1. Introduction
Granulomatous cutaneous T-cell lymphoma (GCTCL) represents a rare variant of cutaneous lymphomas, accounting for only 2% of all cases [
1,
2]. First described in 1970 by Ackerman and Flaxman, it is characterized histologically by a predominantly histiocytic and/or granulomatous infiltrate that often obscures the underlying malignant lymphocytes, therefore often leading to misdiagnosis as well as delay in diagnosis and treatment [
3,
4,
5]. GCTCL presents a diagnostic challenge due to its rarity and clinicopathological heterogeneity. While the granulomatous slack skin variant of GCTCL is a distinct clinical subtype with bulky pendulous skin folds, the granulomatous mycosis fungoides (GMF) subtype has no distinct clinical presentation [
4,
5].
The exact pathogenic mechanisms of granuloma formation in GCTCL remains poorly understood. Specifically, there is limited data describing the microenvironment composition of granulomas in GCTCL. Macrophages present in granulomas are polarized into either a pro-inflammatory (M1) or an anti-inflammatory (M2) subtype. M1 macrophages are associated with a cytotoxic TH1 response and secrete reactive oxygen species that have microbicidal, inflammatory, and anti-tumor functions [
6]. Conversely, M2 macrophages are associated with an immunoregulatory TH2 response responsible for the secretion of growth factors and inhibition of cell death pathways, which can shield tumor cells from the effects of chemotherapy [
6]. Prior studies have associated a higher M1/M2 ratio with a favorable outcome and better response to treatment when compared to a lower M1/M2 ratio in pediatric classical Hodgkin Lymphoma, ovarian cancer, locally advanced cervical cancer, breast cancer, lung cancer, hepatocellular carcinoma, and gastric cancer [
6]. While the M1/M2 ratio of tumor associated macrophages (TAMs) has been shown to have prognostic significance in different cancer types with a prominent granulomatous component, similar studies are lacking in GCTCL [
6,
7].
Herein, we aim to characterize the TAMs that predominate in GCTCL to better understand the significance of this histologic finding.
2. Materials and Methods
This study was approved by the Mayo Clinic Institutional Review Board. Within a thirteen-year period from 2007 to 2020, a total of six cases with a definitive diagnosis of granulomatous cutaneous T-cell lymphoma (GCTCL) were identified.
Clinical information was extracted from the electronic medical record including age, gender, race, date of presentation at our practice, date of GCTCL diagnosis, lesion morphology, clinical images, treatment and follow up data. All available pathology slides and ancillary studies including molecular study by polymerase chain reaction for T cell receptor gene rearrangement were reviewed by a dermatopathologist (OS) and hematopathologist (LJ) with expertise in the diagnosis of cutaneous lymphomas. Histological features were assessed including predominant patterns, composition of cellular infiltrates, distribution and density of the atypical lymphocytic infiltrates, and dermal stromal changes.
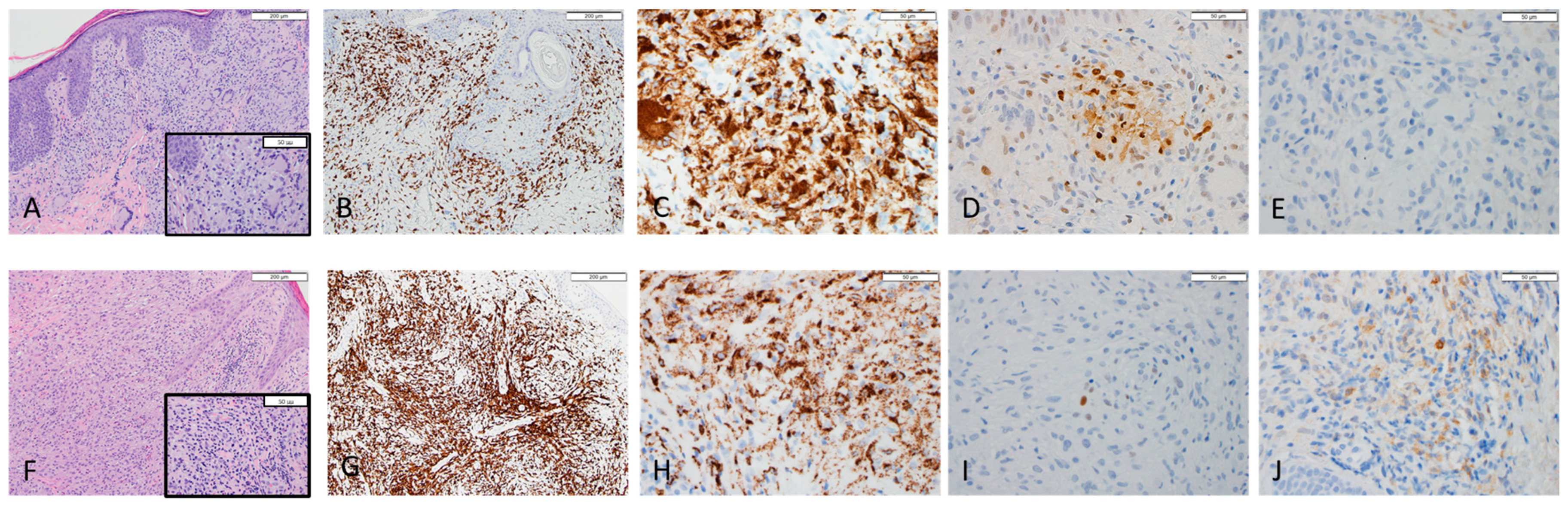
Besides the diagnostic immunostaining markers (CD3, CD20, CD4, CD5, CD7, CD8), additional immunohistochemistry (IHC) studies were performed on Leica and Ventana platforms to characterize M1 and M2 macrophage polarization. CD68 (PGM1) (clone PG-M1, DAKO), pSTAT1 (clone 58D6, Cell Signaling), and CD163 (clone EPR19518, Abcam) were used as pan macrophage, M1, and M2 markers, respectively. Appropriate positive and negative controls were used to ensure the quality of IHC stain and antibody titration. PGM1, pSTAT1, and CD163 immunostaining slides were reviewed by the hematopathologist (LJ); the macrophages with positive staining at hot spot per high power field were counted and recorded for data analysis.
Despite the small sample size for any significant biostatistical conclusion, we summarized the variables to describe the pattern of association between M1–M2 difference and patient age, race, and stage. Categorical variables were summarized as frequency (percentage) and continuous variables were reported as median (range). M1 predominance is defined as M1 positive cells more than M2 with a difference of ≥10 cells/HPF; and M2 predominance is defined as M2 more than M1 with a difference of ≥10 cells/HPF. Stage I to IIa was grouped as early stage mycosis fungoides, and stage IIb to IV as advanced stage mycosis fungoides. The analysis was done using R3.6.2.
3. Results
3.1. Clinical Features
A summary of the clinical features in the six GCTCL patients studied are reviewed in
Table 1. Five patients (83%) were women and one (17%) was a man. The average age was 60.5 years [range, 21–78]. Five patients were Caucasian and one was Black/African American. Three patients presented with early stage GCTCL (Stage Ia-IIa) and three with advanced disease (stage IIb-IVa). The lesion morphology varied and included macules (2/6 cases), patches (5/6 cases), plaques (2/6 cases), and papules (1/6 cases). Distribution of lesion included generalized with involvement of the face (3/6), trunk, arms, and legs (2/6), and trunk and legs (1/6). Patients received a variety of standard therapies including topical corticosteroids, topical retinoids, methotrexate, imiquimod, radiation therapy, and phototherapy. The mean follow-up time after presentation was 7 years. One patient was lost to follow up (patient 1). This patient was recommended CHOP therapy by an outside hematologist/oncologist; however, treatment completion status and outcome data are not available. One patient (patient 5) died with their disease; no data is available regarding the cause of death. This patient had a progressive disease course despite trying six different therapies over the course of four years. Only one out of the six patients achieved complete tumor regression, and three out of six experienced disease progression despite being on multiple treatment modalities.
3.2. Pathological Features
Histopathologic and immunohistochemical findings in cutaneous specimens studied are presented in
Table 1. Representative images of cases #2 and #3 are shown in
Figure 1. Histologic patterns observed consisted of diffuse (2/6), perivascular (2/6), interstitial (1/6), and folliculotropic (2/6) infiltrate. Immunohistochemistry of all pathology slides was positive for PGM1 marker with cytoplasmic staining pattern confirming the presence of a histiocytic and/or granulomatous response in all cases evaluated. There are two patients (case #1 and #3) with M2 predominance (positive for CD163 with cytoplasmic stain) and the other three (case #2, #4, and #6) with M1 predominance (positive for pSTAT1 with nuclear stain). One case (case #5) has both M1 and M2 cells less than 10/HPF; therefore, we have eliminated that case from the final statistical analysis. The clinical correlation with macrophage polarization is summarized in
Table 2.
4. Discussion
Macrophages are present in most tissues as professional phagocytic and immunomodulating cells essential for maintaining host homeostasis. Their role includes antimicrobial activity against various pathogens, tissue repair, and even tumor modulation [
8]. Macrophages exist in tissues as a heterogenous cell population influenced by their environment and differing in their response to inflammatory cytokines. In the early 2000s, Mills et al. proposed the polarization of macrophages into M1 and M2 [
9]. M1 polarized macrophages, also known as classically activated macrophages, are described as pro-inflammatory with a role in pathogen resistance and tissue destruction [
9]. M1 macrophages are activated by INF y, lipopolysaccharide (LPS, a component of the outer membrane of bacteria), and granulocyte macrophage colony stimulating factor (GCSF [
6]. On the other hand, M2 polarized macrophages, also known as alternatively activated macrophages, are anti-inflammatory and respond to IL-4 and TGF-B1 [
6]. M2 macrophages have a role in host immune response against parasites and in repair after acute tissue damage [
6].
Tumor associated macrophages (TAMs) has also been described as having an important role in tumor modulation [
9]. TAMs that exhibit an M2 phenotype are linked to tumor proliferation, invasion, metastasis, and inhibition of anti-tumor immune response of T cells. [
9]. A recent meta-analysis of Non-Hodgkin lymphoma found that the type of TAMs found within a tumor microenvironment served as a prognostic indicator [
10]. Tumors with high density of CD163+, a specific marker for M2 polarized macrophages, were associated with lower overall survival (OS) and a low progression-free survival (PFS) [
10]. Increased number of CD163+ cells and serum soluble CD163 were observed in CTCL, atopic dermatitis, and psoriasis comparing with normal skin; increased lesion macrophages also been associated with disease progression and an overall worse prognosis in CTCL [
11]. Moreover, although the exact mechanisms remain unknown, chemotherapy agents targeted on TAMs via reprograming M2 macrophages or promoting antitumor activities have proven effective in various hematopoietic malignancies including multiple myeloma and CNS lymphomas [
12,
13,
14,
15].
While several tumors with a high density of M2 polarized macrophages have been associated with worse prognosis, there are no studies in the literature that examine the macrophage polarization in granulomatous mycosis fungoides. The GMF cases studied here showed a predominant M2 macrophage polarization in 2/6 cases and M1 macrophage predominant in 3/6 cases. All cases with M1 polarization had an early disease stage, whereas cases with M2 polarization had advanced disease. Our study found that M2 polarization was only present in patients with more advanced disease stage. One possible explanation is that, as seen in previously reported malignancies, patients with an M2 predominance have a more aggressive disease course [
6,
10]. As such, M1 macrophages in GMF likely represent a pro-inflammatory response that leads to suppression of T-cell lymphoma. Another possibility is that in the early stages GMF might be associated with a pro-inflammatory M1 infiltrate that is then altered into an anti-inflammatory, pro-tumor M2 response as the disease progresses. More studies are needed to further explore this relationship and elucidate the prognostic value of macrophage polarization. Larger studies are needed to examine how disease progression might differ in patients with M1 versus M2 predominance.
We found no relationship between patient’s age and macrophage predominance. Additionally, patients presented with varied lesion location and morphology, which was not related to the type of macrophage predominance observed within the granulomas. No relationship was observed between gender or race and macrophage predominance.
This study is limited by the small number of cases given the rarity of GMF. Larger studies are needed to understand how the microenvironment found within the infiltrate of GMF might have important implications for the treatment and prognostication of this condition. In addition, future development of treatment for GMF might focus on targeting the microenvironment of the tumor to either accentuate or attenuate the M1 or M2 response, respectively.
5. Conclusions
Our study suggests that macrophage polarization present in GMF tends to be M1 in early stage and M2 in advanced stages. Additional studies are needed to further elucidate the microenvironment of macrophages present in GMF and to learn how they impact the progression of disease. Such findings will lead to important prognostic and therapeutic advances in GMF.
Author Contributions
Conceptualization, O.S. and L.J.; methodology, L.D.J., M.M.H., O.S. and L.J.; validation, O.S. and L.D.J.; formal analysis, L.D.J., M.M.H., O.S. and L.J.; investigation, L.D.J., M.M.H., O.S. and L.J.; resources, L.D.J., M.M.H., O.S. and L.J.; data curation, O.S., and L.J.; writing—original draft preparation, L.D.J., M.M.H., O.S. and L.J.; writing—review and editing, L.D.J., M.M.H., O.S. and L.J.; visualization, L.D.J., M.M.H., O.S. and L.J.; supervision, O.S. and L.J.; project administration, O.S. and L.J.; funding acquisition, L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Mayo Clinic Institutional Review Board, IRB number 20-012654.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, and patients’ ID are not disclosed and can’t not be identified.
Data Availability Statement
Data is available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gallardo, F.; García-Muret, M.P.; Servitje, O.; Estrach, T.; Bielsa, I.; Salar, A.; Abella, E.; Barranco, C.; Pujol, R.M. Cutaneous lymphomas showing prominent granulomatous component: Clinicopathological features in a series of 16 cases. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Scarabello, A.; Leinweber, B.; Ardigó, M.; Rütten, A.; Feller, A.C.; Kerl, H.; Cerroni, L. Cutaneous lymphomas with prominent granulomatous reaction: A potential pitfall in the histopathologic diagnosis of cutaneous T- and B-cell lymphomas. Am. J. Surg. Pathol. 2002, 26, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.B.; Flaxman, B.A. Granulomatous mycosis fungoides. Br. J. Dermatol. 1970, 82, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Pulitzer, M.P.; Myskowski, P.L.; Dusza, S.W.; Horwitz, S.; Moskowitz, A.; Querfeld, C. A case-control study of clinicopathologic features, prognosis, and therapeutic responses in patients with granulomatous mycosis fungoides. J. Am. Acad. Dermatol. 2013, 69, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kempf, W.; Ostheeren-Michaelis, S.; Paulli, M.; Lucioni, M.; Wechsler, J.; Audring, H.; Assaf, C.; Rüdiger, T.; Willemze, R.; Meijer, C.J.L.M.; et al. Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer. Granulomatous mycosis fungoides and granulomatous slack skin: A multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer (EORTC). Arch. Dermatol. 2008, 144, 1609–1617. [Google Scholar] [PubMed] [Green Version]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, M.H.M.; Segges, P.; Vera-Lozada, G.; Hassan, R.; Niedobitek, G. Macrophage Polarization Reflects T Cell Composition of Tumor Microenvironment in Pediatric Classical Hodgkin Lymphoma and Has Impact on Survival. PLoS ONE 2015, 10, e0124531. [Google Scholar]
- Lee, K.Y. M1 and M2 polarization of macrophages: A mini-review. Med. Biol. Sci. Eng. 2019, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Galán, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages. Front. Immunol. 2015, 6, 263. [Google Scholar] [PubMed]
- Xu, X.; Li, Z.; Liu, J.; Zhu, F.; Wang, Z.; Wang, J.; Zhang, J.; Wang, H.; Zhai, Z. The prognostic value of tumour-associated macrophages in Non-Hodgkin’s lymphoma: A systematic review and meta-analysis. Scand. J. Immunol. 2019, 91, e12814. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, M.; Miyagaki, T.; Ohmatsu, H.; Suga, H.; Kai, H.; Kamata, M.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; et al. Association of the numbers of CD163+ cells in lesional skin and serum levels of soluble CD163 with disease progression of cutaneous T cell lymphoma. J. Dermatol. Sci. 2012, 68, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiu, Y.; Personett, D.; Huang, P.; Edenfield, B.; Katz, J.; Babusis, D.; Tang, Y.; Shirely, M.A.; Moghaddam, M.F.; et al. Pomalidomide Shows Significant Therapeutic Activity against CNS Lymphoma with a Major Impact on the Tumor Microenvironment in Murine Models. PLoS ONE 2013, 8, e71754. [Google Scholar] [CrossRef] [PubMed]
- Busch, L.; Mougiakakos, D.; Büttner-Herold, M.; Müller, M.J.; Volmer, D.A.; Bach, C.; Fabri, M.; Bittenbring, J.T.; Neumann, F.; Boxhammer, R.; et al. Lenalidomide enhances MOR202-dependent macrophage-mediated effector functions via the vitamin D pathway. Leukemia 2018, 32, 2445–2458. [Google Scholar] [CrossRef]
- Gutiérrez-González, A.; Martínez-Moreno, M.; Samaniego, R.; Arellano-Sánchez, N.; Salinas-Muñoz, L.; Relloso, M.; Valeri, A.; Martínez-López, J.; Corbí, L.; Hidalgo, A.; et al. Evaluation of the potential therapeutic benefits of macrophage reprogramming in multiple myeloma. Blood 2016, 128, 2241–2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronte, V.; Murray, P.J. Understanding Local Macrophage Phenotypes In Disease: Modulating macrophage function to treat cancer. Nat. Med. 2015, 21, 117–119. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).