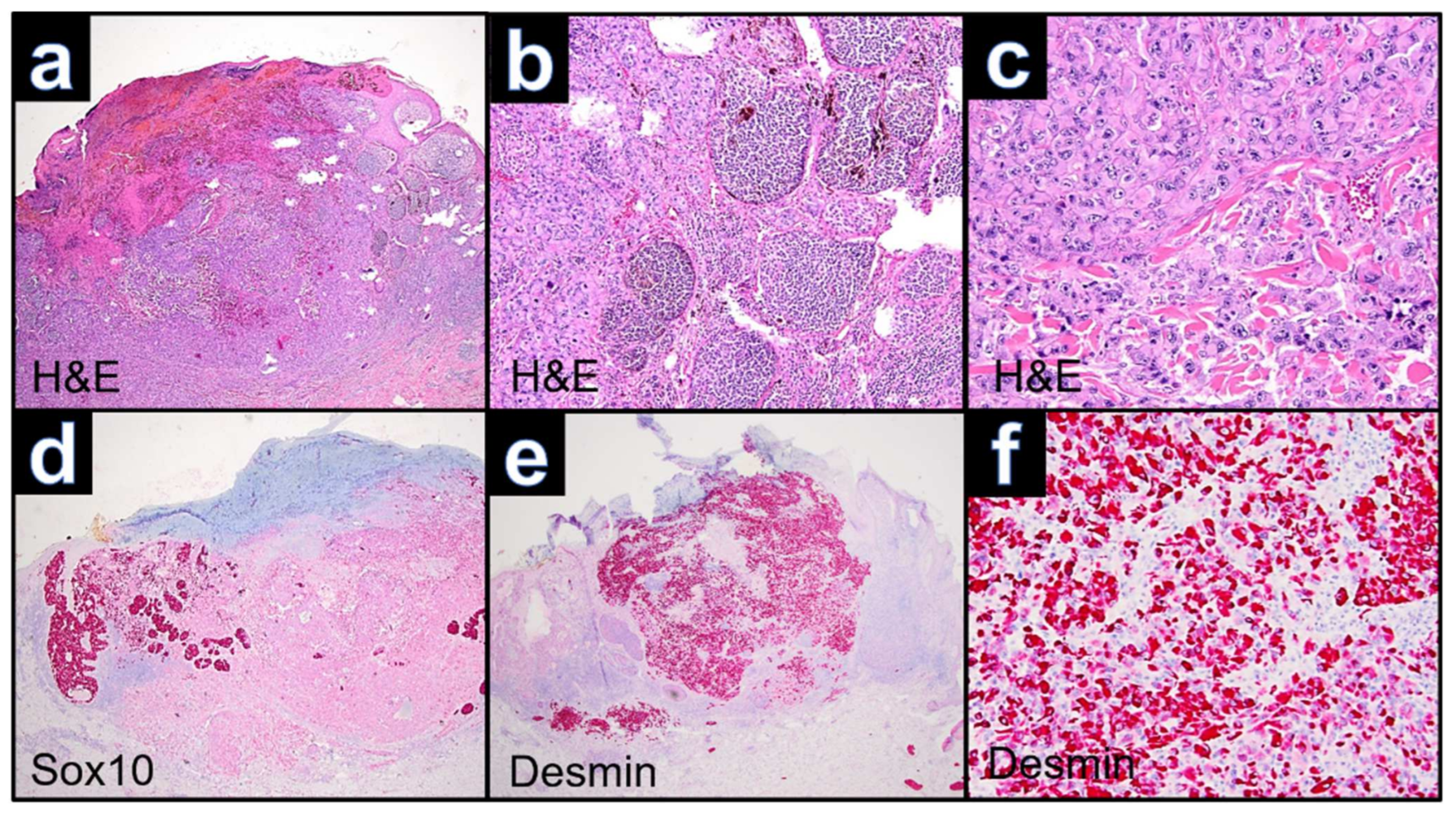
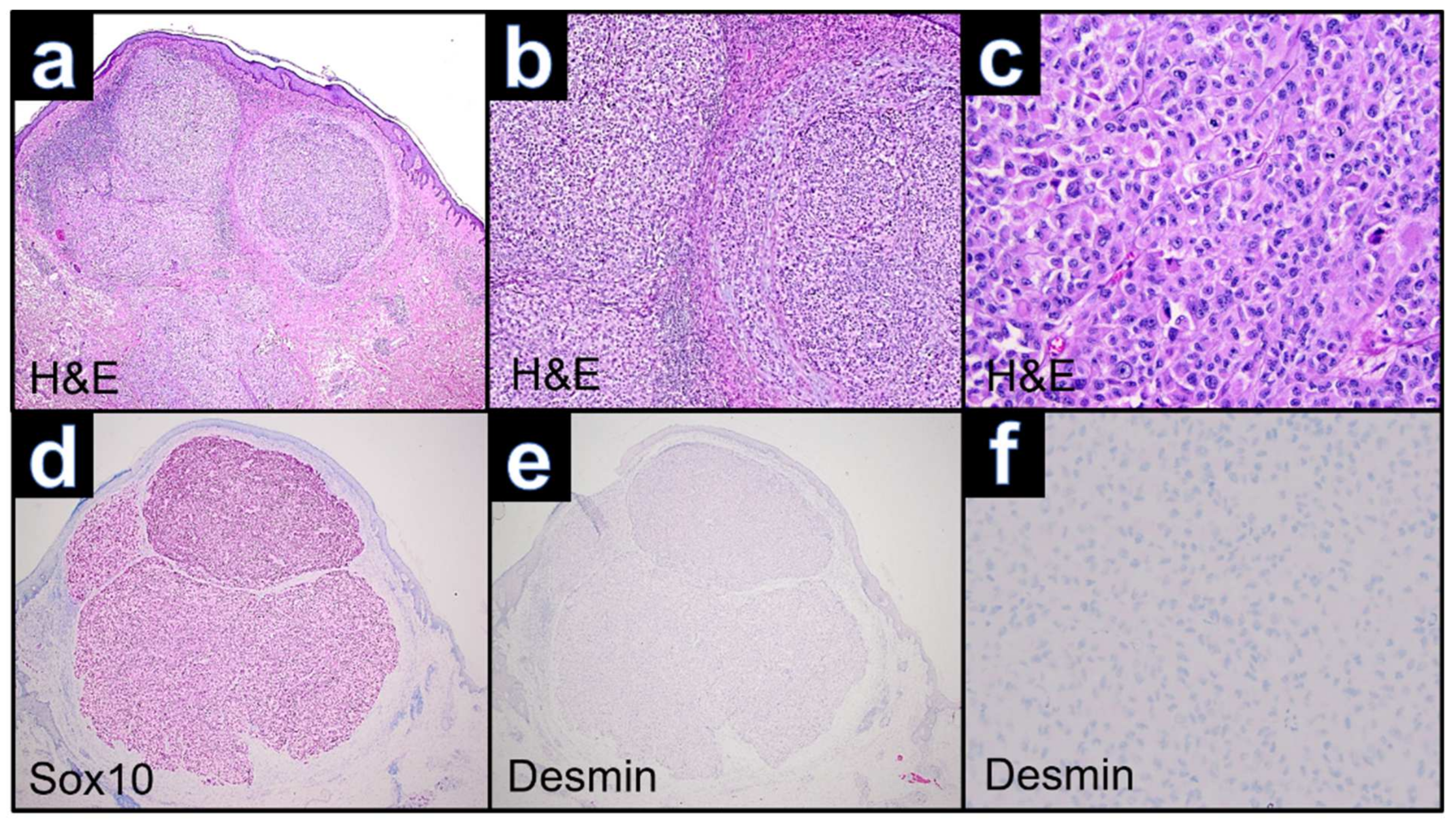
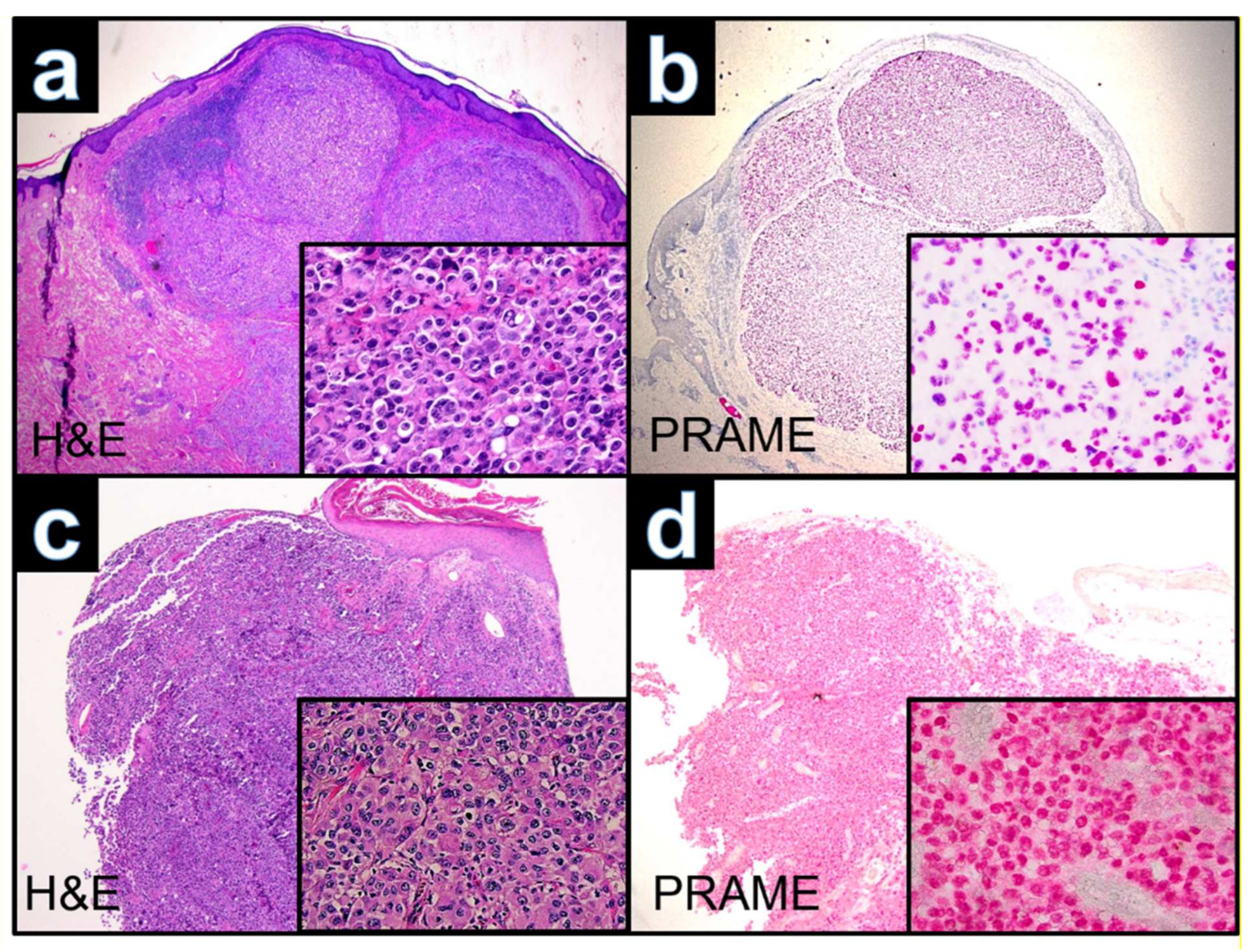
3.1. Case WUE1
3.2. Case WUE2
3.3. Case WUE3
3.4. Case WUE4
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, S.S.; Harris, M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology 2000, 36, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Bittesini, L.; Dei Tos, A.P.; Fletcher, C.D. Metastatic malignant melanoma showing a rhabdoid phenotype: Further evidence of a non-specific histological pattern. Histopathology 1992, 20, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Korekawa, A.; Akasaka, E.; Rokunohe, D.; Nakano, H.; Sawamura, D. Primary amelanotic rhabdoid melanoma: A case report with review of the literature. Case Rep. Derm. 2015, 7, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Gattenlohner, S.; Brocker, E.B.; Muller-Hermelink, H.K. Malignant melanoma with metastatic rhabdomyosarcomatoid transdifferentiation. N. Engl. J. Med. 2008, 358, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Borek, B.T.; McKee, P.H.; Freeman, J.A.; Maguire, B.; Brander, W.L.; Calonje, E. Primary malignant melanoma with rhabdoid features: A histologic and immunocytochemical study of three cases. Am. J. Derm. 1998, 20, 123–127. [Google Scholar] [CrossRef]
- Parham, D.M.; Weeks, D.A.; Beckwith, J.B. The clinicopathologic spectrum of putative extrarenal rhabdoid tumors. An analysis of 42 cases studied with immunohistochemistry or electron microscopy. Am. J. Surg. Pathol. 1994, 18, 1010–1029. [Google Scholar] [CrossRef]
- Tallon, B.; Bhawan, J. Primary rhabdoid melanoma with clonal recurrence. Am. J. Derm. 2009, 31, 200–204. [Google Scholar] [CrossRef]
- Chung, B.Y.; Ahn, I.S.; Cho, S.I.; Kim, H.O.; Kim, K.H.; Park, C.W.; Lee, C.H. Primary malignant rhabdoid melanoma. Ann. Derm. 2011, 23, S155–S159. [Google Scholar] [CrossRef] [Green Version]
- Ishida, M.; Iwai, M.; Yoshida, K.; Kagotani, A.; Okabe, H. Rhabdoid melanoma: A case report with review of the literature. Int. J. Clin. Exp. Pathol. 2014, 7, 840–843. [Google Scholar]
- Fernandez-Vega, I.; Santos-Juanes, J.; Fresno-Forcelledo, M.F. Primary amelanotic rhabdoid melanoma of the forehead. Br. J. Derm. 2016, 174, 1156–1158. [Google Scholar] [CrossRef]
- Murakami, T.; Ogata, D.; Arai, E.; Tsuchida, T. Case of primary hypomelanotic rhabdoid melanoma on the forehead. J. Derm. 2019, 46, e278–e279. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.J.; Folpe, A.L.; Giannini, C.; Perry, A. Pathology of peripheral nerve sheath tumors: Diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012, 123, 295–319. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.B.; Stevens, L.; Patterson, J.; Tannenbaum, M. Cutaneous epithelioid malignant nerve sheath tumor with rhabdoid features: A histologic, immunohistochemical, and ultrastructural study of three cases. J. Cutan. Pathol. 2000, 27, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Panse, G.; Subtil, A.; McNiff, J.M.; Glusac, E.J.; Ko, C.J.; Galan, A.; Myung, P.; Xu, M.L. Cutaneous involvement in plasma cell myeloma. Am. J. Clin. Pathol. 2021, 155, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Q.; Stanek, A.E.; Teichberg, S.; Shepard, B.; Kahn, E. Malignant rhabdoid tumor of the kidney in an adult: A case report and review of the literature. Arch. Pathol. Lab. Med. 2003, 127, e371–e373. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Z.; Wu, Y. Prame is a potential carcinogenic biomarker that correlates with patient prognosis and tumor immunity based on pan-cancer analysis. Ann. Clin. Lab. Sci. 2022, 52, 185–195. [Google Scholar]
- Lezcano, C.; Jungbluth, A.A.; Nehal, K.S.; Hollmann, T.J.; Busam, K.J. Prame expression in melanocytic tumors. Am. J. Surg. Pathol. 2018, 42, 1456–1465. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sipp, D.; Enomoto, H. Tissue interactions in neural crest cell development and disease. Science 2013, 341, 860–863. [Google Scholar] [CrossRef]
- Haas, J.E.; Palmer, N.F.; Weinberg, A.G.; Beckwith, J.B. Ultrastructure of malignant rhabdoid tumor of the kidney. A distinctive renal tumor of children. Hum. Pathol. 1981, 12, 646–657. [Google Scholar] [CrossRef]
- Gavino, A.C.; Gillies, E.M. Metastatic rhabdoid melanoma: Report of a case with a comparative review of the literature. J. Cutan. Pathol. 2008, 35, 337–342. [Google Scholar] [CrossRef]
- Fanburg-Smith, J.C.; Hengge, M.; Hengge, U.R.; Smith, J.S., Jr.; Miettinen, M. Extrarenal rhabdoid tumors of soft tissue: A clinicopathologic and immunohistochemical study of 18 cases. Ann. Diagn. Pathol. 1998, 2, 351–362. [Google Scholar] [CrossRef]


| Case | WUE1 | WUE2 | WUE3 | WUE4 |
|---|---|---|---|---|
| Age, years | 74 | 72 | 79 | 75 |
| Sex | male | female | male | male |
| MM subtype | NM | ALM | NM | NM |
| Location | chest | ankle | arm | scalp |
| Tumor thickness, mm | 3.8 | 5 | 5.8 | 8 |
| Metastasis at primary diagnosis | satellite metastasis | SLN and in-transit metastasis | no | in-transit metastasis |
| Follow-up | ||||
| Time to (further) metastasis (PFS) | 3 weeks until lymph node and distant metastases | 3 months until lung metastases, 6 months until brain metastases | no metastasis | no metastasis |
| Localization of metastases | sub-/cutaneous, lymph node, pleura | lung, brain | na | na |
| Treatment for metastatic disease | no | chemotherapy | na | na |
| OS after diagnosis | 2 months | 11 months | ||
| Time of follow-up without additional metastasis | - | - | lost to follow-up | 3 months |
| Case (Ref.) | 1 [4] | 2 [7] | 3 [3] | 4 [10] |
|---|---|---|---|---|
| Age, years | 41 | 74 | 63 | 80 |
| Sex | male | male | male | male |
| MM subtype | na | NM | NM | na |
| Location | scalp | back | sole | forehead |
| Tumor thickness, mm | 6 | 9 | 4 | 12 |
| Metastasis at primary diagnosis | no | satellite metastasis | SLN metastasis | in-transit metastasis |
| Follow-up | ||||
| Time to (further) metastasis (PFS) | 3 months until lymph node metastases | 10 months to local recurrence | 44 months until lung metastases | 1 month until lymph node metastases |
| Localization of metastases | lymph node | cutaneous, lung | lymph node, lung | cutaneous, lymph node |
| Treatment for metastatic disease | dacarbazine, radiotherapy | na | nivolumab | radiotherapy |
| OS after diagnosis | 6 months | na | na | 2 months |
| Case (Ref.) | WUE1 | WUE2 | WUE3 | WUE4 | 1 [4] | 2 [7] | 3 [3] | 4 [10] | 5 [5] | 6 [5] | 7 [5] | 8 [6] | 9 [8] | 10 [11] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amelanotic | no | no | no | no | na | na | yes | yes | yes | yes | yes | yes | no | yes |
| IHC— melanocytic markers in the rhabdoid areas of the primary | ||||||||||||||
| S100 | - | + | + | + | + | + | + | (+) | + | + | + | + | + | + |
| MART-1 | - | + | - | + | + | - | + | nd | nd | nd | nd | nd | nd | nd |
| SOX-10 | - | + | + | + | nd | nd | nd | (+) | nd | nd | nd | nd | nd | nd |
| HMB-45 | + (focal) | + | - | + | nd | nd | + | - | - | - | - | nd | + | - |
| IHC — mesenchymal markers in the rhabdoid areas of the primary | ||||||||||||||
| Vimentin | + | + | + | + | nd | + | + | + | + | + | + | + | + | + |
| Desmin | + | - | - | - | + | nd | - | (+) | - | - | - | nd | - | - |
| IHC — additional staining of the primary | ||||||||||||||
| PRAME | + | + | + | + | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glutsch, V.; Wobser, M.; Schilling, B.; Gesierich, A.; Goebeler, M.; Kneitz, H. PRAME Expression as Helpful Immunohistochemical Marker in Rhabdoid Melanoma. Dermatopathology 2022, 9, 148-157. https://doi.org/10.3390/dermatopathology9020019
Glutsch V, Wobser M, Schilling B, Gesierich A, Goebeler M, Kneitz H. PRAME Expression as Helpful Immunohistochemical Marker in Rhabdoid Melanoma. Dermatopathology. 2022; 9(2):148-157. https://doi.org/10.3390/dermatopathology9020019
Chicago/Turabian StyleGlutsch, Valerie, Marion Wobser, Bastian Schilling, Anja Gesierich, Matthias Goebeler, and Hermann Kneitz. 2022. "PRAME Expression as Helpful Immunohistochemical Marker in Rhabdoid Melanoma" Dermatopathology 9, no. 2: 148-157. https://doi.org/10.3390/dermatopathology9020019
APA StyleGlutsch, V., Wobser, M., Schilling, B., Gesierich, A., Goebeler, M., & Kneitz, H. (2022). PRAME Expression as Helpful Immunohistochemical Marker in Rhabdoid Melanoma. Dermatopathology, 9(2), 148-157. https://doi.org/10.3390/dermatopathology9020019






